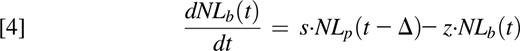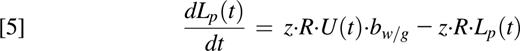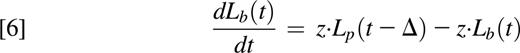Key Points
Mechanistic modeling of stable isotope labeling verifies human neutrophil half-lives of 13-19 h in contrast to recent estimates of >3 days.
Human neutrophil kinetics can be measured using a single-dose deuterium-labeled glucose protocol.
Abstract
Human neutrophils have traditionally been thought to have a short half-life in blood; estimates vary from 4 to 18 hours. This dogma was recently challenged by stable isotope labeling studies with heavy water, which yielded estimates in excess of 3 days. To investigate this disparity, we generated new stable isotope labeling data in healthy adult subjects using both heavy water (n = 4) and deuterium-labeled glucose (n = 9), a compound with more rapid labeling kinetics. To interpret results, we developed a novel mechanistic model and applied it to previously published (n = 5) and newly generated data. We initially constrained the ratio of the blood neutrophil pool to the marrow precursor pool (ratio = 0.26; from published values). Analysis of heavy water data sets yielded turnover rates consistent with a short blood half-life, but parameters, particularly marrow transit time, were poorly defined. Analysis of glucose-labeling data yielded more precise estimates of half-life (0.79 ± 0.25 days; 19 hours) and marrow transit time (5.80 ± 0.42 days). Substitution of this marrow transit time in the heavy water analysis gave a better-defined blood half-life of 0.77 ± 0.14 days (18.5 hours), close to glucose-derived values. Allowing the ratio of blood neutrophils to mitotic neutrophil precursors (R) to vary yielded a best-fit value of 0.19. Reanalysis of the previously published model and data also revealed the origin of their long estimates for neutrophil half-life: an implicit assumption that R is very large, which is physiologically untenable. We conclude that stable isotope labeling in healthy humans is consistent with a blood neutrophil half-life of less than 1 day.
Introduction
Maintenance of adequate numbers of circulating neutrophils is critical for survival, but our understanding of how this is achieved is incomplete. Some aspects of neutrophil development, such as the origins, development, and maturation of bone marrow precursors,1-3 are well characterized, whereas others, such as the dynamics of cell proliferation and blood turnover, are more controversial.4 It is known that there is a clear separation between the precursor expansion phase, which terminates when cells become metamyelocytes,1 and the maturation phase, which normally lasts about 5 to 6 days before release of cells into the circulation,5 but the fate of cells after being released into the circulation is more controversial.
Traditional dogma maintains that cells circulate for less than a day before leaving the circulation; this may be even faster during infection or trauma. Estimated half-lives in healthy individuals vary between 4.3 and 17.5 hours.4-6 However, this dogma dates back to early experiments with cell transfer models and/or toxic or radioactive tracers.5,7-9 Interpretation of such experiments may have been confounded by cell death (particularly because neutrophils are very susceptible to apoptosis with any ex vivo manipulation) or direct effects from high-energy radioactive tracers.4
A recent study by Pillay et al using in vivo stable isotope labeling with heavy (deuterium-labeled) water (2H2O), which is nontoxic and does not require cell manipulation, concluded that the previous estimates were incorrect; their estimate for the in vivo half-life of circulating neutrophils was about 3.7 days (corresponding to a life span of 5.4 days), an order of magnitude higher than previous estimates.10 If true, this would represent a major paradigm shift in our understanding of neutrophil kinetics. One potential explanation for this discrepancy between historic and recent studies might be excess cell toxicity in historic data leading to an overestimate of cell disappearance in these earlier studies. A second potential explanation is the mathematical model used to interpret the recent stable-isotope labeling curves.11 The 1-compartment model of Pillay et al may have failed to adequately describe the behavior of a complex population that proliferates in a remote (unmeasured) compartment (ie, bone marrow) and that is lost from the sampled compartment (ie, blood). Furthermore, rapid cell fluxes may be difficult to capture with heavy water, which labels and delabels slowly (turnover rate, ∼5%/d).
One way to resolve this uncertainty would be to sample bone marrow after deuterium labeling12 ; although undoubtedly revealing, this would require repeated marrow aspirations. We propose here an alternative, less-invasive approach, combining a novel mathematical model of granulopoiesis with the acquisition of new complementary data using the short-lived label, deuterium-labeled glucose. To investigate why Pillay et al10 obtained such large estimates, we acquired new data using their label of choice (heavy water) and performed a reanalysis of their published data.
The aims of this study were to (1) test whether a short-pulse labeling protocol with deuterium-labeled glucose could be developed for analysis of in vivo human neutrophil kinetics, (2) develop a physiological yet parsimonious model of neutrophil proliferation and loss, and (3) reevaluate quantitative parameters of neutrophil kinetics (proliferation rate, marrow transit time, and blood half-life) using data from both published water-labeling data and new glucose- and heavy water-labeling studies.
Methods
In vivo glucose labeling
Nine healthy adult subjects (aged 20-52 years; 6 men, 3 women; Table 1) received deuterium-labeled glucose ([6,6-2H2]-glucose; Cambridge Isotopes, Cambridge MA) as an oral solution. In initial studies, 60 g was given over 10 hours as half-hourly aliquots, preceded by a priming dose to rapidly achieve steady-state labeling. Subsequent studies were modified to develop the simplest workable protocol; dosing was progressively reduced (Table 1) to a single oral dose of 20 g. Blood glucose enrichment was monitored during and after oral labeling, as previously described.13 At selected time points after labeling, neutrophils were isolated from blood by gradient centrifugation with Polymorphprep (Alere Technologies AS, Oslo, Norway); purity and yield were confirmed by flow cytometry. In 2 subjects, C59 and C60, aliquots were further purified by CD16 antibody-coated magnetic bead adhesion (Miltenyi Biotec, Bisley, United Kingdom) and analyzed in parallel with cells purified by gradient separation alone. DNA was extracted, and deuterium enrichment was measured by gas chromatography–mass spectrometry as previously described.13,14
Participants and dosing for human deuterium-labeled glucose and heavy water studies
| Subject identifier . | Age, y . | Sex . | Label . | Total dose* . | Labeling time . |
|---|---|---|---|---|---|
| C36R | 52 | Male | Deuterium-labeled glucose | 60 g | 10 h |
| C41 | 28 | Female | Deuterium-labeled glucose | 60 g | 10 h |
| C42 | 44 | Female | Deuterium-labeled glucose | 30 g | 4 h |
| C46 | 28 | Female | Deuterium-labeled glucose | 20 g | 3 h |
| C48 | 36 | Male | Deuterium-labeled glucose | 20 g | 3 h |
| C49 | 30 | Male | Deuterium-labeled glucose | 20 g | 3 h |
| C50 | 27 | Male | Deuterium-labeled glucose | 20 g | 3 h |
| C59 | 20 | Male | Deuterium-labeled glucose | 20 g | Bolus |
| C60 | 21 | Male | Deuterium-labeled glucose | 20 g | Bolus |
| DW04 | 83 | Male | Heavy water | 5250 mL | 7 wk |
| DW09 | 47 | Male | Heavy water | 5250 mL | 7 wk |
| DW10 | 34 | Male | Heavy water | 5250 mL | 7 wk |
| DW11 | 29 | Female | Heavy water | 5250 mL | 7 wk |
| Subject identifier . | Age, y . | Sex . | Label . | Total dose* . | Labeling time . |
|---|---|---|---|---|---|
| C36R | 52 | Male | Deuterium-labeled glucose | 60 g | 10 h |
| C41 | 28 | Female | Deuterium-labeled glucose | 60 g | 10 h |
| C42 | 44 | Female | Deuterium-labeled glucose | 30 g | 4 h |
| C46 | 28 | Female | Deuterium-labeled glucose | 20 g | 3 h |
| C48 | 36 | Male | Deuterium-labeled glucose | 20 g | 3 h |
| C49 | 30 | Male | Deuterium-labeled glucose | 20 g | 3 h |
| C50 | 27 | Male | Deuterium-labeled glucose | 20 g | 3 h |
| C59 | 20 | Male | Deuterium-labeled glucose | 20 g | Bolus |
| C60 | 21 | Male | Deuterium-labeled glucose | 20 g | Bolus |
| DW04 | 83 | Male | Heavy water | 5250 mL | 7 wk |
| DW09 | 47 | Male | Heavy water | 5250 mL | 7 wk |
| DW10 | 34 | Male | Heavy water | 5250 mL | 7 wk |
| DW11 | 29 | Female | Heavy water | 5250 mL | 7 wk |
Total volume of heavy water is shown as milliliters of 70% 2H2O; dosing was 50 mL 3 times daily for 1 week and then twice daily for 6 weeks.
In vivo heavy water labeling
Four further healthy subjects (aged 29-83 years; 3 men, 1 woman) received heavy water for 7 weeks: 50 mL 70% 2H2O (Cambridge Isotopes) 3 times daily for 1 week, then twice daily for 6 weeks, as previously described.14 During labeling and delabeling periods, saliva samples were collected to measure the deuterium content in body water. Neutrophils were prepared from the buffy coat layer below a Ficoll density gradient, and DNA was processed and analyzed as above.
All subjects gave written informed consent after Ethical Review Board approval (Ref 10/H0803/102 and 13/LO/0022); all interventions were performed according to the principles of the Declaration of Helsinki.
Analysis of previously published in vivo heavy water labeling data
Previously published15 in vivo heavy water labeling data from 5 subjects were reanalyzed using the new model (see “Modeling”). Experimental details have been published elsewhere.15 Briefly, healthy young humans (aged 20-25 years) received heavy water for 9 weeks: a prime of 10 mL 2H2O/kg body weight (BW) over 24 h, followed by 1.25 mL 2H2O/kg BW daily. Cell samples were taken at 14 time points between week 0 and 16; urine samples were measured for body water enrichment. DNA enrichments in neutrophils were measured by gas chromatography–mass spectrometry.
Modeling
To interpret the experimental data, we constructed a new model based on the known physiology of the granulopoietic system (Figure 1).16-22 Implicit are two premises: (1) that mature circulating neutrophils acquire label as a consequence of cell division during the mitotic stage in the bone marrow (ie, as myelocytes, promyelocytes, myeloblasts, and so forth) and (2) that after the last mitosis, metamyelocytes go through a maturation/transit process before release into the circulation, resulting in a delayed appearance of labeled cells in blood. Model equations are as follows:
where Np is the number of proliferating bone marrow neutrophils and Nb is the number of blood neutrophils at time t. Np proliferate at a rate p and leave the proliferating pool at rate q into the postmitotic maturation/transit pool where they reside for time Δ. After the transit time Δ, neutrophils enter into the circulation at rate s and exit the circulation at rate z.
Physiology of granulocyte turnover translated to a model. We consider mitotic neutrophil precursors as a single pool, Np (proliferating neutrophils). The Np pool consists mainly of myelocytes, promyelocytes, and myeloblasts but also contains earlier precursors. Cells in the Np population proliferate at a mean rate p. After the last mitosis, cells enter the postmitotic maturation/transit pool at a rate q. Transit neutrophils remain in the postmitotic pool for a period of 4 to 6 days,5,16,17 referred to as the transit time (Δ), before egressing from bone marrow into the blood pool. Most egressing cells are segmented neutrophils, but some cells leave as band neutrophils. In the blood, neutrophils exist both in a freely circulating pool and a marginal pool (cells retained in proximity to the endothelium). Because the circulating and marginal pool are considered to be in rapid dynamic equilibrium,7,8 we consider them a single kinetically homogeneous pool, NB. Blood neutrophils are lost from the circulation at a rate z primarily to the bone marrow, liver, and spleen.18-20 This loss is generally considered to be a random, irreversible process.5,8,17,21,22 GMP, granulocyte-monocyte progenitor cells; HSC, hematopoietic stem cell.
Physiology of granulocyte turnover translated to a model. We consider mitotic neutrophil precursors as a single pool, Np (proliferating neutrophils). The Np pool consists mainly of myelocytes, promyelocytes, and myeloblasts but also contains earlier precursors. Cells in the Np population proliferate at a mean rate p. After the last mitosis, cells enter the postmitotic maturation/transit pool at a rate q. Transit neutrophils remain in the postmitotic pool for a period of 4 to 6 days,5,16,17 referred to as the transit time (Δ), before egressing from bone marrow into the blood pool. Most egressing cells are segmented neutrophils, but some cells leave as band neutrophils. In the blood, neutrophils exist both in a freely circulating pool and a marginal pool (cells retained in proximity to the endothelium). Because the circulating and marginal pool are considered to be in rapid dynamic equilibrium,7,8 we consider them a single kinetically homogeneous pool, NB. Blood neutrophils are lost from the circulation at a rate z primarily to the bone marrow, liver, and spleen.18-20 This loss is generally considered to be a random, irreversible process.5,8,17,21,22 GMP, granulocyte-monocyte progenitor cells; HSC, hematopoietic stem cell.
In the presence of label, deuterium is incorporated into neutrophil DNA during division of the Np pool in the bone marrow. Assuming that the amount of DNA per cell is conserved (amount of DNA proportional to the number of neutrophils), the model equations can be written as follows:
where NL are labeled neutrophils, bw/g is the normalizing factor of either water or glucose, respectively,13,14,16,23 and U(t) represents label enrichment (instantaneous exposure to label) at time t approximated by the experimental plasma label enrichment. U(t) was described by an empirical curve for water labeling studies15 ; by a square-pulse approach with exponential tail for the 3-hour, 4-hour, and 10-hour labeling protocols; and by interpolation between data points for the single oral bolus approach (C59 and C60) (supplemental Text 1, available on the Blood Web site).
Assuming a constant number of cells in each pool, the steady-state constraint requires that the net flow of the system equals 0 (p·Np(t) = q·Np(t) = s·Np(t − Δ) = z·Nb(t), from Equations 1 and 2). Using these constraints to eliminate p, q, and s and dividing Equations 3 and 4 by the number of cells in each pool gives Equations 5 and 6, where L is the fraction of labeled neutrophils in the proliferating pool (Lp) and in blood (Lb), and R is the ratio of blood neutrophils to mitotic neutrophil precursors in marrow (R = Nb/Np).
This model, therefore, has up to 3 free parameters (z, R, and Δ; see “Estimation of the ratio of blood neutrophils to bone marrow mitotic precursors (R)”).
Estimation of the ratio of blood neutrophils to bone marrow mitotic precursors (R).
We defined R as the ratio of the number of neutrophils in blood to the number of mitotic neutrophils in marrow. Initially, we fixed R using estimates derived from the literature (R = 0.26; Box 1). This value was used when fitting each individual separately. In later analysis, we allowed R to be a free parameter, which we estimated from fitting the model to all individuals simultaneously (R, z, and Δ were population parameters).
Fitting procedure and expression of results.
Equations 5 and 6 were fitted to the experimental data by minimizing the sum of squared residuals between prediction and observation using the pseudorandom algorithm in the R-package Flexible Model Environment (FME).24-26 Standard errors on estimates were calculated using the asymptotic covariance matrix method.27
When comparing our conclusions with other estimates in the literature, the reader should be aware that we have quoted half-life values (t1/2= ln(2)/z), which are a factor of 1.44 less than life span values (1/z).
Results
Experimental exploration of neutrophil kinetics using deuterium-labeled glucose
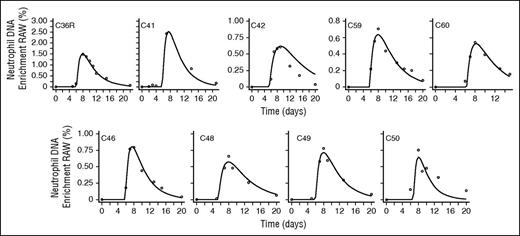
Neutrophil labeling after dosing with deuterium-labeled glucose showed a consistent pattern (Figure 2) characterized by a lag period of 5 to 7 days with no appreciable labeling, a rapid increase in the proportion of labeled cells, and then a slower disappearance. Because deuterium enrichments in the DNA of circulating neutrophils in initial experiments with a 60 g/10 h dosing schedule were high in the measurement range, we sought to develop the simplest, least-intrusive, and most cost-effective protocol by progressively reducing the dose and duration of labeling. Our final protocol, 20 g given as a single dose (Table 1), yielded enrichments in the readily measurable range, typically 0.5 atom percent enrichment; further dosage reductions may be feasible while still obtaining reliable results. Subject acceptability was good, although repeated postlabeling measurements (we estimate at least 6 time points) were required to capture the lag, peak, and disappearance phases adequately.
Best model fits to neutrophil DNA enrichment data in subjects labeled with deuterium-labeled glucose. Subjects were labeled with deuterium-labeled glucose (6,6-2H2-glucose) for 10 hours (C36R and C41), 4 hours (C42), 3 hours (C46, C48, C49, and C50), or as a single oral dose (C59 and C60). Circles represent measured fractional enrichment of deoxyadenosine in blood neutrophil DNA; lines represent best fits of the model (Equations 5 and 6) to the data.
Best model fits to neutrophil DNA enrichment data in subjects labeled with deuterium-labeled glucose. Subjects were labeled with deuterium-labeled glucose (6,6-2H2-glucose) for 10 hours (C36R and C41), 4 hours (C42), 3 hours (C46, C48, C49, and C50), or as a single oral dose (C59 and C60). Circles represent measured fractional enrichment of deoxyadenosine in blood neutrophil DNA; lines represent best fits of the model (Equations 5 and 6) to the data.
Modeling of heavy water–derived and deuterium-labeled glucose–derived data sets
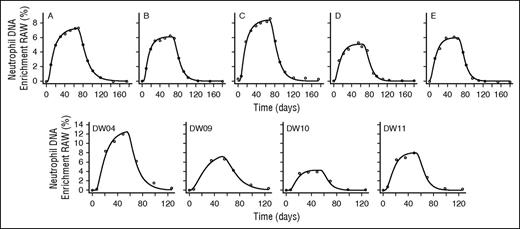
We developed a 2-compartment model in which neutrophils acquire label as a consequence of cell division during the mitotic stage in the bone marrow (ie, as myelocytes, promyelocytes, myeloblasts, and so forth) and then go through a maturation/transit phase (metamyelocytes) before release into the circulation (Figure 1). The ratio of the size of the total blood neutrophil pool to the bone marrow neutrophil precursor mitotic pool (ie, R) was initially fixed at a value derived from published literature (0.26; Box 1); later, we explored the implications of allowing R to be a free parameter. We fitted this model to the experimental data to estimate the rate of neutrophil disappearance from the blood (z) and the bone marrow transit time (Δ). The model fitted the new water and glucose data and the published heavy water data well, with the exception of 1 individual (C42) whose data were not consistent with other data sets (Figures 2 and 3). Parameters estimates are given in Table 2. Glucose-labeling experiments yielded values for the median marrow transit time of 5.72 days (mean ± SD, 5.80 ± 0.42 days), with a mean ± SD neutrophil half-life in blood of 0.79 ± 0.25 days (19 ± 6 hours, equivalent to a life span of 1.05 days). Heavy water studies gave not dissimilar values for the half-life in blood of 1.11 ± 0.36, days but parameter estimates were poorly resolved, especially the postmitotic transit time, which had a wide interindividual range and large standard errors (range, 0.41-5.91 days; mean ± SD, 3.4 ± 2.09 days; supplemental Figure 1, subjects A-E and DW04-DW11); similarly a wide range of values of z fitted the data equally well (supplemental Figure 2, subjects A-E and DW04-DW11). In contrast, estimates from glucose labeling were better resolved (Table 2, C36R-C60; supplemental Figures 1 and 2, C36R-C50).
Best model fits to neutrophil DNA enrichment data in subjects labeled with heavy water. Subjects were labeled with heavy water (2H2O) for 9 weeks (subjects A, B, C, D, E from Vrisekoop et al15 ) or 7 weeks (subjects DW04, DW09, DW10, and DW11 from the current study’s data). Circles represent measured fractional enrichment of deoxyadenosine in blood neutrophil DNA; lines represent best fits of the model (Equations 5 and 6) to the data.
Best model fits to neutrophil DNA enrichment data in subjects labeled with heavy water. Subjects were labeled with heavy water (2H2O) for 9 weeks (subjects A, B, C, D, E from Vrisekoop et al15 ) or 7 weeks (subjects DW04, DW09, DW10, and DW11 from the current study’s data). Circles represent measured fractional enrichment of deoxyadenosine in blood neutrophil DNA; lines represent best fits of the model (Equations 5 and 6) to the data.
Best fit parameters derived from deuterium labeling experiments
| Subject identifier . | Normalization factor, bg/w* . | Transit time, Δ, d† . | Loss rate from blood, z, d−1‡ . | Proliferation rate, p, d−1§ . | Blood half-life, d‖ . |
|---|---|---|---|---|---|
| Deuterium-labeled glucose | |||||
| C36R | 0.73¶ | 5.91 ± 0.07 | 1.03 ± 0.04 | 0.268 | 0.67 |
| C41 | 0.73¶ | 5.56 ± 0.00 | 1.06 ± 0.19 | 0.276 | 0.65 |
| C42 | 0.73¶ | 5.38 ± 0.00 | 0.52 ± 0.08 | 0.135 | 1.34 |
| C46 | 0.73¶ | 5.72 ± 0.00 | 1.09 ± 0.05 | 0.283 | 0.63 |
| C48 | 0.73¶ | 5.29 ± 0.07 | 0.72 ± 0.12 | 0.187 | 0.97 |
| C49 | 0.73¶ | 5.88 ± 0.00 | 0.92 ± 0.06 | 0.239 | 0.76 |
| C50 | 0.73¶ | 6.60 ± 0.72 | 1.42 ± 0.29 | 0.369 | 0.49 |
| C59 | 0.73¶ | 5.61 ± 0.00 | 0.82 ± 0.00 | 0.213 | 0.85 |
| C60 | 0.73¶ | 6.24 ± 0.01 | 0.98 ± 0.00 | 0.255 | 0.71 |
| Mean ± SD | 5.80 ± 0.42 | 0.95 ± 0.29 | 0.247 ± 0.066 | 0.79 ± 0.25 | |
| Median | 5.72 | 0.98 | 0.255 | 0.71 | |
| Heavy water | |||||
| A | 4.32 ± 0.03 | 4.49 ± 0.00 | 0.74 ± 0.02 | 0.192 | 0.94 |
| B | 4.74 ± 0.05 | 4.69 ± 0.62 | 0.77 ± 0.09 | 0.200 | 0.90 |
| C | 4.89 ± 0.07 | 5.25 ± 0.35 | 0.78 ± 0.00 | 0.203 | 0.89 |
| D | 3.69 ± 0.11 | 1.77 ± 0.07 | 0.47 ± 0.04 | 0.122 | 1.49 |
| E | 3.98 ± 0.06 | 0.41 ± 0.00 | 0.53 ± 0.03 | 0.138 | 1.31 |
| DW04 | 4.40 ± 0.31 | 5.91 ± 0.00 | 1.18 ± 0.09 | 0.307 | 0.59 |
| DW09 | 3.83 ± 0.19 | 0.84 ± 2.82 | 0.40 ± 0.11 | 0.104 | 1.74 |
| DW10 | 4.38 ± 0.00 | 5.07 ± 1.97 | 0.73 ± 0.00 | 0.190 | 0.96 |
| DW11 | 3.18 ± 0.14 | 2.20 ± 2.29 | 0.57 ± 0.17 | 0.148 | 1.21 |
| Mean ± SD | 4.16 ± 0.54 | 3.40 ± 2.09 | 0.68 ± 0.23 | 0.178 ± 0.060 | 1.11 ± 0.36 |
| Median | 4.32 | 4.49 | 0.73 | 0.190 | 0.96 |
| Subject identifier . | Normalization factor, bg/w* . | Transit time, Δ, d† . | Loss rate from blood, z, d−1‡ . | Proliferation rate, p, d−1§ . | Blood half-life, d‖ . |
|---|---|---|---|---|---|
| Deuterium-labeled glucose | |||||
| C36R | 0.73¶ | 5.91 ± 0.07 | 1.03 ± 0.04 | 0.268 | 0.67 |
| C41 | 0.73¶ | 5.56 ± 0.00 | 1.06 ± 0.19 | 0.276 | 0.65 |
| C42 | 0.73¶ | 5.38 ± 0.00 | 0.52 ± 0.08 | 0.135 | 1.34 |
| C46 | 0.73¶ | 5.72 ± 0.00 | 1.09 ± 0.05 | 0.283 | 0.63 |
| C48 | 0.73¶ | 5.29 ± 0.07 | 0.72 ± 0.12 | 0.187 | 0.97 |
| C49 | 0.73¶ | 5.88 ± 0.00 | 0.92 ± 0.06 | 0.239 | 0.76 |
| C50 | 0.73¶ | 6.60 ± 0.72 | 1.42 ± 0.29 | 0.369 | 0.49 |
| C59 | 0.73¶ | 5.61 ± 0.00 | 0.82 ± 0.00 | 0.213 | 0.85 |
| C60 | 0.73¶ | 6.24 ± 0.01 | 0.98 ± 0.00 | 0.255 | 0.71 |
| Mean ± SD | 5.80 ± 0.42 | 0.95 ± 0.29 | 0.247 ± 0.066 | 0.79 ± 0.25 | |
| Median | 5.72 | 0.98 | 0.255 | 0.71 | |
| Heavy water | |||||
| A | 4.32 ± 0.03 | 4.49 ± 0.00 | 0.74 ± 0.02 | 0.192 | 0.94 |
| B | 4.74 ± 0.05 | 4.69 ± 0.62 | 0.77 ± 0.09 | 0.200 | 0.90 |
| C | 4.89 ± 0.07 | 5.25 ± 0.35 | 0.78 ± 0.00 | 0.203 | 0.89 |
| D | 3.69 ± 0.11 | 1.77 ± 0.07 | 0.47 ± 0.04 | 0.122 | 1.49 |
| E | 3.98 ± 0.06 | 0.41 ± 0.00 | 0.53 ± 0.03 | 0.138 | 1.31 |
| DW04 | 4.40 ± 0.31 | 5.91 ± 0.00 | 1.18 ± 0.09 | 0.307 | 0.59 |
| DW09 | 3.83 ± 0.19 | 0.84 ± 2.82 | 0.40 ± 0.11 | 0.104 | 1.74 |
| DW10 | 4.38 ± 0.00 | 5.07 ± 1.97 | 0.73 ± 0.00 | 0.190 | 0.96 |
| DW11 | 3.18 ± 0.14 | 2.20 ± 2.29 | 0.57 ± 0.17 | 0.148 | 1.21 |
| Mean ± SD | 4.16 ± 0.54 | 3.40 ± 2.09 | 0.68 ± 0.23 | 0.178 ± 0.060 | 1.11 ± 0.36 |
| Median | 4.32 | 4.49 | 0.73 | 0.190 | 0.96 |
Parameter estimates are shown with their standard error estimated from the asymptotic covariance matrix; mean values are shown with their standard deviation (SD).
Normalization factors are bg and bw for glucose and water, respectively.
Transit time, Δ, is the time from the last mitosis in marrow until cell egression to blood.
Loss rate from blood, z, is the loss rate of blood neutrophils.
The proliferation rate of mitotic neutrophils, p, is not a free parameter; it is calculated from the other model parameters: p = z⋅R.
Half-life is calculated from the loss rate z, as ln2/z.
Denotes a fixed value.
Box 1: Estimation of R from the published literature
We define R as the ratio of the number of neutrophils in blood to the number of mitotic neutrophil precursors in bone marrow. We assume R is constant for all individuals. We estimate R by estimating each pool size from values in the published literature.
Total blood neutrophil pool size
The mean count of circulating blood neutrophils for the white population has been estimated to be 4.2 × 109 cells per liter.28 The blood volume of the 73-kg reference man is 5.3 L.29 This gives an estimate for the circulating pool of 3.05 × 108 cells per kilogram BW. In addition to the circulating pool, the total blood pool also includes the marginal pool; because this is in fast equilibrium with the circulating pool, we consider them to be a single kinetically homogeneous population.8 The size of the marginal pool has been estimated by different methods. Labeling studies suggest a value of between 43% and 58% of the total blood granulocyte pool,5,7,8 but these studies were performed under conditions that might affect neutrophil kinetics. Studies using epinephrine (which mobilizes the marginal blood pool without affecting the total blood pool size7,30 ), suggest that the marginal pool size accounts for 50% to 55% of the total neutrophil blood pool.31,32 Taking the more conservative value (50%) gives an estimate of the total blood granulocyte pool (marginal and circulating) of 6.1 × 108 cells per kilogram BW, the value used in this study. Notably, it agrees closely with the estimate of 7 × 108 cells per kilogram BW published by Cartwright et al.8
Bone marrow mitotic neutrophil pool size
Mitotic neutrophils include myeloblasts, promyelocytes, and myelocytes, with a minor contribution from the stem cell pool.33 Flow cytometry and microscopy studies suggest that mitotic neutrophils in marrow account for 17% to 19% of total marrow cellularity.2,34,35 The cellularity of bone marrow has been extensively studied in mice36-38 and humans39-43 ; the median of these different estimates is 13.05 × 109 cells per kilogram (supplemental Table 1). Taking a value of 17% for the marrow neutrophil precursor mitotic pool gives an estimate of 2.35 × 109 cells per kilogram (the value used in this study), in line with the estimate provided by Dancey et al of 2.11 × 109 cells per kilogram.5
Estimation of the ratio of blood neutrophils to bone marrow mitotic precursors (R) from published values
Combining these values, a total blood neutrophil pool of 6.1 × 108 cells per kilogram and a marrow neutrophil precursor mitotic pool of 2.35 × 109 cells per kilogram yields an estimation of R of 0.26.
We hypothesized that the slightly longer half-life estimates for the water data set could be an artifact of the poor estimates of the transit times. Hence, we repeated the fits to the water data sets with a fixed transit time of 5.7 days, a value consistent both with historical data5,16,44 and our estimates from glucose labeling. The resulting estimates for the half-life of neutrophils (mean ± SD, 0.77 ± 0.14 days), were not only better resolved but also remarkably consistent with the glucose-based estimates (Table 3). The quality of the fit (deviation between observation and prediction) with free and fixed transit times was very similar. Therefore, if R is fixed, based on estimates from the literature, and if a glucose/literature-derived value for transit time is used for heavy water modeling, then both approaches give almost identical estimates of the key kinetics of neutrophil homeostasis and provide good fits to the experimental data.
Revised parameter estimates for water data sets after fixing the transit time
| Identifier . | Normalization factor, bw . | Transit time, Δ, d* . | Loss rate from blood, z, d−1 . | Proliferation rate, p, d−1 . | Blood half-life, d . |
|---|---|---|---|---|---|
| Heavy water | |||||
| A | 4.30 ± 0.03 | 5.7* | 0.93 ± 0.00 | 0.242 | 0.75 |
| B | 4.72 ± 0.02 | 5.7* | 0.96 ± 0.01 | 0.250 | 0.72 |
| C | 4.88 ± 0.07 | 5.7* | 0.84 ± 0.00 | 0.218 | 0.82 |
| D | 3.59 ± 0.12 | 5.7* | 0.84 ± 0.00 | 0.218 | 0.82 |
| E | 3.89 ± 0.04 | 5.7* | 1.34 ± 0.16 | 0.348 | 0.52 |
| DW04 | 4.40 ± 0.15 | 5.7* | 1.13 ± 0.24 | 0.294 | 0.62 |
| DW09 | 3.65 ± 0.20 | 5.7* | 0.68 ± 0.00 | 0.177 | 1.01 |
| DW10 | 4.37 ± 0.25 | 5.7* | 0.80 ± 0.19 | 0.208 | 0.87 |
| DW11 | 3.14 ± 0.14 | 5.7* | 0.92 ± 0.19 | 0.239 | 0.75 |
| Mean ± SD | 4.10 ± 0.57 | 0.94 ± 0.19 | 0.244 ± 0.051 | 0.77 ± 0.14 | |
| Median | 4.30 | 0.92 | 0.239 | 0.75 |
| Identifier . | Normalization factor, bw . | Transit time, Δ, d* . | Loss rate from blood, z, d−1 . | Proliferation rate, p, d−1 . | Blood half-life, d . |
|---|---|---|---|---|---|
| Heavy water | |||||
| A | 4.30 ± 0.03 | 5.7* | 0.93 ± 0.00 | 0.242 | 0.75 |
| B | 4.72 ± 0.02 | 5.7* | 0.96 ± 0.01 | 0.250 | 0.72 |
| C | 4.88 ± 0.07 | 5.7* | 0.84 ± 0.00 | 0.218 | 0.82 |
| D | 3.59 ± 0.12 | 5.7* | 0.84 ± 0.00 | 0.218 | 0.82 |
| E | 3.89 ± 0.04 | 5.7* | 1.34 ± 0.16 | 0.348 | 0.52 |
| DW04 | 4.40 ± 0.15 | 5.7* | 1.13 ± 0.24 | 0.294 | 0.62 |
| DW09 | 3.65 ± 0.20 | 5.7* | 0.68 ± 0.00 | 0.177 | 1.01 |
| DW10 | 4.37 ± 0.25 | 5.7* | 0.80 ± 0.19 | 0.208 | 0.87 |
| DW11 | 3.14 ± 0.14 | 5.7* | 0.92 ± 0.19 | 0.239 | 0.75 |
| Mean ± SD | 4.10 ± 0.57 | 0.94 ± 0.19 | 0.244 ± 0.051 | 0.77 ± 0.14 | |
| Median | 4.30 | 0.92 | 0.239 | 0.75 |
Parameters are as explained in Table 2.
Values denote the fixed value of transit time, Δ, in this model.
Validation of the ratio of blood neutrophils to bone marrow mitotic precursors (R)
Our initial approach relied on estimating R from the published literature. To investigate the accuracy of this estimate, we simultaneously fitted the data from the previously published 9-week heavy water labeling and our 3-hour and 10-hour oral glucose labeling data sets; we let z, transit time, and R be free population parameters; C42 was excluded at this point because this subject’s data were not consistent with other individuals and we were concerned that this may have a distorting effect on the estimate of R. To minimize the number of free parameters, bg and bw were fixed, bg to 0.7313 and bw to 1, by normalizing the water data to 100%.15 The latter normalization could not be carried out for the 7-week labeling data set due to the uncertainty in having reached 100% of the attainable label in neutrophil DNA15 ; for this reason, the 7-week data set was excluded from estimates of R. We found 2 scenarios that fitted the data equally well (supplemental Figure 3), as previously hypothesized11 ; one with R = 0.19 ± 0.00 (transit time, 5.72 days; half-life, 13 hours), and another with R = 5.49 ± 1.26 (transit time, 5.73 days; half-life, 3 days). In the former scenario, marrow maturation/transit time is the rate-limiting step, whereas in the latter, the cell disappearance from the blood is also slow, as previously described.11 Focusing on the first scenario, which produces estimates of R similar to those in the literature, we see that our estimates of neutrophil half-life are reasonably robust to the assumed value of R, being only slightly lower with a free value of R than with a fixed value. This analysis also showed that a second scenario was numerically possible but physiologically unlikely because an R on the order of 5 is incompatible with what is known about the relative sizes of the marrow and blood compartments.
Comparison of our estimates of neutrophil half-life with those obtained by Pillay et al
Our estimates of neutrophil half-life are consistent with a short half-life of less than 1 day, whether we fit to our newly obtained data or the data presented by Pillay et al10 and whichever label we use. Thus, the difference in estimated half-life must be attributable to model choice, not data. Comparison of our model with that of Pillay et al (supplemental Text 2) shows that Pillay et al implicitly assume that R is large, an assumption that is at odds with current knowledge of the system. Furthermore, it can be seen (supplemental Figure 3) that as R is increased, the estimate of the circulating half-life increases, explaining why Pillay et al obtained such large estimates for the half-life. We suggest that because R is thought to be small (on the order of 0.2), our shorter estimates of neutrophil half-life are more likely to be accurate.
Discussion
Neutrophil proliferation and maturation/transit in marrow, egression to blood, and loss rates from blood have recently received unprecedented attention. Revised estimates for neutrophil life span in blood, generated from heavy water experiments (mean life span of 5.4 days, corresponding to a half-life of 3.7 days) conflict with the widely accepted dogma that neutrophils spend only a very short period in blood (generally considered to be about 11 hours, corresponding to a half-life of 7.6 hours5 ). Li et al11 observed that this disparity could result from data interpretation using a single-compartment model. Applying a multicompartment model to the tritium-labeled thymidine data of Dancey et al,5 they showed that 2 distinct scenarios are mathematically plausible: one where the time taken for proliferation and transit in marrow is rate limiting, and another in which the circulatory half-life is the rate-limiting step.11 We confirmed the mathematical plausibility of these 2 scenarios using a 2-compartment model with deuterium-labeling experimental data, albeit with slightly longer half-lives in both situations. Crucially, we observed that a pivotal difference between the 2 scenarios correlates with different relative sizes of the marrow precursor and blood neutrophil pools. We hypothesized that focusing on this aspect might allow resolution of the dilemma without recourse to invasive bone-marrow sampling experiments. We also hypothesized that parameter estimates should be independent of the kinetics of the tracer used and so should yield similar conclusions whether derived from heavy water or deuterium-labeled glucose when interpreted using a 2-compartment model. Our studies have enabled us to draw several conclusions.
First, in terms of experimental approaches, we found that deuterium-labeled glucose labeling allowed estimation of the key parameters of neutrophil kinetics with a simple, short, readily executed protocol. Administration of a single oral dose achieves adequate enrichment for quantitation, although repeated follow-up blood sampling is required. Because neutrophils are highly labeled, minor cellular impurities have a relatively small effect and cell sorting by dual-gradient density centrifugation appears adequate; parallel studies with and without further magnetic bead purification in 2 subjects yielded almost identical enrichment results (supplemental Figure 4). The single-dose deuterium-labeled glucose approach with 2-compartment modeling appears to represent a potentially widely applicable method for the study of human neutrophil kinetics in vivo.
Second, in terms of modeling, we confirmed that estimation of parameters is critically related to the relationship between the size of the marrow precursor and blood neutrophil pools. Fixing this ratio of pool sizes at 0.26 (from published values for physiological variables; Box 1) allowed us to obtain unique best fits for key parameters in neutrophil kinetics. Poor parameter estimates for heavy water could be attributed to the difficulty in estimating the marrow transit time using a tracer with a slow “on” and “off” dynamic. When externally derived values for transit time were fixed for the heavy water data, much more precise estimates for the key parameters were obtained, which were remarkably close to those obtained from glucose-labeling experiments.
In addition, we were able to demonstrate the source of the very high estimates of circulatory half-life previously calculated by Pillay et al (supplemental Text 2). It seems that the major problem of the previously described 1-compartment model is the implicit assumption that the number of proliferating neutrophil precursors in marrow is small compared with the number of neutrophils in blood, leading to a very high estimate of the neutrophil half-life. This assumption clashes with current knowledge of neutrophil cell population sizes in bone marrow and blood.
Third, in terms of the physiological implications of these findings, we found that, for fixed R, stable isotope labeling data were consistent with the traditional dogma of a rapid turnover of blood neutrophils. Mean values for the circulatory half-lives were 19 and 18 hours for glucose and water, respectively. We further confirmed that in healthy individuals, the postmitotic transit time is about 5.7 days. When R is allowed to be a free parameter, we estimate a slightly shorter neutrophil half-life of 13 hours. These estimates are in good agreement with previous half-life estimates obtained both by labeling5,7-9 and an independent (label-free) method based on fitting a model of myelosuppression to antitumor treatment data sets.45 When the model presented here, with R = 0.26, is fitted to tritium-labeled thymidine data available in the literature,1,5 a blood half-life of 13 to 16 hours is obtained, which is slightly smaller than the estimates obtained from heavy water and glucose using the same value of R. It is well known that ex vivo manipulation of neutrophils or the use of toxic compounds such as radioactive diisopropyl fluorophosphate, 5-bromo-2′-deoxyuridine, and tritium-labeled thymidine may affect the viability of cells, affect their kinetics and plasticity, and/or increase their sequestration in organs.46-50 As discussed elsewhere,4 this may explain these remaining discrepancies.
Although more complex models can readily be developed, they did not change our basic conclusion that neutrophil half-life is short, although precise estimates did vary (3-16 hours, depending on model). None of the models provided a better fit to the labeling data than our current model choice (data not shown). Other scenarios considered included concatenated series of mitotic pools in marrow, a discrimination between marginal/circulating blood pools, egression from marrow defined by different probability density functions, and age-dependent loss in blood. The current model assumes a steady state; application to non-steady-state situations such as trauma and sepsis may be attained by the inclusion of a feedback loop affecting proliferation, as has been done before in studies of myelosuppression upon antitumor treatment.45,51,52 Similarly, circadian oscillations are not included within the steady-state model. Blood neutrophil counts follow a circadian rhythm,53,54 which may be due to changes in proliferation, transit/release, or death/disappearance. Heavy water labels throughout the 24-hour cycle while glucose labeling was always performed in daylight hours (photophase); samples were consistently taken in the early morning (8:00-10:00 am). The fact that photophase labeling with glucose and continual (photophase and scotophase) labeling with water gave similar estimates for neutrophil proliferation and dwell time suggests that there are probably not very large variations in these parameters with phase of the circadian clock. A more detailed analysis of the potential impact of circadian oscillations is, however, beyond the scope of this paper.
In summary, application of a mechanistic, biologically meaningful model demonstrates that in vivo deuterium-labeling data, whether obtained using heavy water or glucose, is consistent with a half-life for neutrophils in blood of less than a day. We have shown how longer half-lives may be mathematically consistent with the data, but only when the relative sizes of the blood and marrow pools are allowed to assume nonphysiological values. This model has wider potential applications, including investigation of cells such as some T-cell populations, leukemic cells, and monocytes, in which proliferation and sampling occur in different compartments.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The Imperial College High Performance Computing Service (http://www.imperial.ac.uk/ict/services/teachingandresearchservices/highperformancecomputing) was used for this work.
This work was supported by Medical Research Council UK grants J007439 (B.A.) and G1001052 (B.A. and D.M.), the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement 317040 (QuanTI) (B.A. and J.L.-B.), and the Leukemia and Lymphoma Research (15012) (B.A.). B.A. is a Wellcome Trust Investigator (103865).
Authorship
Contribution: D.M., B.A., M.E., J.L.-B., and C.N. designed the research; J.L.-B., M.E., R.A., A.S., and Y.Z. performed the research; R.A., A.S., and Y.Z. collected the data; D.M., B.A., J.L.-B., and M.E. analyzed and interpreted the data; and B.A., D.M., J.L.-B., M.E., C.N., and M.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Becca Asquith, Division of Infectious Diseases, Theoretical Immunology Group, Faculty of Medicine, Imperial College London, London W2 1PG, United Kingdom; e-mail: b.asquith@imperial.ac.uk.
References
Author notes
B.A. and D.M. contributed equally to this study.