To the editor:
Arsenic trioxide (ATO) and all-trans retinoic acid (ATRA) have demonstrated notable success in the treatment of acute promyelocytic leukemia (APL).1-3 However, few studies have reported the adverse effects (AEs), long-term toxicity, and secondary carcinogenesis of ATO. The Shanghai Institute of Hematology has focused on the long-term survival and safety of ATO in patients with APL. In a 5-year follow-up study, Hu et al2 demonstrated high efficacy of ATRA/ATO combination therapy with no reports of chronic AEs or secondary tumors, but ATO retention was slightly greater than in healthy controls. In this study, we followed 265 patients with newly diagnosed APL treated with ATRA and ATO to assess the long-term survival, chronic AE profile, and total arsenic (TAs) retention.
Between January 2001 and June 2012, 265 patients with newly diagnosed APL were included in this study. Diagnosis was performed as previously described.1 All patients received ATRA and ATO with or without chemotherapy, according to the protocol previously reported.1,2 By July 1, 2012, 217 patients had completed treatment. Between July 1 and August 31, 2012, 112 patients participated in an assessment with informed consent. In addition, an age- and gender-matched group of 112 healthy individuals was enrolled as healthy controls. AEs were reported according to the Common Terminology Criteria for Adverse Events Version 4.0 (National Cancer Institute; http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf). Patient quality of life (QoL) was evaluated using the European Organization for Research and Treatment of Cancer (EORTC) core QoL questionnaire (QLQ-C30, version 3.0).4 Skin lesions and secondary cancer, if already recovered, were recorded according to the patients’ description or previous discharge summary. Blood, urine, hair, and nail samples of patients (n = 112), healthy controls (n = 112), and patients with APL undergoing ATO treatment (n = 7) were collected. Inductively coupled plasma mass spectrometry was used for the detection of TAs at the Analysis Center of Tsinghua University (see supplemental Data, available on the Blood Web site).
Event-free survival (EFS), overall survival (OS), and disease-free survival (DFS) were estimated using the Kaplan-Meier method and compared by log-rank test. Questionnaires for QoL were scaled using the EORTC QLQ-C30 Scoring Manual.5 The χ2 test or Fisher’s exact test were used to compare the incidence of chronic AEs between patients and healthy controls. The concentration of arsenic was compared by Kolmogorov-Smirnov test. All statistical analyses were performed using the Statistical Package for the Social Science v20.0.
Of the 265 patients enrolled, 178 received chemotherapy during the induction period. Eighteen patients (6.8%) died during induction, including 12 cases of intracranial hemorrhage, 3 of disseminated intravascular coagulation, 2 of differentiation syndrome, and 1 of cerebral infarction. Another 2 patients failed to reach complete remission (CR). Both of these patients were PML-RARA positive; 1 had extramedullary infiltration, including the central nervous system and the auricle at diagnosis, and the other had severe pulmonary infection during bone marrow suppression, leading to cessation of ATRA and ATO on day 18. They died at month 6 and month 2, respectively. A total of 245 (92.5%) patients achieved CR. In the consolidation and maintenance phases, 4 patients relapsed and died. By September 30, 2015, another 17 patients had relapsed, including 4 relapses that occurred 5 years after initial treatment. Five of the patients died after a median of 5 months (range, 0-43 months) from relapse.
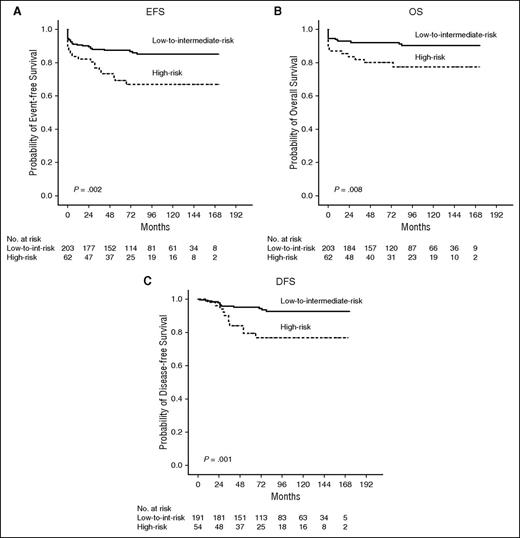
With a median follow-up of 83 months (range, 0-173 months), the estimated 12-year EFS, OS, and DFS were 80.9%, 87.4%, and 89.1%, respectively. White blood cell (WBC) count was the only unfavorable prognostic factor for DFS (P = .002; see supplemental Table 1). In the low-to-intermediate–risk group (WBC count ≤10 × 109/L), the 12-year EFS, OS, and DFS were 85.2%, 90.4%, and 92.6%, respectively, whereas in the high-risk group (WBC count >10 × 109/L), they were 67.0%, 77.5%, and 76.8%, respectively (P = .002, .008, and .001, respectively; Figure 1).
Major abnormalities and arsenic retention of patients
| Abnormalities . | Patients, n (%) . | Healthy controls, n (%) . | P value . |
|---|---|---|---|
| Lower WBC count | 1 (0.9)* | 0 | 1.000 |
| Cardiovascular events | |||
| Elevated myocardial enzymes | 5 (4.5)† | 1 (0.9) | .212 |
| Long Q-T interval | 0 | 0 | NA |
| T wave change | 14 (12.5) | 15 (13.4) | .842 |
| Echocardiogram abnormality | 1 (0.9)‡ | 0 | 1.000 |
| Liver dysfunction | |||
| Liver dysfunction, grade 1¶ | 17 (15.2) | 2 (1.8) | <.001 |
| Hepatic steatosis | 48 (42.9) | 20 (17.9) | <.001 |
| Kidney and GI dysfunction | |||
| Elevated creatinine | 0 | 0 | NA |
| Albuminuria | 1 (0.9)‡ | 0 | 1.000 |
| Fecal occult blood test | 0 | 0 | NA |
| Diabetes | 6 (5.4) | 5 (4.5) | .757 |
| Neurological disorders | 1 (0.9)§ | 1 (0.9)‖ | 1.000 |
| Potential secondary tumor | |||
| Elevated serum tumor markers | 3** | NA | NA |
| Thoracic neoplasm on CXR | 0 | 0 | NA |
| Abdominal neoplasm on BUS | 0 | 0 | NA |
| Skin lesion | 8 (7.1)†† | 5 (4.5)‡‡ | .391 |
| Breast cancer | 1 (0.9) | 0 | 1.000 |
| Arsenic retention, mean ± SD | |||
| Plasma, ng/g | 6.43 ± 1.56 | 8.99 ± 1.14 | <.001 |
| Urine, ng/mL | 45.28 ± 33.28 | 57.55 ± 33.61 | .004 |
| Hair, ng/g¶¶ | 195.43 ± 202.25 | 198.23 ± 133.16 | .340 |
| Nails, ng/g¶¶ | 294.65 ± 158.94 | 338.80 ± 212.27 | .761 |
| Abnormalities . | Patients, n (%) . | Healthy controls, n (%) . | P value . |
|---|---|---|---|
| Lower WBC count | 1 (0.9)* | 0 | 1.000 |
| Cardiovascular events | |||
| Elevated myocardial enzymes | 5 (4.5)† | 1 (0.9) | .212 |
| Long Q-T interval | 0 | 0 | NA |
| T wave change | 14 (12.5) | 15 (13.4) | .842 |
| Echocardiogram abnormality | 1 (0.9)‡ | 0 | 1.000 |
| Liver dysfunction | |||
| Liver dysfunction, grade 1¶ | 17 (15.2) | 2 (1.8) | <.001 |
| Hepatic steatosis | 48 (42.9) | 20 (17.9) | <.001 |
| Kidney and GI dysfunction | |||
| Elevated creatinine | 0 | 0 | NA |
| Albuminuria | 1 (0.9)‡ | 0 | 1.000 |
| Fecal occult blood test | 0 | 0 | NA |
| Diabetes | 6 (5.4) | 5 (4.5) | .757 |
| Neurological disorders | 1 (0.9)§ | 1 (0.9)‖ | 1.000 |
| Potential secondary tumor | |||
| Elevated serum tumor markers | 3** | NA | NA |
| Thoracic neoplasm on CXR | 0 | 0 | NA |
| Abdominal neoplasm on BUS | 0 | 0 | NA |
| Skin lesion | 8 (7.1)†† | 5 (4.5)‡‡ | .391 |
| Breast cancer | 1 (0.9) | 0 | 1.000 |
| Arsenic retention, mean ± SD | |||
| Plasma, ng/g | 6.43 ± 1.56 | 8.99 ± 1.14 | <.001 |
| Urine, ng/mL | 45.28 ± 33.28 | 57.55 ± 33.61 | .004 |
| Hair, ng/g¶¶ | 195.43 ± 202.25 | 198.23 ± 133.16 | .340 |
| Nails, ng/g¶¶ | 294.65 ± 158.94 | 338.80 ± 212.27 | .761 |
BUS, B-ultrasound; CA125, serum carbohydrate antigen 125; CEA, carcino embryonic antigen; CXR, chest X-ray; GI, gastrointestinal; NA, not available; NSE, neuron specific enolase; SD, standard deviation; WBC, white blood cells.
One patient was later diagnosed as the third relapse of APL.
No acute myocardial infarction. 2 had histories of heart diseases before APL.
One patient had rheumatic heart disease 1 wk after initial therapy, who also had albuminuria probably as a result of diabetes.
Only grade 1 liver dysfunction was observed in patients and healthy controls.
One patient had depression before APL that was well controlled by medication.
One patient had essential tremor.
Three patients had a mild and transient elevation in NSE, CEA, and CA125, respectively, and retests were normal.
Two patients had hyperpigmentation, 1 had hypopigmentation, and 5 had hyperkeratosis/hyperplasia.
Two had hyperpigmentation and 3 had hyperkeratosis.
Patients within 6 mo off ATO were not included because of high retention of arsenic.
Risk-adapted survival analysis. The estimated 12-year EFS (A), OS (B) for all 265 patients, and the 12-year DFS (C) for 245 patients in CR, showing significant difference between low-to-intermediate–risk and high-risk patients (P = .002, .008, and .001, respectively).
Risk-adapted survival analysis. The estimated 12-year EFS (A), OS (B) for all 265 patients, and the 12-year DFS (C) for 245 patients in CR, showing significant difference between low-to-intermediate–risk and high-risk patients (P = .002, .008, and .001, respectively).
The assessment of 112 patients is presented in Table 1. Seventeen (15.2%) patients had grade 1 liver dysfunction, and 48 (42.9%) had hepatic steatosis; none of them had previous hepatitis. Only 1 patient documented breast cancer 3 years after termination of ATO. Eight patients developed hyperpigmentation, hypopigmentation, or hyperkeratosis/hyperplasia. All these skin lesions occurred during maintenance therapy or within 6 months after treatment, and patients recovered within 2 to 18 months. In terms of patient QoL, the mean scores (±95% confidence interval) for Global Health Status/QoL, functional scale, and symptom scale were 79.2 (76.0-82.5)/100, 92.7 (91.0-94.5)/100, and 6.9 (5.3-8.5)/100, respectively. The chief complaints were mild to moderate weakness (55.4%), degenerated memory (41.1%), and financial difficulties (33.0%).
Plasma and urine TAs levels were significantly elevated during ATO infusion. On cessation of ATO, they rapidly turned normal within 0 to 6 months, which was no higher and even lower than healthy controls (6.43 ± 1.56 vs 8.99 ± 1.14 ng/g in plasma [P < .001] and 45.28 ± 33.28 vs 57.55 ± 33.61 ng/mL in urine [P = .004]). However, TAs levels in hair and nails revealed a delayed increase, and decreased to normal and stable levels after 6 months, which was comparable with the healthy controls (195.43 ± 202.25 vs 198.23 ± 133.16 ng/g in hair and 294.65 ± 158.94 vs 338.80 ± 212.27 ng/g in nails; both P > .05; see supplemental Figure 1).
ATRA and ATO combination therapy for APL has brought significant advantages in laboratory6 and clinical studies.2 In our study, high WBC count was the only unfavorable factor for relapse. When compared with the long-term survival rates of ATRA plus chemotherapy, DFS of the low-to-intermediate–risk group in our study appeared to be higher (92.6% vs 85.9% in AIDA20007 and 82.1% in a German study8 ), which might be benefited from ATO therapy. However, ATO appears to have a limited contribution to the high-risk group, indicating that the current regimen may not be sufficient to completely eliminate APL-initiating cells.
ATO is also considered a potential toxicant and carcinogen.9-14 In our study, the common signs of chronic arseniasis, such as cardiovascular events, chronic renal insufficiency, diabetes, or neurological dysfunction, were not observed. Skin lesions might be associated with ATO, but were reversible. Only 1 patient developed breast cancer, which is not a typical type of secondary cancer caused by ATO. However, the incidence of mild liver dysfunction (15.2%) and hepatic steatosis (42.9%) was significantly higher. In fact, acute liver dysfunction was as high as 33% to 75%2,15,16 during induction, which could be ameliorated by decreasing or suspending ATO. This may lead to chronic liver steatosis and fibrosis, which is also common in chronic arseniasis.17,18 No liver fibrosis is noted in our follow-up, but long-term monitoring is warranted. Furthermore, the involvement of chemotherapy, the significantly higher dose of ATO in our regimen, and other conditions including dietary habits might also contribute to hepatic disorders. Laboratory studies should be performed to evaluate the role of ATO in hepatic disorders, so we can modify the dose of ATO.
Presence of arsenic in the plasma and urine reveals short-term arsenic exposure, whereas arsenic in the hair and nails represents long-term retention.19,20 In our 5-year follow-up study, Hu et al2 reported slightly higher TAs in patients compared with controls. In this study, TAs in the plasma, urine, hair, and nails of patients all reached normal levels, indicating no significant retention in these patients.
In summary, patients with APL treated with ATRA/ATO combination therapy demonstrated encouraging long-term survival, particularly in the low-to-intermediate–risk group, with good QoL. ATO was generally safe for patients with APL, with no major chronic AEs, secondary carcinoma, or arsenic retention. However, the study showed a higher incidence of hepatic disorders in patients, and the mechanisms of this effect are yet to be elucidated.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank Zhi Xing and Xiu Huang (Analysis Center of Tsinghua University) for technical assistance in arsenic tests. The authors also thank Shumin Xiong, Bin Chen, and Chunlei Jiang (Rui Jin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine) for morphological, cytogenetic, and molecular analysis.
This work was supported by the National Natural Science Foundation of China (81270621 to J.L.; 81300451 to H. Zhao; 81300406 to A.W.; and 81500159 to H. Zhu), the National High-tech Research and Development Program (863 Program) of China (2012AA02A505) (S.C. and J.L.), Special Grant of Ministry of Health of China (2012105) (J.L.), the National Public Health Grand Research Foundation of China (201202017) (S.C. and J.L.), the Shanghai Health System Advanced and Appropriate Technology Promotion Project (2013SY001) (J.L.), and the 2015 Shanghai Production-Study-Research Practice Project for College Teacher (X.L.).
Contribution: Z.W., Z.C., S.C., Z.S., and J.L. designed the study and served as principal investigators; J.H., H. Zhao, A.W., Yú C., H.S., Q.C., Yù C., W. Zhao, and J.M. carried out the research, enrolled patients, included the initial data, and reviewed the manuscript; J.L., J.H., and H. Zhu designed the protocol for arsenic tests; H. Zhu, W. Zhou, X.L., L.W., X.Z., and Y.Z. carried out the arsenic tests; H. Zhu and L.C. collected, analyzed, and interpreted the data; H. Zhu, L.C., and X.L. carried out statistical analysis; H. Zhu and J.H. wrote the manuscript; and all authors contributed to the final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Junmin Li, Shanghai Institute of Hematology, Rui Jin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, 197 Rui Jin Rd II, Shanghai, 200025, China; e-mail: drlijunmin@126.com; and Saijuan Chen, Shanghai Institute of Hematology, Rui Jin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, 197 Rui Jin Rd II, Shanghai, 200025, China; e-mail: sjchen@stn.sh.cn.
References
Author notes
H. Zhu, J.H., and L.C. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal