In this issue of Blood, Nagata et al reported that different Ras homolog gene family, member A (RHOA) hotspot mutations among the adult T-cell leukemia/lymphoma (ATLL) category have opposite biochemical activities, which are linked to different T-cell phenotypes.1
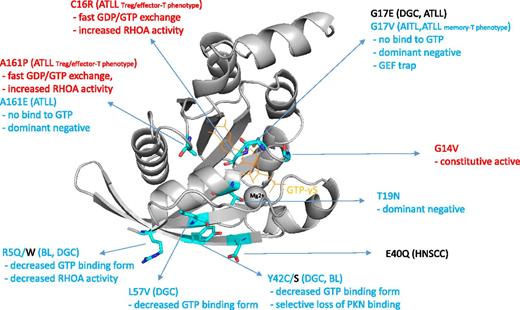
The RHOA hotspot mutations reported so far in various tumor subtypes.1-9 The disease categories, mutations, and biochemical properties linked to increased and decreased RHOA activity are indicated by red and blue color, respectively. Image of 1A2B10 created with PyMOL (Schrödinger, LLC). GDP, guanosine diphosphate; GEF, guanine nucleotide exchange factor; Treg, T-regulatory cell.
The RHOA hotspot mutations reported so far in various tumor subtypes.1-9 The disease categories, mutations, and biochemical properties linked to increased and decreased RHOA activity are indicated by red and blue color, respectively. Image of 1A2B10 created with PyMOL (Schrödinger, LLC). GDP, guanosine diphosphate; GEF, guanine nucleotide exchange factor; Treg, T-regulatory cell.
RHOA is a well-known protein which was extensively characterized in the context of cell movement, cytokinesis, and stress fiber formation. Although RHOA’s involvement in cancer has been reported by many researchers so far, its critical role as a tumor driver gene has recently attracted attention through cancer genome sequencing. Several groups reported recurrent somatic RHOA mutations in angioimmunoblastic T-cell lymphoma (AITL), a subtype of lymphoma with follicular helper T-cell phenotype.2,3 The mutation distribution among AITL cases constitutes a clear hotspot G17V. Rohde et al also reported recurrent mutations of RHOA in pediatric Burkitt lymphoma (BL), and they found that the mutation distribution is biased with several hotspots, like R5W.4 RHOA somatic mutations have also been identified in solid tumors. Large-scale pan-cancer genome analysis identified recurrent hotspot E40Q mutations in head-and-neck squamous cell carcinoma (HNSCC).5 Independent groups discovered RHOA mutations in diffuse-type gastric carcinoma (DGC), and, among several hotspots, Y42C/S mutations are identified recurrently across independent cohorts in these studies.6,7
The recurrent hotspot nature of the mutations gives rise to a simple notion that these mutations increase the biochemical RHOA activity, like typical tyrosine kinase mutations, therefore, small-molecule inhibitors targeting the RHOA mutant should be effective. However, the story is unlikely to be so simple. In the report about AITL and BL, G17V and R5Q mutations reduce the guanosine triphosphate (GTP)-binding form of RHOA, and the downstream effector activities like serum response factor transcription and the stress fiber formation significantly decreased.2,3,8 In addition, it has been shown that the loading of GTP does not occur by G17V mutation. In the case of Y42C mutations in gastric cancer, it has also been shown that the adenosine triphosphate–bound active form has decreased, whereas another report showed selective loss of binding of RHOA to protein kinase N (PKN) effector protein.7,9 It is inferred from these facts that, unlike typical kinase mutations, RHOA mutants are not biochemically activated and so-called “RHOA inhibitor” would not be effective.
There are 2 major questions about RHOA functions. First, why are the sites of hotspot mutations different depending on the tumor type? Second, how do the biochemical properties of each mutation differ (see figure1-10 )?
The study reported by Nagata et al in this issue suggests important clues for these questions.1 Based on the RHOA mutations they found in ATLL, they analyzed in detail the biochemical activity and the cellular counterpart of the RHOA mutant cases with different hotspot positions. In their analysis, RHOA mutations of ATLL constitute C16R, G17V/E, and A161P/V hotspots, and these positions are partially shared by G17V and A161E found in AITL. In detailed biochemical analysis, it was shown that C16R and A161P/V result in fast GTP/GDP exchange and are biochemically highly active, whereas G17V and A161E do not incorporate the GTP and are biochemically inactive.
Why are the gain-of-function and the loss-of-function mutations observed in the same disease category? Nagata et al have focused on the difference in cellular phenotype/lineage as a factor correlated with these biochemical differences. They performed surface marker analyses and immunohistochemical assays and found that, whereas ATLL cells with activating C16R and A161P/V mutations have Treg or effector T-cell phenotype, ATLL cells with inactivating G17V have memory T-cell phenotype. It is supposed that, in human T-lymphotropic virus type infection, proliferation and survival of these T cells are differentially dependent on the active and inactive RHOA signals by their cellular lineages. Alternatively, an active or inactive RHOA signal is an upstream trigger for the cellular differentiation of T cells into Treg/effector T-cell or memory T-cell lineages.
In any case, this report clearly shows that the RHOA mutations with opposite biochemical activities are mixed within the ATLL category, and these are associated with the cellular lineages of the tumors. Comprehensive understanding of the mutant RHOA function (including various mutations found in BL, HNSCC, and DGC) are future challenges, but this report gives us important hints as to why different hotspot mutations dominate in different tumor subtypes, and how these biochemical functions are.
Conflict-of-interest disclosure: The author declares no competing financial interests.