Abstract
Human α2-antiplasmin (α2AP, also called α2-plasmin inhibitor) is the main physiological inhibitor of the fibrinolytic enzyme plasmin. α2AP inhibits plasmin on the fibrin clot or in the circulation by forming plasmin-antiplasmin complexes. Severely reduced α2AP levels in hereditary α2AP deficiency may lead to bleeding symptoms, whereas increased α2AP levels have been associated with increased thrombotic risk. α2AP is a very heterogeneous protein. In the circulation, α2AP undergoes both amino terminal (N-terminal) and carboxyl terminal (C-terminal) proteolytic modifications that significantly modify its activities. About 70% of α2AP is cleaved at the N terminus by antiplasmin-cleaving enzyme (or soluble fibroblast activation protein), resulting in a 12-amino-acid residue shorter form. The glutamine residue that serves as a substrate for activated factor XIII becomes more efficient after removal of the N terminus, leading to faster crosslinking of α2AP to fibrin and consequently prolonged clot lysis. In approximately 35% of circulating α2AP, the C terminus is absent. This C terminus contains the binding site for plasmin(ogen), the key component necessary for the rapid and efficient inhibitory mechanism of α2AP. Without its C terminus, α2AP can no longer bind to the lysine binding sites of plasmin(ogen) and is only a kinetically slow plasmin inhibitor. Thus, proteolytic modifications of the N and C termini of α2AP constitute major regulatory mechanisms for the inhibitory function of the protein and may therefore have clinical consequences. This review presents recent findings regarding the main aspects of the natural heterogeneity of α2AP with particular focus on the functional and possible clinical implications.
Introduction
α2-antiplasmin (α2AP, also called α2-plasmin inhibitor) is a key player in the fibrinolytic system (Figure 1). The fibrinolytic system is crucial for dissolving fibrin clots, facilitating tissue repair, and preventing clots from occluding vessels.1 Recent clinical studies have shown that reduced fibrinolysis (eg, due to an increase in α2AP level) is associated with an increase in both venous and arterial thrombotic risk.2 In contrast, an increase in fibrinolysis due to α2AP deficiency is associated with hemophilia-like bleeding symptoms, which typically occur after initial hemostasis as a result of the premature dissolution of fibrin. The phenotype of α2AP deficiency is heterogeneous. Complete congenital α2AP deficiency leads to severe bleeding with hemophilia-like bleeding symptoms such as joint bleeding, whereas heterozygous α2AP-deficient patients typically have mild or no bleeding symptoms.3,4 In addition, acquired α2AP deficiency may also occur in liver disease5 and amyloidosis6 or during fibrinolytic therapy.7
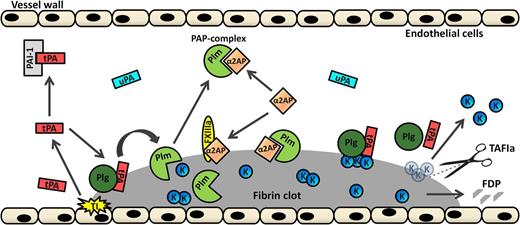
Cartoon representation of the fibrinolytic system. After tissue injury (TI, yellow explosion), tPA (red rectangle) is extensively released from endothelial cells. If not cleared from the circulation or inhibited by plasminogen activator inhibitor 1 (PAI-1; light gray), tPA can, alongside plasminogen (Plg, dark green sphere), bind to fibrin. This results in the generation of plasmin (Plm, light green PacMan figure), which will degrade fibrin into fibrin degradation products (FDPs; gray pieces). Additionally, plasminogen can be activated by fibrin-independent uPA (marine blue rectangle). Plasmin is inhibited by circulating α2AP (orange diamond) by the formation of PAP complexes. Furthermore, α2AP can be crosslinked to the fibrin surface by activated FXIIIa (yellow oval), thereby inhibiting plasmin on the fibrin surface. C-terminal lysines (K; small blue spheres) that are exposed on partially degraded fibrin form binding sites for plasminogen and tPA. Activated thrombin-activatable fibrinolysis inhibitor (TAFIa; scissors) inhibits plasmin-mediated fibrin degradation by cleaving C-terminal lysine residues from partially degraded fibrin, thereby inhibiting binding of plasminogen and tPA to fibrin and thus inhibiting generation of plasmin. Adapted and extended from Wiman and Hamsten.1
Cartoon representation of the fibrinolytic system. After tissue injury (TI, yellow explosion), tPA (red rectangle) is extensively released from endothelial cells. If not cleared from the circulation or inhibited by plasminogen activator inhibitor 1 (PAI-1; light gray), tPA can, alongside plasminogen (Plg, dark green sphere), bind to fibrin. This results in the generation of plasmin (Plm, light green PacMan figure), which will degrade fibrin into fibrin degradation products (FDPs; gray pieces). Additionally, plasminogen can be activated by fibrin-independent uPA (marine blue rectangle). Plasmin is inhibited by circulating α2AP (orange diamond) by the formation of PAP complexes. Furthermore, α2AP can be crosslinked to the fibrin surface by activated FXIIIa (yellow oval), thereby inhibiting plasmin on the fibrin surface. C-terminal lysines (K; small blue spheres) that are exposed on partially degraded fibrin form binding sites for plasminogen and tPA. Activated thrombin-activatable fibrinolysis inhibitor (TAFIa; scissors) inhibits plasmin-mediated fibrin degradation by cleaving C-terminal lysine residues from partially degraded fibrin, thereby inhibiting binding of plasminogen and tPA to fibrin and thus inhibiting generation of plasmin. Adapted and extended from Wiman and Hamsten.1
The main enzyme of the fibrinolytic system is the serine protease plasmin, which is predominantly responsible for the degradation of fibrin into rapidly cleared soluble fibrin degradation products (Figure 1). The inactive proenzyme plasminogen can be converted to plasmin by two different plasminogen activators (PAs), tissue-type PA (tPA) and urokinase-type PA (uPA). tPA-mediated plasminogen activation is fibrin dependent and is mainly involved in the dissolution of fibrin deposits in the circulation, whereas uPA is fibrin independent and binding of uPA to a specific cellular receptor (uPAR) results in enhanced activation of cell-bound plasminogen.8
To prevent the fibrin clot from being dissolved before restoration of the injured vessel, the activity of plasmin (ie, fibrinolysis) needs to be tightly controlled. Different mechanisms are responsible for inhibition of the fibrinolytic system. Plasminogen activators can be inhibited by plasminogen activator inhibitors 1 and 2. Plasmin itself is directly inhibited by α2AP by the formation of plasmin-antiplasmin (PAP) complexes. α2AP accounts for about 90% of plasmin inhibition in vivo.9 When the capacity of α2AP is exceeded by a high concentration of plasmin, plasmin will also be inhibited by α2-macroglobulin.10 Furthermore, active thrombin-activatable fibrinolysis inhibitor cleaves off carboxyl terminal (C-terminal) lysines from partially plasmin-degraded fibrin, reducing binding of plasminogen and tPA to fibrin and thus reducing plasmin generation.11
α2AP
Protein and function
Native α2AP circulates at a concentration of approximately 70 μg/mL (∼1 μM) and is primarily synthesized and secreted by the liver,14,15 although the kidney and more recently the brain have also been described as sites of α2AP production.16,17 The mean absolute catabolic (synthetic) rate of α2AP has been estimated at 2.1 mg/kg per day.18 The in vivo half-life of α2AP is about 2.6 days, whereas PAP complexes are cleared much faster from the circulation with a half-life of approximately 0.5 days.19
α2AP is a member of the serine protease inhibitor (serpin) family clade F2. The main physiological function of α2AP, as mentioned above, is the rapid inhibition of plasmin, which occurs when α2AP forms a 1:1 stable complex with plasmin, either in the circulation or on the fibrin surface.20 The reaction proceeds via a 2-step mechanism (Figure 2). Initially, in a reversible second-order reaction, the C-terminal end of α2AP, which contains 6 lysine residues, binds noncovalently to the lysine binding sites (LBSs) of plasmin(ogen).21 This step of the reaction can be competitively inhibited by plasmin(ogen) fragments containing LBSs or by lysine analogs such as ε-amino caproic acid.21 The recently crystallized structure of native plasminogen showed that in Glu-plasminogen (closed conformation), the initial binding of α2AP would actually be primarily LBS independent, whereas in Lys-plasminogen (open conformation), the LBS of kringle 2 is mainly involved in this binding.22 Earlier studies showed that kringle 4 is important in the interaction of plasmin with α2AP, but that kringles 1, 2, 3, and 5 are also involved.23,24
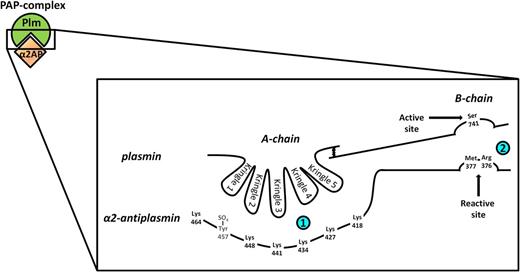
Schematic representation of plasmin inhibition by α2AP. α2AP inhibits plasmin by forming a 1:1 stable complex with plasmin. The reaction proceeds via a 2-step mechanism. Initially, in a reversible second-order reaction (small upper left box), the C-terminal end of α2AP, which contains 6 lysine residues, binds noncovalently to the LBSs present in the kringle domains of plasmin. On the basis of structural studies, it has been suggested that the α2AP C-terminal lysines interact with the kringle domains of plasmin in a cooperative zipper-like manner, although it has not yet been determined which lysines bind to which LBS.46 Kringle 3, the only non-lysine binding plasmin kringle, possibly adds to the binding between plasmin and α2AP by interacting with the highly electronegative sulfotyrosine (Tyr457-SO4) present in the α2AP C terminus.23 In the second step (large rightmost box), which is an irreversible first-order reaction, the arginine residue at position 376 of α2AP in the reactive center loop forms a covalent bond with the active site serine residue at position 741 of plasmin. This results in formation of the PAP complex accompanied by complete loss of plasmin activity. The scribble between the A-chain and B-chain of plasmin represents 2 disulfide bridges.
Schematic representation of plasmin inhibition by α2AP. α2AP inhibits plasmin by forming a 1:1 stable complex with plasmin. The reaction proceeds via a 2-step mechanism. Initially, in a reversible second-order reaction (small upper left box), the C-terminal end of α2AP, which contains 6 lysine residues, binds noncovalently to the LBSs present in the kringle domains of plasmin. On the basis of structural studies, it has been suggested that the α2AP C-terminal lysines interact with the kringle domains of plasmin in a cooperative zipper-like manner, although it has not yet been determined which lysines bind to which LBS.46 Kringle 3, the only non-lysine binding plasmin kringle, possibly adds to the binding between plasmin and α2AP by interacting with the highly electronegative sulfotyrosine (Tyr457-SO4) present in the α2AP C terminus.23 In the second step (large rightmost box), which is an irreversible first-order reaction, the arginine residue at position 376 of α2AP in the reactive center loop forms a covalent bond with the active site serine residue at position 741 of plasmin. This results in formation of the PAP complex accompanied by complete loss of plasmin activity. The scribble between the A-chain and B-chain of plasmin represents 2 disulfide bridges.
In the second step, which is an irreversible first-order reaction, the arginine residue in position 376 of α2AP (numbering in this manuscript is according to methionine in position 1) in the reactive center loop (RCL) forms a covalent bond with the active site serine of plasmin.14,20 This results in the PAP complex, accompanied by complete loss of plasmin activity and cleavage of a scissile peptide bond of α2AP.26
Early biochemical studies showed that the interaction between α2AP and plasminogen is under the strong influence of N-glycosylation of the asparagine residue in position 289 in plasminogen kringle 3.26 Furthermore, it was shown that the interaction between α2AP and plasminogen may additionally hinder plasmin generation by competitively inhibiting the adsorption of plasminogen to fibrin.27 In contrast, another study reported that the α2AP-plasminogen interaction did not affect the binding of plasminogen to fibrin, the dissociation of the plasminogen-fibrin complex, or the generation of plasmin.28 The latter study postulated that inhibition of plasmin by α2AP inhibits the plasmin-mediated generation of C-terminal lysines, and in this way reduces the binding sites for plasminogen and tPA on fibrin and thus reduces plasmin generation.
During fibrin clot formation, activated coagulation factor XIII (FXIIIa) covalently incorporates (crosslinks) α2AP into the fibrin clot where it can also directly inhibit plasmin.29 The actual crosslink is formed between the glutamine residue at position 14 of α2AP and the lysine residue at position 303 of the fibrin α chain.30 The functional importance of crosslinking of α2AP to fibrin was illustrated in experiments using plasma from α2AP-deficient patients, who since early childhood usually suffered from mild or severe bleeding tendencies depending on the residual levels of α2AP.31 Clot lysis was accelerated in α2AP-deficient plasma, and when α2AP-deficient plasma was reconstituted with α2AP, the rate and extent of lysis was inversely proportional to the concentration of exogenous α2AP added. Subsequent studies in whole blood and plasma showed that the formation of fibrin-fibrin crosslinks do not contribute significantly to the resistance of the clot to fibrinolysis, and thus that the stability of thrombi to lysis depends predominantly on crosslinking of α2AP into the thrombus.32,33 Other studies showed that α2AP may also noncovalently bind to fibrin and fibrinogen, which could potentially serve to provide the proper orientation of the crosslinking sites of α2AP and fibrin to facilitate crosslinking of α2AP to fibrin.34
Gene and protein structure
The gene coding for human α2AP, SERPINF2, is located on chromosome 17p13.337 and encodes a protein of 491 amino acid residues that is expressed as a single-chain protein of 464 amino acid residues with a 27-amino-acid residue signal peptide.10,36-40 The gene contains 10 exons and 9 introns and spans approximately 16 kb of DNA.41 The complete complementary DNA sequence shows 23% to 28% homology with other members of the serpin family and 73% to 81% homology with α2AP from other species, such as rat, mouse, cow, and rabbit.42,43 Exon 1 of the human SERPINF2 gene consists entirely of untranslated sequences. The 27-amino-acid residue signal peptide is encoded by exon 2 and part of exon 3, whereas the mature protein is encoded by exons 3 to 10. The amino terminal (N-terminal) region of the protein comprising the glutamine involved in crosslinking to fibrin is encoded by exon 4, whereas both the RCL containing the reactive site arginine and the plasminogen-binding site in the C-terminal region are encoded by exon 10.41 An alternatively spliced isoform of α2AP has been reported that lacks exon 5 (NM_001165921), although the expressed amount of this variant or any consequences of its function have not yet been described.
Native α2AP molecules contain 11% to 14% carbohydrate (four putative N-linked glycosylation sites at asparagine residues 99, 268, 282, and 289) and a sulfated tyrosine residue at position 457.44 This leads to a molecular weight of approximately 67 000.10,14,36 Furthermore, α2AP contains 1 disulfide bridge (between the cysteine residues in positions 43 and 116) that does not appear to have a profound influence on the structure or function of α2AP.45 This is consistent with the fact that most serpins do not contain disulfide bridges. To date, there is no 3D protein structure available for intact human α2AP, but using x-ray diffraction, the crystal structure of a 43-amino-acid N-terminally truncated form of murine α2AP was determined, which exhibited the typical fold of the serpin core structure with its β sheets and α helices.46 In this structure, parts of the RCL (residues 368-376) and the C-terminal end (residues 420-464) could not be modeled into electron density, suggesting that these regions of the molecule are flexible in the absence of plasmin. The structure did reveal that the interactions between C-terminal residues 410-419 and the serpin body positioned the C terminus less than 30 A from the RCL, suggesting that the C-terminal region may function as a hook that accelerates the interaction with plasmin into the physiological range. The I-TASSER predicted structure of intact human α2AP, based on the structure of murine a2APΔ43, is shown in Figure 3.47 It will be interesting to solve the actual structure of intact human α2AP to see how the N and C termini are situated.
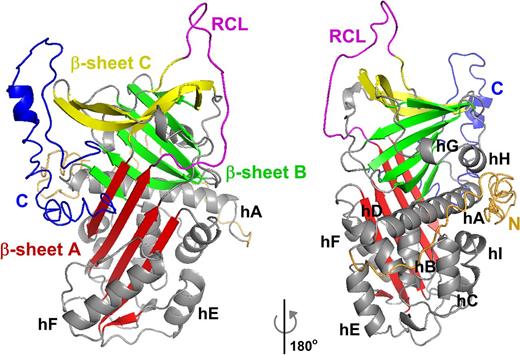
Predicted structure of intact human α2AP. Schematic representation of the structure of intact human α2AP, as predicted by I-TASSER.47 The structure is based on 2R9Y.pdb of murine α2APΔ43.46 Colors and orientation were adjusted using PyMOL (Schrödinger LLC, The PyMOL Molecular Graphics System, Version 1.7.4). The A sheet is shown in red, the B sheet in green, and the C sheet in yellow, the RCL in magenta, and the 9 helices (labeled hA-hI) and loops in gray. The N terminal region is orange, and the C terminal region is blue.
Predicted structure of intact human α2AP. Schematic representation of the structure of intact human α2AP, as predicted by I-TASSER.47 The structure is based on 2R9Y.pdb of murine α2APΔ43.46 Colors and orientation were adjusted using PyMOL (Schrödinger LLC, The PyMOL Molecular Graphics System, Version 1.7.4). The A sheet is shown in red, the B sheet in green, and the C sheet in yellow, the RCL in magenta, and the 9 helices (labeled hA-hI) and loops in gray. The N terminal region is orange, and the C terminal region is blue.
Posttranslational heterogeneity of α2AP
N-terminal variation
After expression, full-length α2AP is proteolytically modified, leading to a variety of circulating α2AP molecules (Figure 4). About 70% of circulating α2AP is N-terminally cleaved between the proline residue at position 12 and the asparagine residue at position 13.48 This results in an α2AP molecule with an asparagine (Asn) residue at the N terminus (Asn-α2AP).39,40 The other 30% of the protein circulates in plasma with a methionine (Met) residue at the N terminus (Met-α2AP). This N-terminal cleavage has been shown to affect the ability of α2AP to become crosslinked to fibrin by FXIIIa. Recombinant studies showed that the amount of crosslinked recombinant Met-α2AP at 5 minutes incubation was less than one-third that of plasma α2AP (ie, 70% Asn-α2AP),38 suggesting that the crosslinking site (the glutamine at position 2 in Asn-α2AP, which corresponds to position 14 in Met-α2AP) could be hindered by the additional 12 amino acids present in Met-α2AP. In line with this, Holmes et al49 reported that recombinant Asn-α2AP with an extension of 3 additional N-terminal amino acids could no longer be crosslinked into fibrin. More recently, these findings were clarified and extended in studies by Lee et al,48 who showed that during the initial 10 minutes of clot formation, plasma-purified Asn-α2AP became crosslinked to fibrin approximately 13 times faster than plasma purified Met-α2AP. Furthermore, they observed that urokinase-induced plasma clot lysis rates were delayed in direct proportion to the ratio of Asn-α2AP to Met-α2AP in human plasma, with a maximally delayed clot lysis when only Asn-α2AP was present.48,50,51 These studies suggested that, because of the greater efficiency of Asn-α2AP incorporation into fibrin, Asn-α2AP inhibits plasmin-mediated fibrin digestion more effectively than Met-α2AP, which may have clinical consequences as described below. Other studies investigated the importance of the N-terminal sequence of α2AP in the interaction with FXIIIa. They showed that both Asn1 in Asn-α2AP (corresponding to Asn13 in Met-α2AP) and amino acids 7 through 12 in Asn-α2AP (corresponding to amino acids 19-24 in Met-α2AP) as a secondary binding site are essential for an effective enzyme-substrate interaction.52,53
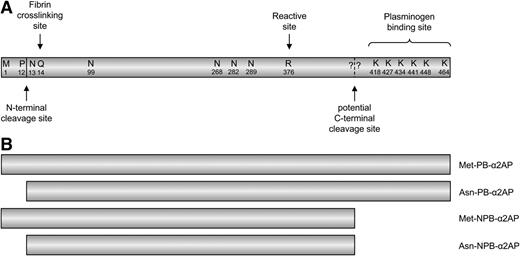
Schematic representation of the α2AP protein and its different molecular forms. (A) Natively expressed α2AP and amino acid residues with their respective locations in the α2AP protein (not to scale). N-terminal cleavage, which results in the conversion of Met-α2AP to Asn-α2AP, occurs between proline at position 12 and asparagine at position 13. The glutamine residue at position 14 (position 2 in Asn-α2AP) is the fibrin crosslinking site. The dashed line represents the potential C-terminal cleavage site. The question marks represent the fact that the actual cleavage site is still unknown. The plasminogen-binding site of α2AP is located at the C-terminal end. (B) The different molecular forms of α2AP, 2 plasminogen-binding (PB) forms, and 2 non-plasminogen-binding forms.
Schematic representation of the α2AP protein and its different molecular forms. (A) Natively expressed α2AP and amino acid residues with their respective locations in the α2AP protein (not to scale). N-terminal cleavage, which results in the conversion of Met-α2AP to Asn-α2AP, occurs between proline at position 12 and asparagine at position 13. The glutamine residue at position 14 (position 2 in Asn-α2AP) is the fibrin crosslinking site. The dashed line represents the potential C-terminal cleavage site. The question marks represent the fact that the actual cleavage site is still unknown. The plasminogen-binding site of α2AP is located at the C-terminal end. (B) The different molecular forms of α2AP, 2 plasminogen-binding (PB) forms, and 2 non-plasminogen-binding forms.
Lee et al48 also purified and identified the plasma proteinase that is responsible for the in vitro conversion of Met-α2AP to Asn-α2AP and named it antiplasmin-cleaving enzyme. The enzyme appeared to be a soluble, circulating derivative of fibroblast activation protein (FAP) that lacked the cytoplasmic tail and transmembrane part of FAP.54 FAP is a homodimeric type II integral membrane protein of the prolyl oligopeptidase family mainly expressed on activated fibroblasts in areas of tissue remodeling,55 and its intrahepatic expression significantly correlates with fibrosis severity.56 We recently showed in patients with different stages of liver cirrhosis and thus different severities of fibrosis that, besides intrahepatic expression, circulating levels of soluble FAP (sFAP) also increased with the severity of fibrosis.57 Consequently, cirrhotic patients had an increased percentage of α2AP N-terminal cleavage (ie, more Asn-α2AP).
On basis of the in vitro data mentioned above, we also expected to find more Asn-α2AP in patients suffering from arterial thrombosis, because earlier studies showed that a reduced fibrinolytic capacity is a risk factor for myocardial infarction and stroke.58,59 But in our studies of sFAP levels in relation to α2AP N-terminal cleavage in patients suffering from arterial thrombosis (coronary heart disease, ischemic stroke, or peripheral arterial disease) and in age- and sex-matched healthy control individuals, we unexpectedly found less Asn-α2AP and reduced sFAP levels in the coronary heart disease patients compared with the levels in the healthy control individuals.60,61 In addition, we did not find an association between α2AP N-terminal cleavage and plasma clot lysis time. Thus, although the in vitro data on the effect of α2AP N-terminal cleavage on crosslinking to fibrin and clot lysis seem convincing, we have not yet been able to show these effects in population studies. Further studies are needed to find out whether these discrepancies are the result of methodologic issues or whether the in vitro studies overestimate the in vivo effects of α2AP N-terminal cleavage.
There is evidence that the N-terminal heterogeneity of α2AP is influenced by genetic variation in the SERPINF2 gene.62 Christiansen et al62 showed that the arginine-to-tryptophan (Trp) polymorphism (p.Arg6Trp, rs2070863) at position 6 is a functional polymorphism affecting the conversion of Met-α2AP to Asn-α2AP and thereby the rate of α2AP incorporation into fibrin. They showed that plasma-purified Met-α2AP(Arg6) was cleaved approximately eightfold faster by antiplasmin-cleaving enzyme/sFAP than Met-α2AP(Trp6), relating the polymorphism to α2AP N-terminal cleavage with the highest percentage of N-terminal cleavage in homozygous Arg carriers. These individuals also tended to have the shortest plasma clot lysis times, indicating that the polymorphism may influence fibrinolysis, although the results did not reach statistical significance and were not controlled for other determinants of plasma clot lysis time such as plasminogen activator inhibitor 1 or fibrinogen levels.
There have been only a few studies that investigated the p.Arg6Trp polymorphism in relation to clinical outcomes. In a Hungarian study in 100 ischemic stroke patients, the polymorphism affected the outcome of thrombolytic therapy using recombinant tPA as the Trp allele had a protective effect against the development of an ischemic lesion.63 In another study, the same research group showed that the presence of the Trp allele did not influence the risk of coronary atherosclerosis or myocardial infarction.64 Furthermore, 1 study investigated whether the polymorphism was associated with the development of abdominal aortic aneurism (AAA).65 The authors found that Trp allele carriers had a slight (18%) but nonsignificant decrease in risk. They actually showed that another polymorphism in α2AP (p.Arg407Lys, rs1057335), which has a high degree of linkage disequilibrium with p.Arg6Trp, was significantly associated with the development of AAA with a 23% reduced risk for Lys407 carriers. Their data suggest that p.Arg407Lys might be the functional α2AP variant that influences fibrinolysis and AAA formation. It will also be interesting to determine whether any of these polymorphisms has an influence on venous thrombotic risk as α2AP might play a more significant role in the stabilization of fibrin-rich venous clots, but this has not yet been investigated.
C-terminal variation
In addition to the N terminus, the C terminus of α2AP is also posttranslationally modified. The C-terminal region of α2AP is unique because it contains approximately 55 additional amino acid residues when compared with the C termini of other serpins present in the hemostatic system (Figure 5).66,67 This C-terminal extension is conserved among species, with 67% identity between human and bovine and 61% identity between human and mouse species.68
Alignment of serpin C termini. C termini of serpins present in the hemostatic system and (*) identical, (:) conserved, or (.) semiconserved substitutions are shown. The C terminus of α2AP extends approximately 55 amino acids beyond the C termini of the other hemostatic serpins. The 6 lysine residues (K) in the α2AP C terminus are shown in bold, and the RGD sequence is underlined. The alignment was made with ClustalW2.67 (●) Sulfated tyrosine residue (Y). α1AT, α-1-antitrypsin; ATIII, antithrombin III; HCII, heparin cofactor II; PCI, protein C inhibitor; PZRPI, protein Z–related protease inhibitor.
Alignment of serpin C termini. C termini of serpins present in the hemostatic system and (*) identical, (:) conserved, or (.) semiconserved substitutions are shown. The C terminus of α2AP extends approximately 55 amino acids beyond the C termini of the other hemostatic serpins. The 6 lysine residues (K) in the α2AP C terminus are shown in bold, and the RGD sequence is underlined. The alignment was made with ClustalW2.67 (●) Sulfated tyrosine residue (Y). α1AT, α-1-antitrypsin; ATIII, antithrombin III; HCII, heparin cofactor II; PCI, protein C inhibitor; PZRPI, protein Z–related protease inhibitor.
Several studies regarding the purification of human α2AP found that 2 molecular species of α2AP exist in plasma of which only 1 can bind to the LBS of plasminogen.21,69 The form that can bind to plasminogen was therefore called plasminogen-binding α2AP (PB-α2AP) and comprises approximately 65% of circulating α2AP. The form that does not bind to plasminogen was called non-plasminogen-binding α2AP (NPB-α2AP) and comprises the remaining 35% of circulating α2AP.69,70 Kluft et al71,72 demonstrated the presence of the 2 variants in plasma and in serum by modified crossed immunoelectrophoresis and showed that PB-α2AP is produced by the liver and that NPB-α2AP is formed in the circulation. These 2 forms of α2AP were also found in rat α2AP.73
Studies on the mechanism and kinetics of the reaction between α2AP and plasmin showed that the very fast-acting inhibitor of plasmin was actually PB-α2AP; in all these studies α2AP was purified by using a column with plasminogen or plasminogen kringles 1 to 3 attached to it and thus only PB-α2AP was purified.74-76 It has also been reported that the portion of α2AP that did not absorb to the plasminogen LBS (ie, NPB-α2AP) was still functional because it could form a complex with plasmin,69,75 although this is a very slow reaction. C-terminal cleavage of α2AP may actually have significant clinical consequences, because removal of the α2AP C terminus strongly regulates α2AP activity. We showed this in a recent study of C-terminal variation of α2AP in which we found the highest αAP activity in individuals with the highest percentage of PB-α2AP.77 On average, 38% of all circulating α2AP was cleaved at its C terminus, with a wide interindividual range from 10% to 60%. However, we did not find a difference in the percentage α2AP C-terminal cleavage in a small population of male myocardial infarction patients and healthy male controls.77 Furthermore, we showed that the antiplasmin activity assay, as currently used for diagnostic purposes, is indeed mainly based on PB-α2AP. Although studies of crosslinking of α2AP to fibrin by FXIIIa have mainly focused on the N terminus and N-terminal variation of the protein, there is evidence that the C terminus could also be involved, because it was shown that crosslinking of α2AP to fibrin primarily involved PB-α2AP.78 Thus, the enzyme or mechanism responsible for loss of the α2AP C terminus may be a strong regulator of fibrinolysis.
In addition to the in vivo presence of NPB-α2AP, it has also been shown that in vitro purified PB-α2AP can spontaneously convert to an NPB-α2AP.79 Trypsin digestion of both purified PB-α2AP and in vitro formed NPB-α2AP localized the plasminogen-binding site of α2AP in the C terminus of the molecule.80 A 26-amino-acid peptide cleavage product representing the C-terminal 26 amino acids of the molecule was found in PB-α2AP but was not present in NPB-α2AP formed in vitro, which indicates its participation in plasminogen binding.81 Although some subsequent studies have reported this trypsin cleavage site as the position where PB-α2AP is truncated into NPB-α2AP, to date it is unknown where and how the conversion happens in the circulation. An actual in vivo cleavage site and the protease responsible have not yet been identified, despite previous attempts to inhibit NPB-α2AP formation during plasma incubation by a spectrum of protease inhibitors directed to various serine and thiol proteases.71 Several investigations have identified enzymes that can cleave PB-α2AP in vitro, such as elastase82-84 and MMP-3,85 but it has to be confirmed whether one of these enzymes is actually responsible for PB-α2AP cleavage in vivo. Furthermore, it has been suggested that the in vivo and in vitro conversions of PB-α2AP to NPB-α2AP in plasma have different mechanisms and possibly involve different cleavage sites.86 Using a monoclonal antibody against the C terminus of α2AP in a crossed immunoelectrophoresis technique, Leebeek et al86 showed that NPB-α2AP formed in vivo lost its capacity to bind to both plasminogen and to the antibody, whereas NPB-α2AP formed in vitro lost its plasminogen binding capacity but could still bind to the antibody. This indicated that in vivo and in vitro NPB-α2AP are not the same molecules but may differ in their C termini or may have a different structure.
All studies of PB-α2AP stress the importance of the α2AP C terminus in the inhibiting reaction with plasmin(ogen), a reaction that occurs through the interaction of the lysine (K) residues present in the C terminus (K418, K427, K434, K441, K448, and K464; Figure 5) with the LBS in the kringle domains of plasmin(ogen), as described above. Several studies have investigated the role of these lysine residues in the interaction with the LBS of plasmin(ogen), although results have been somewhat inconsistent. Initial studies showed that the association of α2AP with plasmin was reduced in the presence of synthetic peptides mimicking the α2AP C terminus, with K464 and K448 being major sites of interaction with plasmin.87,88 Subsequent peptide studies indicated that the α2AP C terminus is flexible, with the most important role for K464 in the binding of the C terminus to isolated plasminogen kringles, whereas the internal K residues may strengthen this binding in a sequential zipper-like way.23,89 However, using recombinant intact α2AP, Wang et al90,91 showed that K448, not K464, played the most important role in the LBS-mediated interaction between intact plasmin(ogen) or kringles 1 to 3 and α2AP. They explain the discrepancies between the findings by the different proteins used (peptides vs intact α2AP and isolated kringles vs full-length plasminogen), and they believe that K464 may be more involved in interactions with smaller molecules but may be involved to a lesser extent with the complete plasmin(ogen) molecule. More recently, Lu et al92 performed a comprehensive study in which they systematically and sequentially mutated the 5 most conserved C-terminal lysines (K427, K434, K441, K448, and K464) in recombinant human α2AP. They investigated the plasmin inhibition rate and the binding affinity of full-length recombinant α2AP and plasmin and showed that each conserved K residue participated in the binding and inhibition of plasmin, with K464 being the most important K residue. They further suggested that additional interactions may exist between the serpin core domain of α2AP and plasmin. Other possibly electrostatic interactions could exist between the α2AP C terminus and the plasmin(ogen) kringle domains, because at physiological pH, the kringle domains carry an estimated net positive charge,23 whereas the α2AP C terminus has a net negative charge, with 9 aspartic acid and glutamic acid residues and a sulfated tyrosine residue (Figure 5).44
Besides the lysine residues, the C-terminal region of human α2AP also contains an arginine-glycine-aspartic acid (RGD) sequence (Figure 5), a sequence crucial for cell recognition and adhesion via integrins.93 The functionality of this RGD sequence in α2AP has only marginally been studied. It was shown that a hybrid peptide (RGDF coupled with the 26 C-terminal amino acids of α2AP) could simultaneously inhibit platelet activation and accelerate in vitro fibrinolysis.94 The same peptide was used to show that the binding of plasminogen to platelets via integrin α2bβ3 was enhanced in the presence of the peptide.95 Furthermore, there has been 1 study that showed that an α2AP C-terminal peptide could bind to human umbilical vein endothelial cells via the RGD sequence, mediated mainly by integrins α5β1 and ανβ3.96 It is interesting to speculate whether the interaction of α2AP with integrins at cell surfaces may play a role in the control of cellular fibrinolysis (eg, by regulating local plasmin activity on cell surfaces and inhibiting plasmin-induced migration of endothelial cells).97 So although there are some clues, the actual biological relevance of the RGD sequence in the α2AP C terminus remains to be determined and could be questioned, because the RGD sequence is present only in human and cow α2AP but is not conserved in other mammalian species such as rats, mice, and rabbits.
Summary and prospects
α2AP, a main inhibitor of fibrinolysis, circulates in the blood in different molecular forms. Posttranslational modifications of the 2 termini of α2AP constitute major regulatory mechanisms for the inhibitory function of the protein. N-terminal cleavage leads to increased crosslinking of α2AP to fibrin, and C-terminal cleavage leads to loss of its plasmin inhibitory capacity. The importance of α2AP in the pathogenesis of thrombotic disorders was recently re-emphasized, because a study in mice showed that, compared with treatment with tPA, inhibition of α2AP using an inhibiting antibody led to a markedly reduced stoke mortality,98 which highlights the potential of modulating α2AP activity as antithrombotic treatment. Increasing the knowledge about the mechanisms of α2AP posttranslational modification that modulate α2AP function will profoundly improve our understanding of the action of α2AP and the fibrinolytic system, which may lead to novel means of antithrombotic therapy.
Acknowledgment
This research was funded partly by a grant from the Dutch Thrombosis Foundation (TSN 2009-1) and partly by a fellowship awarded by the European Haematology Association and the International Society of Thrombosis and Haemostasis.
Authorship
Contribution: All authors critically reviewed the literature and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shirley Uitte de Willige, Erasmus University Medical Center Rotterdam, Department of Hematology, Room Nb-847, Wytemaweg 80, 3015 CN Rotterdam, The Netherlands; e-mail: s.uittedewillige@erasmusmc.nl.