In this issue of Blood, Nagler et al present a systematic review and meta-analysis on the diagnostic accuracy of immunoassays for heparin-induced thrombocytopenia (HIT). Their data, when combined with the 4T score, provide an easy-to-use, evidence-based framework for estimating the probability of HIT.1
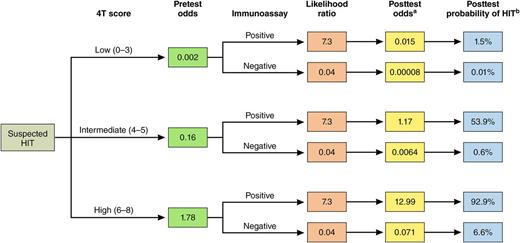
Bayesian approach to estimating the probability of HIT. The 4T score in conjunction with an immunoassay can be used to estimate the posttest probability of HIT. The pretest odds of HIT, based on the 4T score, is derived from Cuker et al.3 For likelihood ratios for various immunoassays, see Table 2 in the article by Nagler et al that begins on page 546.1 The likelihood ratios for the polyspecific ELISA (low threshold) are shown as an example. These values can be used to calculate the posttest odds and posttest probability of HIT using the equations below. aPosttest odds = pretest odds × likelihood ratio; bPosttest probability = posttest odds/(posttest odds + 1). Professional illustration by Patrick Lane, ScEYEnce Studios.
Bayesian approach to estimating the probability of HIT. The 4T score in conjunction with an immunoassay can be used to estimate the posttest probability of HIT. The pretest odds of HIT, based on the 4T score, is derived from Cuker et al.3 For likelihood ratios for various immunoassays, see Table 2 in the article by Nagler et al that begins on page 546.1 The likelihood ratios for the polyspecific ELISA (low threshold) are shown as an example. These values can be used to calculate the posttest odds and posttest probability of HIT using the equations below. aPosttest odds = pretest odds × likelihood ratio; bPosttest probability = posttest odds/(posttest odds + 1). Professional illustration by Patrick Lane, ScEYEnce Studios.
The hematology fellow’s phone rings. A call from the surgical intensive care unit (SICU). Another consult for thrombocytopenia, rule out HIT. The fellow looks down at the floor and follows the well-worn path to the SICU. She has done so many consults like this before and yet still finds them difficult. Should she recommend cessation of heparin and initiation of a nonheparin anticoagulant while awaiting confirmatory laboratory testing? Or is the probability of HIT sufficiently low that heparin may be continued? She recognizes the high stakes inherent in this decision. Delays in implementing appropriate therapy in patients with serologically confirmed HIT are associated with an initial 6.1% daily risk of thromboembolism, amputation, and death.2 Misdiagnosis, conversely, may result in exposure of postoperative, thrombocytopenic patients without HIT to costly alternative anticoagulants and their attendant ∼1% daily risk of major bleeding. She wishes for a diagnostic tool that will help her make the right call.
The 4T score, a clinical or pretest scoring system with well-characterized operating characteristics, is one such tool. In a systematic review and meta-analysis, the negative predictive value of a low probability 4T score was 99.8% (95% confidence interval, 97-100). The positive predictive value of an intermediate and high probability 4T score was 14% (9-22) and 64% (40-80), respectively.3 Although helpful, the 4T score is hampered by interobserver variability4 and modest positive predictive value, and may be difficult to calculate or unreliable if clinical information is missing.5
In light of these limitations, clinicians rely heavily on laboratory testing to assist with diagnosis. Laboratory assays fall into 2 categories: platelet factor 4/heparin immunoassays and washed platelet functional assays. The latter are more specific than immunoassays and are considered the “gold standard” among HIT laboratory tests, but are highly specialized and are performed only at select reference laboratories, often with turnaround times of several days. Immunoassays, by contrast, are the mainstay of HIT laboratory testing in most centers. The prototypical immunoassay, the polyspecific enzyme-linked immunosorbent assay (ELISA), is encumbered by frequent false-positive results and slow turnaround time. Recent years have witnessed a proliferation of immunoassays designed to overcome these limitations. Modifications to the ELISA including immunoglobulin G-specific detection, addition of a high heparin confirmatory step, and increases in the optical density cutoff have been implemented to enhance specificity. No less than 6 rapid immunoassays, which provide results in 30 minutes or less, have entered the market to shorten turnaround time and provide laboratory diagnostic information at the point of care.6
The operating characteristics of many of these immunoassays have been described only in small single center studies and have not been compared with one another. Nagler et al present the results of a well-designed systematic review and meta-analysis on the diagnostic accuracy of immunoassays for HIT. Their analysis provides the best available estimates of the performance of these assays. They observed important differences between tests based on type of assay, antibody specificity, and cutoff. Only 5 of the 20 tests they investigated met criteria for high sensitivity (>95%) and specificity (>90%).1
The results of Nagler et al provide clinicians with critical information to assist in test interpretation, patient evaluation, and management. The authors calculated positive and negative likelihood ratios for each of the immunoassays that they investigated. These likelihood ratios may be combined with the pretest odds of HIT, as determined by the 4T score, to estimate the posttest odds of HIT (see figure).6-8 This Bayesian approach provides clinicians with a systematic framework for estimating the probability of HIT in their patients, information that can be used to guide management decisions. In the example highlighted in the figure, a positive polyspecific ELISA (low threshold) in a patient with an intermediate 4T score would increase the probability of HIT to 53.9%, supporting empiric treatment of HIT while awaiting confirmation with a functional assay. Conversely, a negative ELISA in the same patient would reduce the posttest probability of HIT to 0.6%, suggesting that heparin could be safely continued. Similar analyses can be conducted for other immunoassays by inserting their corresponding likelihood ratios, as determined by Nagler et al.1
The approach illustrated in the figure has limitations. Estimates of the diagnostic accuracy of both the 4T score and immunoassays were derived, in part, from small studies of low or moderate methodologic quality.1,3 Sound clinical judgment remains central to evaluation of the patient with suspected HIT. Nevertheless, a Bayesian strategy that combines information from the 4T score and laboratory assay result provides a much-needed, evidence-based structure to this evaluation.
Nagler et al have provided the key data. A logical next step is the development of an app to facilitate estimation of the posttest probability of HIT at the point of care. Such a tool could improve clinical decision-making, outcomes, and cost-effectiveness of care, and perhaps diminish the sense of dread instilled in the hematology fellow the next time the SICU calls.
Conflict-of-interest disclosure: The author declares no competing financial interests.