In this issue of Blood, Estevez et al propose a model of platelet activation induced by low levels of thrombin and requiring 2 different platelet receptors that demonstrate a mutually dependent cooperativity.1
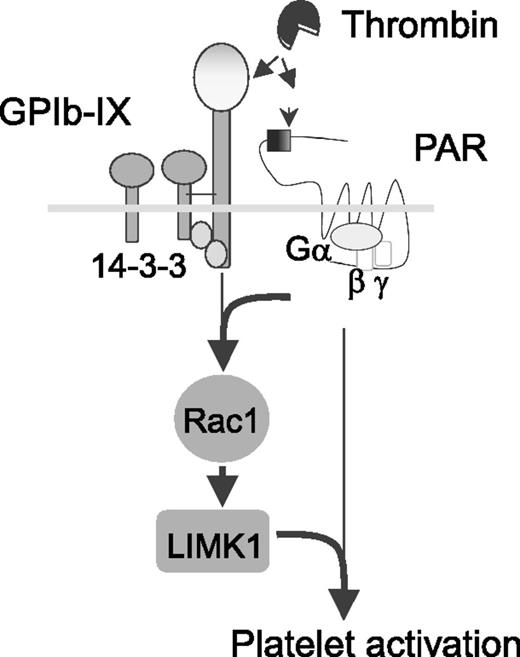
Signaling-mediated cooperativity between platelet GPIb-IX and PAR1. Depicted is a model for independent thrombin binding to 2 different platelet receptors that elicit mutually dependent signals via the 14-3-3-Rac1-LIMK1 pathway. See Figure 6i in the article by Estevez et al that begins on page 626.
Signaling-mediated cooperativity between platelet GPIb-IX and PAR1. Depicted is a model for independent thrombin binding to 2 different platelet receptors that elicit mutually dependent signals via the 14-3-3-Rac1-LIMK1 pathway. See Figure 6i in the article by Estevez et al that begins on page 626.
Thrombin is the central player in hemostasis and thrombosis. As the major platelet agonist, thrombin leads to platelet activation and thrombus formation. There exists a vast literature documenting thrombin binding to platelets and the platelet’s response. The earliest studies identified the platelet glycoprotein Ib-IX complex (GPIb-IX) receptor as a high affinity thrombin binding site on the platelet surface.2 This conclusion has been supported by multiple lines of evidence including inhibitory anti-GPIb monoclonal antibodies, peptide inhibition studies, and site-directed mutagenesis.3 However, the relevance of GPIb-IX as “the thrombin receptor” required rethinking with the important discovery of protease-activated receptors (PARs).4 This elegant work demonstrated that PARs cleaved by thrombin expose a unique tethered ligand sequence that initiates intracellular signaling and platelet activation. However, the situation is more complex when considering the response of platelets to low dose thrombin, as here the presence of GPIb-IX is required. Thus, unresolved questions of precisely how thrombin participates in platelet activation have remained.
Some have suggested the role of thrombin binding to platelet GPIb-IX as a “docking site” or “binding site” on the platelet membrane that facilitates the cleavage and activation of PARs.5 As such, GPIb-IX would be considered a surface-binding site that is unable to transmit activation signals to the intracellular platelet milieu. Alternatively, others have presented data that catalytically inactive thrombin can still activate platelets, suggesting that GPIb-IX is a likely mediator in this pathway.6 The current work of Estevez et al uses heterologous cell lines, mutant GPIb-IX receptors, catalytically inactive thrombin, and a variety of pharmacologic inhibitors targeting thrombin and GPIb-IX to gain insight into the cellular response elicited by thrombin. The data lead to a model whereby GPIb-IX is neither a simple thrombin “docking site” nor a PAR-independent thrombin receptor (see figure). Rather, there is signaling cooperativity between GPIb-IX and PARs in the presence of low levels of thrombin. PAR-dependent responses require GPIb-IX, and GPIb-IX signaling requires cooperativity with PARs. The authors demonstrate the mutually dependent cooperativity signals via the 14-3-3-Rac1-LIMK1 pathway, which is also associated with von Willebrand factor signaling via GPIb-IX.7
The results presented with reconstituted platelet receptors on the surface of heterologous cell lines are compelling, but the important biologic question is whether low levels of thrombin are physiologically relevant during thrombus formation. To address this question, the authors conclude with a determination of in vivo thrombin levels at the site of a growing thrombus. Importantly, these data do document low levels of thrombin surrounding a growing thrombus consistent with the thrombin levels where the GPIb-IX/PAR cooperativity was observed. Thus, the in vivo result validates the importance of the data obtained using the heterologous cell model.
Although the work is performed and presented in a convincing manner, are there still unrecognized complexities that could challenge the proposed model? The experimental model of reconstituted platelet receptors in heterologous cells is certainly far removed from the anucleate platelet. Indeed, in the case of GPIb-IX, the platelet situation includes an additional subunit, GP V, often referred to as the GPIb-IX-V complex. Could the absence of GP V in the heterologous cell model alter the in vivo response to thrombin? It is known that GP V binds thrombin and that GP V-deficient mice have an altered thrombin response.8 Thus, the in vivo situation could be more complex. In addition, how faithfully does the Chinese hamster ovary cell recapitulate the signaling pathways of a circulating platelet? The same group has previously described a membrane permeable peptide of cytoplasmic GPIb sequence that disrupts the 14-3-3-Rac1-LIMK1 signaling pathway in human platelets.9 However, there still could be subtleties between heterologous cells and platelets impacting the major thrombin-centric conclusions in the current work. Yes, the heterologous cell model is a powerful and valuable experimental tool, but it is worth remembering that it is not a platelet. The future direction from this current work will likely be a more rigorous test of the cooperativity model in a platelet setting.
Going forward, could these data support the development of antagonists that selectively target the cooperative pathways of PARs and GPIb-IX? Certainly, this new work from Estevez et al’s laboratory leads us in that direction. Even more widespread interest would be generated if these antagonists were not only beneficial as anti-thrombotics, but also displayed anti-inflammatory and/or anti-cancer benefits.10 More studies will likely follow and the widespread availability of platelet-specific reagents and experimental approaches gives the work reported in this issue of Blood a high level of interest and importance.
Conflict-of-interest disclosure: The author declares no competing financial interests.