Key Points
In tonic BCR signal-dependent DLBCLs, FOXO1 is required for SYK and AKT inhibitor-induced toxicity.
Abstract
Inhibition of spleen tyrosine kinase (SYK) in tonic B-cell receptor (BCR) signal-dependent diffuse large B-cell lymphomas (DLBCLs) inhibits cellular proliferation, decreases cholesterol biosynthesis, and triggers apoptosis, at least in part via a mechanism involving decreased activity of phosphatidylinositol 3-kinase/AKT axis. Because forkhead box O1 (FOXO1) is a major effector of this pathway, we investigated the role of FOXO1 in toxicity of BCR pathway inhibition. Inhibition of SYK in DLBCL cells with tonic BCR signaling decreased phospho-AKT and phospho-FOXO1 levels and triggered FOXO1-driven gene expression. Introduction of constitutively active FOXO1 mutant triggered cell cycle arrest and apoptosis, indicating that increased FOXO1 activity is toxic to these DLBCL cells. Depletion of FOXO1 with short hairpin RNA led to almost complete resistance to chemical SYK inhibitor R406, demonstrating that FOXO1 is also required for R406-induced cell death. FOXO1 in these cells is also involved in regulation of expression of the critical master regulator of cholesterol biosynthesis, SREBP1. Because HRK is the key effector of SYK inhibition, we characterized a mechanism linking FOXO1 activation and HRK induction that involves caspase-dependent cleavage of HRK’s transcriptional repressor DREAM. Because AKT in lymphoma cells can be regulated by other signals than BCR, we assessed the combined effects of the AKT inhibitor MK-2206 with R406 and found markedly synergistic FOXO1-dependent toxicity. In primary DLBCLs, FOXO1 expression was present in 80% of tumors, correlated with SYK activity, and was associated with longer overall survival. These results demonstrate that FOXO1 is required for SYK and AKT inhibitor-induced toxicity.
Introduction
Diffuse large B-cell lymphomas (DLBCLs) are clinically and genetically heterogeneous disorders. Gene expression profiling has allowed functional classification of tumors into distinct subgroups that differ with respect to underlying pathogenetic mechanisms and survival programs.1-7 Detailed analyses of molecular signatures of DLBCL subsets, coupled to functional studies, have led to identification of tumors that are reliant on B-cell receptor (BCR) signaling.1-3,7,8 The BCR consists of an immunoglobulin receptor noncovalently associated with transmembrane proteins, referred to as immunoglobulin (Ig)α and Igβ.8 On BCR engagement, Igα and Igβ immunoreceptor tyrosine-based activation motifs (ITAMs) undergo phosphorylation by the Src family kinases.8 Phosphorylated ITAMs facilitate recruitment and activation of spleen tyrosine kinase (SYK) that through additional adaptor proteins amplifies and propagates the original signal.9-11 However, there are major differences concerning the mechanisms that evoke the BCR signal and utilization of BCR-related survival pathways by DLBCL subgroups. A fraction of BCR-dependent DLBCLs exhibit frequent mutations of CD79B and the presence of BCR surface clustering, termed “chronic active BCR signaling.”7,12 Chronic active BCR signaling is transmitted by Burton tyrosine kinase to enhance activity of the nuclear factor (NF)-κB pathway.7 Certain DLBCL cell lines with chronic active BCR signals also exhibit constitutive phosphatidylinositol 3-kinase (PI3K) activation, which further augments downstream NF-κB signaling.13 More recent studies indicate that certain tumors that do not exhibit a chronic active BCR signal are also addicted to this pathway.2,14,15 These DLBCLs exhibit low-level BCR signals, resembling the tonic signals in naïve, quiescent B cells. Inhibition of the tonic signal with either SYK-silencing short hairpin (sh)RNA or with the adenosine triphosphate–competitive SYK inhibitor R406 decreased the proliferation and induced apoptosis of DLBCL cell lines with intact BCR signaling.2 Consequences of SYK inhibition involved decreased PI3K/AKT signaling, AKT-dependent induction of a proapoptotic BCL2 family member, HRK, and disruption of the metabolic program sustaining cholesterol biosynthesis and lipid raft integrity.3
The subgroup O of forkhead (FOXO) transcription factors are major substrates of AKT. AKT phosphorylates FOXOs at 3 conserved serine/threonine residues, facilitating their association with 14-3-3 chaperone and leading to nuclear export and transcriptional inactivation.16 The FOXO family consists of 4 members, FOXO1, FOXO3, FOXO4, and FOXO6, that share a conserved forkhead DNA-binding domain. FOXOs control diverse cellular processes, such as apoptosis, cell cycle, DNA damage repair, oxidative stress, or glucose metabolism.17,18 FOXO1 plays an essential role in B-cell development by inducing the expression of genes critical for progression through the consecutive steps of differentiation.18 In mature B lymphocytes, BCR-induced activation of PI3K-AKT kinases and subsequent phosphorylation and inactivation of FOXO1 decreases expression of FOXO1 target genes, including proapoptotic BH3-only protein BCL2L11 (BIM) and cell cycle inhibitor CDKN1B (p27Kip1).19 In murine models, conditional deletion of FOXO1 protected quiescent peripheral B cells from apoptosis mediated by inducible loss of the BCR.19 Taken together, these data demonstrate that the PI3K-AKT-FOXO1 axis plays a central role in B-cell homeostasis downstream the BCR pathway.
Given the FOXO1’s role in normal B cells downstream of AKT, we specifically investigated its function in DLBCLs that are reliant on tonic BCR signals. We show that inhibition of these signals leads to transcriptional activation of FOXO1 and induction of FOXO1-dependent gene expression. Depletion of FOXO1 decreased cellular sensitivity to the BCR pathway inhibitors, demonstrating that FOXO1 is an important effector of SYK and AKT inhibition in DLBCLs, and its expression is required for SYK inhibitor-induced toxicity.
Materials and methods
Cell lines, cell culture, and chemicals
Human DLBCL cell lines DHL4, DHL6, DHL10, and U2932 were maintained in RPMI-1640 (Lonza). Ly1, Ly7, Ly19, and HBL-1 cells were maintained in Iscove’s modified Dulbecco’s medium (Lonza), each supplemented with 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer, 50 U/mL penicillin, 50 U/mL streptomycin, and 10% heat-inactivated fetal bovine serum (Lonza). Cells were grown in a humidified atmosphere at 37°C with 5% CO2. SYK inhibitor (R406), AKT inhibitor (MK2206), and pan-caspase inhibitor (Z-VAD-FMK) were purchased from Selleckchem.
BCR crosslinking and SYK inhibition
DLBCL cells DHL4, DHL6, Ly1, and Ly7 (4 × 106) were incubated with R406 (4 µM) or vehicle at 37°C for 30 minutes. Afterward, cells were stimulated with goat anti-human IgG (DHL4) or IgM (DHL6, Ly1, and Ly7) for 10 minutes and lysed in radio-immunoprecipitation assay lysis buffer.
Immunoblotting
After BCR crosslinking and/or incubation in the presence of indicated compounds, proteins were extracted using radio-immunoprecipitation assay buffer, resolved on 10% polyacrylamide gels, and transferred to polyvinyl difluoride membranes (Millipore). Membranes were blocked in 5% bovine serum albumin and incubated overnight at 4°C with indicated primary antibodies (see supplemental Table 1 for details, available on the Blood Web site). Thereafter, membranes were incubated with appropriate horseradish peroxidase–labeled secondary antibody, and blots were visualized with Western Lighting Pro ECL (PerkinElmer) and G:BOX Chemi XT4 (Syngene). When >1 protein was analyzed at the same blot, membranes were stripped and reprobed. Densitometric analyses were performed using ImageJ software (www.imagej.net).
Quantitative real-time polymerase chain reaction
RNA was extracted using GeneMATRIX Universal RNA purification kit (EURx) and reverse-transcribed with Transcriptor First Strand cDNA synthesis kit (Roche). Gene expression levels were measured on 7500 Fast RT-PCR system (Applied Biosystems) with the gene-specific primers (sequences provided in supplemental Table 2) and SYBR Green PCR Master Mix (Life Technologies). All experiments were conducted in triplicate. Obtained CT values for FOXO1, NF-κB target genes, and a housekeeping control (glyceraldehyde-3-phosphate dehydrogenase [GAPDH]) were used to calculate relative transcript abundance using ΔΔCT method.
Vector constructs and retroviral transduction procedures
A pMIG-IRES-green fluorescent protein (GFP), pMIG-mAKT-internal ribosomal entry site (IRES)-GFP, and pSiren-RetroQ-scrambled-shRNA vectors were described previously.3,20 The FOXO1-targeting shRNA sequence (GTGCCCTACTTCAAGGATAAG) was designed using GeneScript siRNA target finder as previously described20 and synthesized by Sigma-Aldrich. The annealed oligonucleotide was ligated into pSIREN-RetroQ backbone using EcoRI and BamHI sites. The pMIG-FOXO1-WT-IRES-GFP and pMIG-FOXO1-3A-IRES-GFP were generated by ligating wild-type (WT) or 3A FOXO1 mutant open reading frames (derived from Addgene vectors 13507 and 13508, deposited by Kunliang Guan21 ) into the pMIG-IRES-GFP vector. The DREAM coding sequence was polymerase chain reaction (PCR)-amplified from the chronic myeloid leukemia cDNA library and cloned into pMIG-IRES-GFP with a human influenza hemagglutinin (HA) tag. All generated plasmids were verified by sequencing. Generation of retroviruses and infection of DLBCL cells were performed as described previously.20 After 24 hours of infection, GFP+ cells were isolated by fluorescence-activated cell sorting (FACS Aria II; BD Biosciences) or subjected to selection with puromycin (0.3 µg/mL).
Viability and apoptosis assays
DLBCL cell lines were incubated with vehicle (DMSO) or R406 (1-4 µM) and/or MK2206 (0.1-0.5 µM) for 72 hours and subsequently assessed with the 3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (Promega). Detection of apoptosis in DHL4 cells transduced with pMIG-FOXO1-WT/3A-IRES-GFP was performed 60 hours after cell sorting. Cells were washed in phosphate-buffered saline, suspended in AnnexinV binding buffer (BD Biosciences), stained with AnnexinV-phycoerythrin and 7-amino-actinomycin (7-AAD) (BD Biosciences), and analyzed using an FACS Canto flow cytometer (BD Biosciences).
Intracellular phospho-specific flow cytometry
Intracellular phospho-specific flow cytometry was performed using the Phosflow Kit (BD Biosciences), as previously described,15 with antibodies listed in supplemental Table 1. Cells were analyzed using the FACS Canto flow cytometer.
Patient samples and immunohistochemistry
Formalin fixed, parafin-embedded diagnostic slides from a retrospective group of 60 DLBCL patients, diagnosed according to the 2008 World Health Organization classification, were assessed for FOXO1 and phospho-SYK expression. Patient characteristics are presented in supplemental Table 3. Immunohistochemistry was performed using an automated immunohistochemical stainer (Dako Denmark A/S) and phospho-SYK Y525/6 (Cell Signaling; cat. 2710, 1:100, pH 6.0) or FOXO1 (Cell Signaling; cat. 2880, 1:50, pH 9.0) antibodies. For detection and visualization, the EnVision Detection System (Dako Denmark A/S) was used, following the manufacturer’s recommendations. Microscopic evaluation (Olympus; BX63, ×200 and ×400 magnification) of phospho-SYK Y525/6 and FOXO1 immunostainings was independently performed by 2 experienced hematopathologists who were blinded to the clinical data. Immunostainings were evaluated with a semiquantitative scoring system based on the combination of intensity (0, no staining; 1, weak; 2, intermediate; and 3, strong staining) and percentage of positive cells. Samples were considered positive for phospho-SYK and/or FOXO1 when >10% of lymphoma cells exhibited intermediate/strong staining. Photographs were taken using the microscope camera DP72 Olympus BX63 (Olympus).
Analysis of microarray data and statistical analyses
The gene set enrichment analysis (GSEA) was performed as previously described,22 using averaged gene expression microarray data from R406-treated cell lines (gene expression omnibus dataset GSE43510)3 and the set of FOXO1 regulated genes,23 separately for GCB and ABC DLBCL cell lines. Leading-edge genes (defined as the genes that appear in the ranked list at or before the point where the GSEA enrichment score running sum reaches its maximum deviation from 0) were visualized with dChip sotware as previously described.22,24 The statistical analyses were performed as indicated with the Gosset’s 2-sided t test or analysis of variance test with Tukey’s post hoc test, using GraphPad QuickCalcs/Prism 6 (GraphPad Software). The categorical data were analyzed with a χ2 test using R v.3.1.1 software. Error bars represent standard deviation. The survival of FOXO1+/− patients was compared using the Kaplan-Meier method and log-rank test with the SPSS, v.17.0 software.
Results
FOXO1 is an effector of SYK inhibition in DLBCL cell lines exhibiting tonic BCR signaling
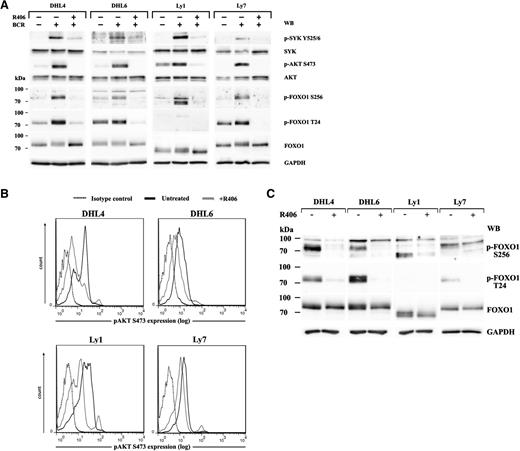
Because FOXO transcription factors are major AKT substrates, we first assessed expression of the individual FOXO family members in DLBCL cells and found FOXO1 to be the most abundantly expressed member of the family (supplemental Figure 1). We next asked whether tonic and antigen-induced BCR signals are transmitted to FOXO1 in DLBCLs reliant on the tonic BCR/SYK activity. To answer this question, we analyzed SYK-AKT-FOXO1 signaling following crosslinking of the BCR receptors in DHL4, DHL6, Ly1, and Ly7 cells, with or without preincubation with the chemical SYK inhibitor R406. BCR stimulation elevated levels of phosphorylated SYK, AKT, and FOXO1 S256 in all investigated cell lines (Figure 1A). T24 FOXO1 phosphorylation increased in DHL4, Ly7, and DHL6 (331%, 148%, and 120% of baseline, respectively; Figure 1A; supplemental Table 4). The lack of T24 phosphorylation in Ly1 cells reflects a Ly1-specific FOXO1 truncating mutation, leading to expression of a shorter protein lacking the N-terminal portion.25 Preincubation with the SYK inhibitor R406 inhibited BCR crosslinking-induced AKT and FOXO1 phosphorylation, demonstrating that the signal transduction in the BCR-SYK-AKT-FOXO1 axis is intact in DLBCL cells dependent on tonic BCR signals, and modulation of SYK activity elicits commensurate changes in FOXO1 phosphorylation (Figure 1A). In line with these findings, the SYK inhibitor also diminished the baseline activities of AKT (S473) and FOXO1 (S256, T24) in unstimulated cells, indicating that tonic BCR signals elicit AKT and FOXO1 phosphorylation that can be decreased with R406 (Figure 1B-C). Because blocking FOXO1 phosphorylation favors its nuclear localization and induces its transcriptional activity, we assessed FOXO1 target gene expression using a previously generated gene expression dataset of vehicle (DMSO) or R406-treated BCR-dependent DLBCL cell lines: DHL4, DHL6, Ly7, U2932, and HBL1.3 We identified concordant upregulation of multiple functionally validated FOXO1 target genes in the DLBCL cell lines with tonic BCR signaling (P = .021, false discovery rate = 0.021; Figure 2A-B). In contrast, in DLBCL cell lines with chronic active BCR signal and high NF-κB activity, there was no coordinated shift in the expression of FOXO1 target genes (P = .441, false discovery rate = 0.443; supplemental Figure 2). Among the 40 most statistically significant FOXO1 target genes upregulated in BCR-dependent DLBCL cells on SYK inhibition, there were those involved in cell cycle arrest and induction of apoptosis, such as CDKN1B (p27Kip1), TNFSFR10 (TRAIL), and GADD45A. We further assessed the transcript abundance of these FOXO1 targets in the series of 4 DLBCL lines treated with vehicle or R406 using quantitative reverse transcription (qRT)-PCR. In these analyses, we also included BIM (BCL2L11) because FOXO1 upregulates its expression in normal and neoplastic B cells.19,26,27 All 4 FOXO1 targets were significantly upregulated in all investigated tonic BCR signal-dependent DLCBL cell lines (Figure 2C). Induction of FOXO1 target genes, p27 and BIM, was also confirmed at the protein level (Figure 2D). Consistent with previous reports,3 SYK inhibition in chronic active BCR signal-dependent cell lines decreased the mRNA abundance of NF-κB target genes, but led to highly variable FOXO1 target gene responses (supplemental Figure 2B). Together, these data demonstrate that transcriptional activity of FOXO1 is attenuated by tonic BCR signaling and that SYK inhibition in these cells restores FOXO1-driven gene expression.
The BCR-SYK-AKT-FOXO1 signaling pathway is operative in tonic BCR signal-dependent DLBCL cell lines and leads to the suppression of FOXO1 activity. (A) The integrity of the BCR-SYK-AKT-FOXO1 axis was determined in BCR-dependent cell lines DHL4, DHL6, Ly1, and Ly7 after crosslinking of their BCRs, with or without R406 pretreatment. Activity of key signaling components of the pathway was assessed with phospho-specific antibodies against SYK(Y525/6), AKT(S473), and FOXO1(T24 and S256). Blots were next stripped and reprobed with antibodies against total-SYK, total-AKT, and total-FOXO1. GAPDH served as a loading control. Densitometric quantification of band intensities is provided in supplemental Table 4. (B) DLBCL cells were treated with R406 (gray line) or vehicle alone (black line) for 18 hours and subjected to single-cell phospho-flow analysis to detect tonic AKT phosphorylation at S473. Dotted line, isotype control. (C) FOXO1 phosphorylation in DLBCL cell lines treated for 18 hours with R406 or vehicle alone was analyzed by immunoblotting. Densitometric quantification of band intensities is provided in supplemental Table 5.
The BCR-SYK-AKT-FOXO1 signaling pathway is operative in tonic BCR signal-dependent DLBCL cell lines and leads to the suppression of FOXO1 activity. (A) The integrity of the BCR-SYK-AKT-FOXO1 axis was determined in BCR-dependent cell lines DHL4, DHL6, Ly1, and Ly7 after crosslinking of their BCRs, with or without R406 pretreatment. Activity of key signaling components of the pathway was assessed with phospho-specific antibodies against SYK(Y525/6), AKT(S473), and FOXO1(T24 and S256). Blots were next stripped and reprobed with antibodies against total-SYK, total-AKT, and total-FOXO1. GAPDH served as a loading control. Densitometric quantification of band intensities is provided in supplemental Table 4. (B) DLBCL cells were treated with R406 (gray line) or vehicle alone (black line) for 18 hours and subjected to single-cell phospho-flow analysis to detect tonic AKT phosphorylation at S473. Dotted line, isotype control. (C) FOXO1 phosphorylation in DLBCL cell lines treated for 18 hours with R406 or vehicle alone was analyzed by immunoblotting. Densitometric quantification of band intensities is provided in supplemental Table 5.
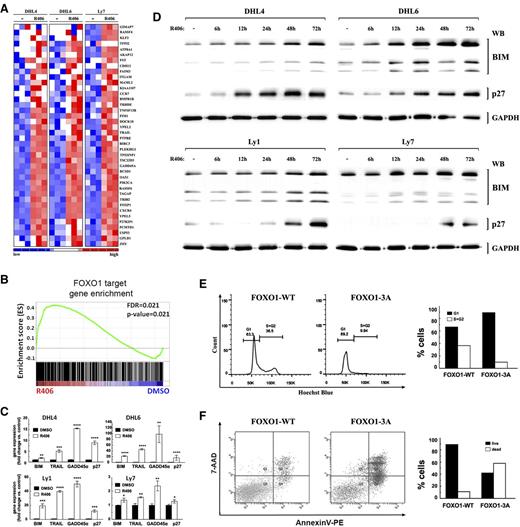
FOXO1 activation in DLBCL cells induces FOXO1-dependent gene expression, cell cycle arrest, and apoptosis. (A) SYK inhibition induces FOXO1 target gene expression. The heat map shows relative abundance of 40 FOXO1 target genes (GSEA leading edge) in tonic BCR signal-dependent DLBCL cell lines following 24-hour treatment with R406. (B) GSEA enrichment plot of FOXO1 target genes in tonic BCR signal-dependent DLBCL cell lines (DHL4, DHL6, Ly7) treated with DMSO or R406 for 24 hours. Note that the positions of the FOXO1 targets (Vogel et al23 ) were significantly skewed toward the left end of the sorted list, reflecting their statistically significant induction in R406-treated lines. (C) FOXO1 target gene expression in 4 tonic BCR signal-dependent cell lines following 24-hour treatment with R406 or vehicle, analyzed by qRT-PCR. (P values were determined using 2-sided Gosset’s t test: *P < .05; **P < .01; ***P < .001; ****P < .0001). (D) Protein abundance of key FOXO1 targets, CDKN1B (p27) and BCL2L11 (BIM), in DLBCL cells following 6- to 72-hour treatment with R406 or vehicle, analyzed by western blot. GAPDH served as a loading control. Densitometric quantification of band intensities is provided in supplemental Table 6. (E) FOXO1 nuclear localization induces cell cycle arrest. DHL4 cells were retrovirally transduced with pMIG-FOXO1-WT-IRES-GFP or pMIG-FOXO1-3A-IRES-GFP to express wild-type (FOXO1-WT) or constitutively nuclear FOXO1 mutant (FOXO1-3A). Cell cycle distribution was measured within the GFP+ population by Hoechst blue staining and FACS analysis. (F) FOXO1 nuclear localization induces apoptosis. Cells were transduced as in E; 48 hours after transduction, GFP+ cells were FACS sorted and after another 60 hours were analyzed by AnnexinV/7-AAD staining. Bar graphs are derived from FACS data.
FOXO1 activation in DLBCL cells induces FOXO1-dependent gene expression, cell cycle arrest, and apoptosis. (A) SYK inhibition induces FOXO1 target gene expression. The heat map shows relative abundance of 40 FOXO1 target genes (GSEA leading edge) in tonic BCR signal-dependent DLBCL cell lines following 24-hour treatment with R406. (B) GSEA enrichment plot of FOXO1 target genes in tonic BCR signal-dependent DLBCL cell lines (DHL4, DHL6, Ly7) treated with DMSO or R406 for 24 hours. Note that the positions of the FOXO1 targets (Vogel et al23 ) were significantly skewed toward the left end of the sorted list, reflecting their statistically significant induction in R406-treated lines. (C) FOXO1 target gene expression in 4 tonic BCR signal-dependent cell lines following 24-hour treatment with R406 or vehicle, analyzed by qRT-PCR. (P values were determined using 2-sided Gosset’s t test: *P < .05; **P < .01; ***P < .001; ****P < .0001). (D) Protein abundance of key FOXO1 targets, CDKN1B (p27) and BCL2L11 (BIM), in DLBCL cells following 6- to 72-hour treatment with R406 or vehicle, analyzed by western blot. GAPDH served as a loading control. Densitometric quantification of band intensities is provided in supplemental Table 6. (E) FOXO1 nuclear localization induces cell cycle arrest. DHL4 cells were retrovirally transduced with pMIG-FOXO1-WT-IRES-GFP or pMIG-FOXO1-3A-IRES-GFP to express wild-type (FOXO1-WT) or constitutively nuclear FOXO1 mutant (FOXO1-3A). Cell cycle distribution was measured within the GFP+ population by Hoechst blue staining and FACS analysis. (F) FOXO1 nuclear localization induces apoptosis. Cells were transduced as in E; 48 hours after transduction, GFP+ cells were FACS sorted and after another 60 hours were analyzed by AnnexinV/7-AAD staining. Bar graphs are derived from FACS data.
FOXO1 activity is sufficient to induce cell cycle arrest and apoptosis in DLBCL cells
After demonstrating that blocking SYK activity in tonic BCR signal-dependent DLBCL cell lines triggers transcription of FOXO1 target genes, we asked whether transcriptional activation of FOXO1 is sufficient to induce growth suppression and cell death. For this purpose, we transduced DHL4 cells with vectors coding either WT FOXO1 (FOXO1-WT), or FOXO1 mutant (FOXO1-3A) that cannot undergo AKT-dependent phosphorylation and thus is constitutively expressed in the nucleus.21 Compared with cells expressing WT protein, expression of the constitutively active FOXO1 mutant induced G1/S cell cycle arrest (Figure 2E). Moreover, FOXO1-3A, but not FOXO1-WT expression markedly increased apoptosis in DHL4 cells as determined by AnexinV/7-AAD staining (Figure 2F). Taken together, our results demonstrate that transcriptionally active FOXO1 is sufficient for G1/S cell cycle arrest and induction of apoptosis in tonic BCR-dependent DLBCL cells.
FOXO1 activity is essential for SYK inhibitor toxicity in tonic BCR signal-dependent DLBCL cells
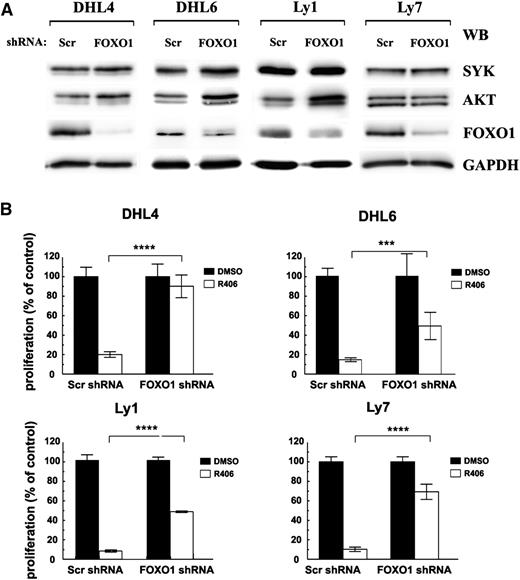
In murine models, conditional deletion of FOXO1 protected quiescent peripheral B cells from apoptosis mediated by inducible loss of the BCR, demonstrating that the AKT-FOXO1 axis plays a central role in B-cell homeostasis downstream of the BCR pathway.13 We therefore asked whether FOXO1 plays similar role in BCR-dependent DLBCL cell lines and is required to convey SYK inhibitor’s toxicity. For this purpose, we silenced FOXO1 with shRNA in tonic BCR signal-dependent cell lines DHL4, DHL6, Ly1, Ly7, Ly19, and DHL10 (Figure 3A; supplemental Figure 3) and assessed the sensitivity of FOXO1-depleted cells to R406 using the MTS assay. To address the role of FOXO1 in chronic active BCR signaling-dependent DLBCLs, we also included in these experiments an ABC-type cell line, HBL-1 (supplemental Figure 3). In contrast to control Scr-shRNA transduced cells, exhibiting similar sensitivity to the SYK inhibitor as parental DLBCL lines, FOXO1 depletion largely protected these tonic BCR signal-dependent DLBCLs from chemical SYK inhibition (Figure 3B). Importantly, although previous studies demonstrated that transient FOXO1 ablation decreased SYK mRNA levels in both DHL4 and Ly7,3 in cells with stable FOXO1 knockdown, expression and activity of upstream pathway components SYK and AKT moderately increased, indicating that lower sensitivity to R406 is not due to loss of the inhibitor’s target expression (Figure 3A). In NF-κB–dependent DLBCL cells with chronic active BCR signaling, FOXO1 depletion did not significantly alter the response to R406 (supplemental Figure 3).
FOXO1 mediates toxicity of SYK inhibitor in tonic BCR signal-dependent DLBCL cells. DHL4, DHL6, Ly1, and Ly7 cells were transduced with pSiren-RetroQ-scrambled-shRNA (Scr shRNA) or pSiren-RetroQ-FOXO1-shRNA (FOXO1 shRNA), and stable transfectants were selected using puromycin. (A) FOXO1, AKT, and SYK protein expression was analyzed by immunoblotting. GAPDH served as a loading control. Densitometric quantification of band intensities is provided in supplemental Table 7. (B) Cells transduced with FOXO1-targeting shRNA vectors were treated with R406 and after 72 hours were analyzed for proliferation using the MTS assay. Please also see supplemental Figure 3 for additional information.
FOXO1 mediates toxicity of SYK inhibitor in tonic BCR signal-dependent DLBCL cells. DHL4, DHL6, Ly1, and Ly7 cells were transduced with pSiren-RetroQ-scrambled-shRNA (Scr shRNA) or pSiren-RetroQ-FOXO1-shRNA (FOXO1 shRNA), and stable transfectants were selected using puromycin. (A) FOXO1, AKT, and SYK protein expression was analyzed by immunoblotting. GAPDH served as a loading control. Densitometric quantification of band intensities is provided in supplemental Table 7. (B) Cells transduced with FOXO1-targeting shRNA vectors were treated with R406 and after 72 hours were analyzed for proliferation using the MTS assay. Please also see supplemental Figure 3 for additional information.
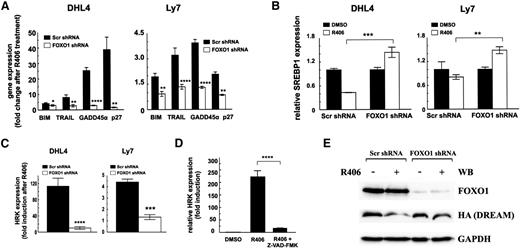
To understand the molecular basis of decreased sensitivity of FOXO1-depleted cells to the SYK inhibitor, we analyzed transcript abundance of BIM, TRAIL, GADD45α, and CDKN1B in these cells following R406 treatment. As expected, SYK inhibition induced expression of all 4 FOXO1 targets in control cells but not in FOXO1-depleted cells (Figure 4A). Consistent with the key role of AKT in FOXO1 regulation, in cells transduced with constitutively active AKT (mAKT), FOXO1 phosphorylation was not decreased by SYK inhibition, and these cells were protected from R406-mediated toxicity (supplemental Figure 4). These results underscore the essential role of AKT in tonic BCR signaling and show that its inhibition on SYK blockade is required to trigger FOXO1-dependent gene expression and toxicity in tonic BCR signal-dependent cell lines.
FOXO1 mediates toxicity of SYK inhibitor in tonic BCR signal-dependent DLBCL cells. (A) Transcript abundance of FOXO1 target genes, BIM, TRAIL, GADD45α, and CDKN1B (p27Kip1) in cell lines transduced with FOXO1-targeting or control shRNA vectors, assessed by qRT-PCR after 24-hour incubation with R406. (B) SREBP1 expression following incubation with R406 was assessed as described above. (C) Transcript abundance of HRK in DHL4 and Ly7 cell lines transduced with scrambled control shRNA (Scr) or FOXO1-targeting shRNA following 24-hour incubation with R406. P values in A to C were determined using a 2-sided Gosset’s t test: *P < .05; **P < .01; ***P < .001; ****P < .0001. (D) HRK expression was determined after 24-hour treatment with vehicle alone (DMSO), R406, or a combination of SYK and pan-caspase inhibitor (Z-VAD-FMK) by qRT-PCR relative to GAPDH. P values were determined using analysis of variance and post hoc Tukey’s test: ****P < .0001. (E) DHL4 stably transduced with indicated shRNAs were further transduced with the pMIG-HA-DREAM-IRES-GFP vector. GFP+ cells were then FACS sorted and treated with R406 or vehicle alone. After 24 hours of incubation, cells were analyzed for FOXO1 and DREAM expression by immunoblotting. GAPDH served as a loading control. Densitometric quantification of band intensities is provided in supplemental Table 8.
FOXO1 mediates toxicity of SYK inhibitor in tonic BCR signal-dependent DLBCL cells. (A) Transcript abundance of FOXO1 target genes, BIM, TRAIL, GADD45α, and CDKN1B (p27Kip1) in cell lines transduced with FOXO1-targeting or control shRNA vectors, assessed by qRT-PCR after 24-hour incubation with R406. (B) SREBP1 expression following incubation with R406 was assessed as described above. (C) Transcript abundance of HRK in DHL4 and Ly7 cell lines transduced with scrambled control shRNA (Scr) or FOXO1-targeting shRNA following 24-hour incubation with R406. P values in A to C were determined using a 2-sided Gosset’s t test: *P < .05; **P < .01; ***P < .001; ****P < .0001. (D) HRK expression was determined after 24-hour treatment with vehicle alone (DMSO), R406, or a combination of SYK and pan-caspase inhibitor (Z-VAD-FMK) by qRT-PCR relative to GAPDH. P values were determined using analysis of variance and post hoc Tukey’s test: ****P < .0001. (E) DHL4 stably transduced with indicated shRNAs were further transduced with the pMIG-HA-DREAM-IRES-GFP vector. GFP+ cells were then FACS sorted and treated with R406 or vehicle alone. After 24 hours of incubation, cells were analyzed for FOXO1 and DREAM expression by immunoblotting. GAPDH served as a loading control. Densitometric quantification of band intensities is provided in supplemental Table 8.
In previous experiments, the tonic BCR-SYK-PI3K signaling pathway triggered a feed-forward mechanism increasing cholesterol biosynthesis and maintaining the integrity of BCRs in lipid rafts in DLBCLs.3 Because the enzymes in the cholesterol synthesis pathway are regulated by a PI3K/AKT/FOXO1-dependent transcription factor SREBP1,28-30 we assessed its transcript abundance in control and FOXO1-depleted cells treated with R406. We observed a marked decrease in SREBP1 mRNA in control cells, whereas in FOXO1-depleted cells, expression of this gene actually increased (Figure 4B). These data indicate that R406-induced FOXO1 activation contributes to decreased SREBP1 expression in DLBCL cells and underscore the role of FOXO1 in integration of survival and metabolic consequences of SYK inhibition.
Induction of HRK after SYK inhibition is FOXO1 dependent
Previous studies highlighted the critical role of the proapoptotic BCL2 family member HRK in R406-induced apoptosis of tonic BCR signal-dependent DLBCLs.3 To find a functional link between FOXO1 and HRK induction, we assessed HRK abundance in FOXO1-expressing and FOXO1-depleted DHL4 and Ly7 cells following SYK inhibition. R406 markedly elevated HRK mRNA levels in control cells but failed to induce HRK expression in FOXO1-depleted DHL4 and Ly7 cells (Figure 4C). Because these results indicate HRK dependence on FOXO1, we analyzed the proximal HRK promoter for potential FOXO binding motifs. This analysis revealed no potential DNA binding sites (data not shown), suggesting that FOXO1 controls HRK expression via an indirect mechanism. In hematopoietic cells, HRK transcription is silenced by a transcriptional repressor DREAM.31 DREAM stability is regulated by caspase-mediated cleavage, releasing HRK repression.32 Because FOXO1 upregulates genes that trigger caspase activation such as TRAIL and BIM, we speculated that FOXO1 induces HRK expression via mechanism that involves caspase-mediated DREAM inactivation. To verify this hypothesis, we first assessed HRK expression in DHL4 cells after inhibition of SYK and/or simultaneous inhibition of caspases. HRK mRNA level was markedly induced in cells exposed to R406, but a pan-caspase inhibitor, Z-VAD-FMK, almost completely blocked this effect (Figure 4D). To determine the role of FOXO1 in DREAM cleavage and HRK induction, we transduced control and FOXO1-depleted DHL4 cells with a vector coding for an HA-tagged DREAM protein and assessed the abundance of DREAM in cells treated with R406 or DMSO. In FOXO1-expressing cells, DREAM expression markedly decreased (0.29 of baseline) after R406 exposure. In contrast, in cells with shRNA-decreased FOXO1 expression, SYK inhibition caused only minor changes of DREAM expression (Figure 4E).
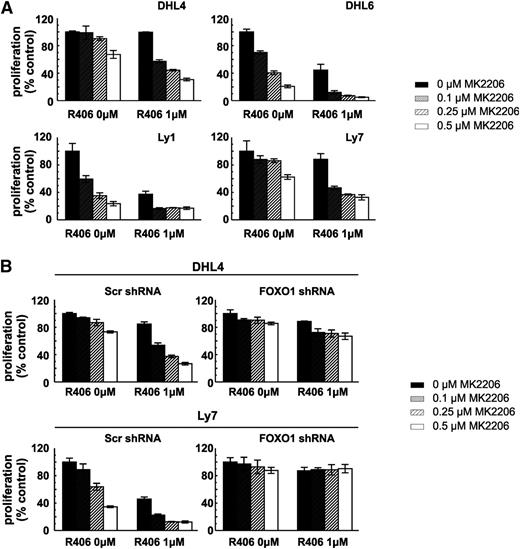
Synergistic effect of simultaneous SYK and AKT inhibition in tonic BCR signal-dependent DLBCL requires FOXO1
Because the key nodes of the SYK-PI3K-AKT-FOXO1 signaling pathway receive and integrate signals from sources other than BCR, we hypothesized that targeting the axis at multiple levels might result in increased toxicity. For this reason, we incubated tonic BCR signal-dependent DLBCL cell lines with SYK (R406) and/or AKT (MK2206) inhibitors and assessed cellular viability after 72 hours. The combined SYK and AKT inhibitors exhibited markedly increased toxicity compared with these compounds used individually (Figure 5A). To formally assess the interaction between these drugs, we computed the combination indices and found that, when used together, the inhibitors produce a markedly synergistic effect in all tested DLBCL cell lines (supplemental Table 9). Next, to determine whether the observed effect depends on FOXO1 activity, we tested toxicity of R406 and MK2206 in combination in the DHL4 and Ly7 cell lines with stable FOXO1 knockdown. As expected, FOXO1-depleted cells were almost completely resistant to simultaneous SYK and AKT inhibition (Figure 5B; supplemental Table 10). Taken together, these data highlight the potential of combinatorial use of SYK and AKT inhibitors in the treatment of tonic BCR signal-dependent DLBCLs and underscore the role of FOXO1 in mediating the synergy of these combinations.
FOXO1 is essential for R406 and the MK2206 synergistic effect in tonic BCR signal-dependent DLBCL cells. (A) Cellular proliferation was assessed in DLBCL cell lines following 72-hour incubation with indicated concentrations of R406 and MK2206 by MTS. (B) DHL4 and Ly7 cells transduced with indicated shRNA vectors were treated and analyzed as described above. For further information please refer to supplemental Tables 9 and 10.
FOXO1 is essential for R406 and the MK2206 synergistic effect in tonic BCR signal-dependent DLBCL cells. (A) Cellular proliferation was assessed in DLBCL cell lines following 72-hour incubation with indicated concentrations of R406 and MK2206 by MTS. (B) DHL4 and Ly7 cells transduced with indicated shRNA vectors were treated and analyzed as described above. For further information please refer to supplemental Tables 9 and 10.
FOXO1 expression in primary DLBCLs
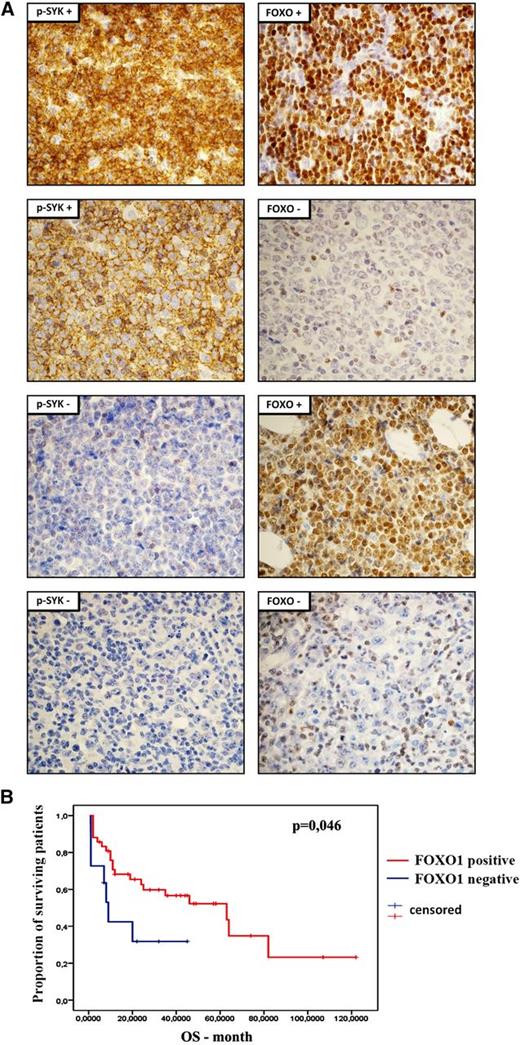
Because our results strongly suggest the essential role of FOXO1 in mediating SYK inhibition toxicity in DLBCL cells, we assessed FOXO1 and phospho-SYK expression by immunohistochemistry in primary DLBCLs (Figure 6A; supplemental Table 11). SYK activity was identified in 35 of 60 (58%) tumors. FOXO1 staining was observed in 48 (80%) cases. In 35 phospho-SYK+ tumors, FOXO1 was present in 32 cases (91%), demonstrating that phospho-SYK and FOXO1 coexpression is not random (P = .008). Importantly, these results indicate that the terminal transcriptional effector of the pathway, FOXO1, is not expressed in 9% of phospho-SYK+ tumors. There was no association between FOXO1 or phospho-SYK expression and cell-of-origin molecular subtype, determined according to the Hans staining algorithm (data not shown). Next, we assessed clinical relevance of FOXO1 expression in the disease outcome. Consistent with the tumor suppressor function of FOXO1, regulating expression of proapoptotic and cell cycle inhibitory genes, patients with FOXO1-positive tumors had longer overall survival (log-rank test, P = .046; Figure 6B).
Expression and clinical significance of FOXO1 in primary DLBCLs. (A) Immunohistochemical analysis of FOXO1 and phospho-SYK (Y525/6) expression in DLBCL samples. Shown are representative cases with positive and negative staining. Pictures were taken with ×60 objective. For the tabular representation of the immunohistochemical analysis of FOXO1 and phospho-SYK expression performed in a panel of 60 DLBCL patients, please see supplemental Table 11. (B) Overall survival of DLBCL patients with respect to FOXO1 expression.
Expression and clinical significance of FOXO1 in primary DLBCLs. (A) Immunohistochemical analysis of FOXO1 and phospho-SYK (Y525/6) expression in DLBCL samples. Shown are representative cases with positive and negative staining. Pictures were taken with ×60 objective. For the tabular representation of the immunohistochemical analysis of FOXO1 and phospho-SYK expression performed in a panel of 60 DLBCL patients, please see supplemental Table 11. (B) Overall survival of DLBCL patients with respect to FOXO1 expression.
Discussion
In this study, we characterize the essential role of FOXO1 in mediating SYK and AKT inhibition toxicity in DLBCL cells that rely on tonic BCR activity for survival. A subset of DLBCLs exhibit dependence on BCR signaling, but the origin of these signals and transduction mechanisms are different in distinct molecular subtypes. DLBCLs reliant on the chronic active BCR signal depend on NF-κB for their survival.5 Constitutive AKT activity is an additional pathogenetic mechanism in these tumors, amplifying NF-κB signaling and correlating with worse prognosis.13,33 Toxicity of BCR signaling pathway inhibition in these tumors is mediated by the decreased expression of NF-κB target genes but not by the activation of FOXO1 (supplemental Figure 5). In contrast, tonic BCR signal-dependent DLBCLs exhibit low baseline NF-κB activity and are reliant on the SYK-AKT pathway.3 SYK inhibition decreases AKT activity and subsequently triggers transcriptional and metabolic effects that eventually lead to cell death.3 The SYK inhibitor triggered coordinated expression of multiple FOXO1 target genes that included cell cycle regulators and mediators of apoptosis. These results are consistent with a proapoptotic/tumor suppressor role of FOXOs in other malignancies, where FOXO-induced apoptosis is typically mediated by an intrinsic, caspase-dependent pathway.2,26,34,35 Importantly, FOXO1 was indispensable for the induction of the proapoptotic BCL2-family protein HRK, the critical mediator of R406 toxicity in tonic BCR signal-dependent DLBCLs. FOXO1 and caspase-dependent DREAM cleavage leads to HRK induction, further caspase activation and execution of an intrinsic apoptotic pathway. Thus, this mechanism reflects a putative self-reinforcing mechanism that leads to massive induction of HRK in the course of a SYK inhibitor-induced apoptosis. FOXO1 in these cells is also involved in regulation of expression of the critical master regulator of cholesterol biosynthesis, SREBP1.3 These data suggest that FOXO1 is the critical mediator of SYK inhibitor toxicity, integrating survival and metabolic effects.
Similar to other tumor suppressor genes, FOXO1 inactivation occurs via structural or functional mechanisms. For example, in classical Hodgkin lymphoma, FOXO1 loss occurs via 13q deletions or is caused by overexpression of FOXO1-targeting micro-RNAs (miR-182, miR-96, and miR-183).26 FOXO1 function is also blocked in primary mediastinal large B-cell lymphoma by a mechanism involving Janus kinase-signal transducer and activator of transcription activation and indirect epigenetic silencing.35 Recent results also indicate that in DLBCLs, FOXO1 is a direct target of oncogenic miR-21.36 This micro-RNA also targets PTEN, indirectly augmenting FOXO1 inhibitory AKT activity.36 Thus, miR-21 exhibits its oncogenic role at least in part by targeting expression and activity of FOXO1. In our DLBCL series, loss of FOXO1’s protein expression occurred in 20% of biopsies and was associated with shorter overall survival. Although characterization of FOXO1’s prognostic role requires larger validation series, these results are consistent with FOXO1’s proapoptotic function and highlight an important biological role of this transcription factor in DLBCL.
In contrast to other tumor suppressors, inactivating mutations of FOXO1 in DLBCLs have not yet been described. Recent results indicate that FOXO1 mutations occur in 8.6% of DLBCLs and are associated with predominant nuclear localization of FOXO1.25 Patients with these FOXO1 mutations exhibit better outcomes and longer overall survival in comparison with those with WT FOXO1, indicating that mutant and WT FOXO1 are transcriptionally and functionally different.25 Predominant nuclear localization of FOXO1 mutants suggests that its transcriptional function is altered, restraining proapoptotic activity. Nevertheless, even mutant FOXO1 is still able to trigger apoptosis, as the hemizygous mutation of FOXO1 in Ly1 cells did not impair its ability to induce proapoptotic genes after SYK inhibition.25 These findings indicate that cells tolerate FOXO1 mutant at steady state, but the overflow of nuclear FOXO1 on SYK inhibition triggers apoptosis.
The key kinases of the tonic BCR pathway (SYK-PI3K-AKT) can be activated by other BCR-independent signals, potentially limiting the activity of the SYK inhibitor.37,38 Consistently, we observed a marked, FOXO1-dependent synergy between the AKT and SYK inhibitors. From a therapeutic standpoint, the combination can potentially limit cellular mechanisms compensating the single inhibitor activity and decrease the probability of resistance. Of note, in the DLBCL cells exposed to the SYK inhibitor, we observed an increased PIP3 signaling signature and overexpression of multiple components of interleukin-7, interleukin-10, and platelet-derived growth factor receptor pathways, likely representing an attempt to compensate R406-induced AKT inhibition.3 These observations can explain a marked synergy between SYK and AKT inhibitors and provide a rationale for their combinatorial use in clinical trials.
Taken together, our results shed new light on the FOXO1 role in DLBCLs, demonstrating that FOXO1 is an important effector of SYK and AKT inhibition in a subset of DLBCLs and its expression is required for SYK/AKT inhibitor-induced toxicity. From a clinical standpoint, these observations indicate that FOXO1 might represent a biomarker of resistance to SYK/AKT inhibitors and its loss precludes their clinical activity in patients with tonic BCR signal-dependent DLBCLs. Therefore, histochemical assessment of FOXO1 expression and pathway integrity in DLBCL patients enrolled in future clinical trials evaluating SYK inhibitors should be considered.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work has been supported by Foundation for Polish Science grant TEAM/2011-7/4, cofinanced by the European Regional Development Fund and Operational Program Innovative Economy 2007-2013.
Authorship
Contribution: M.S. and P.K. designed research, performed research, analyzed data, and wrote the paper; T.S., E.J., and E.B. designed research and performed research; P.G., A.P., S.M., E.N., and M.A.G. performed research; M.P.-S. and A.S.-C. designed research, performed IHC analyses, and analyzed data; E.L.-M. A.M., and K.W. collected clinical information and analyzed data; and P.J. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Przemyslaw Juszczynski, Institute of Hematology and Transfusion Medicine, Department of Diagnostic Hematology, Indiry Gandhi 14, 02-776 Warsaw, Poland; e-mail: pjuszczynski@ihit.waw.pl.
References
Author notes
M.S. and P.K. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal