Key Points
APRIL/BCMA activation promotes MM proliferation, survival, and immunosuppression in vitro and in vivo.
Targeting the APRIL/BCMA pathway represents a promising mechanism-based immunotherapy to target MM and overcome drug resistance.
Abstract
Here we show that overexpression or activation of B-cell maturation antigen (BCMA) by its ligand, a proliferation-inducing ligand (APRIL), promotes human multiple myeloma (MM) progression in vivo. BCMA downregulation strongly decreases viability and MM colony formation; conversely, BCMA overexpression augments MM cell growth and survival via induction of protein kinase B (AKT), MAPK, and nuclear factor (NF)-κB signaling cascades. Importantly, BCMA promotes in vivo growth of xenografted MM cells harboring p53 mutation in mice. BCMA-overexpressing tumors exhibit significantly increased CD31/microvessel density and vascular endothelial growth factor compared with paired control tumors. These tumors also express increased transcripts crucial for osteoclast activation, adhesion, and angiogenesis/metastasis, as well as genes mediating immune inhibition including programmed death ligand 1, transforming growth factor β, and interleukin 10. These target genes are consistently induced by paracrine APRIL binding to BCMA on MM cells, which is blocked by an antagonistic anti-APRIL monoclonal antibody hAPRIL01A (01A). 01A is cytotoxic against MM cells even in the presence of protective bone marrow (BM) myeloid cells including osteoclasts, macrophages, and plasmacytoid dendritic cells. 01A further decreases APRIL-induced adhesion and migration of MM cells via blockade of canonical and noncanonical NF-κB pathways. Moreover, 01A prevents in vivo MM cell growth within implanted human bone chips in SCID mice. Finally, the effect of 01A on MM cell viability is enhanced by lenalidomide and bortezomib. Taken together, these data delineate new molecular mechanisms of in vivo MM growth and immunosuppression critically dependent on BCMA and APRIL in the BM microenvironment, further supporting targeting this prominent pathway in MM.
Introduction
Multiple myeloma (MM) is characterized by the abnormal accumulation of immunoglobulin-producing plasma cells (PCs) in the bone marrow (BM) and the development of osteolytic bone lesions. Although proteasome inhibitors and immunomodulatory drugs have significantly prolonged patient survival, drug resistance develops in the majority of cases due to continuously evolving genetic and molecular changes in tumor cells and their BM milieu. Novel agents targeting MM cells and the tumor-promoting BM microenvironment are urgently needed.
Monoclonal antibody (mAb)-based therapy targets tumor cells and recruits immune effector cells, representing a promising treatment strategy even for MM cells with p53 mutations.1-4 Most recently, elotuzumab and daratumumab targeting cell surface antigens SLAMF7 and CD38, respectively, have been US Food and Drug Administration approved to treat relapsed and refractory MM.1,2,5 Ongoing efforts are focusing on developing more effective immunotherapies targeting selective tumor antigens while simultaneously enhancing immune function in patients. One such selective antigen is B-cell maturation antigen (BCMA), a cell surface glycoprotein6 and non–tyrosine kinase receptor exclusively expressed on all MM cell lines and patient MM cells at high levels.7-9 It is absent on naïve and memory B cells, but is selectively induced during PC differentiation and supports humoral immunity by promoting survival of plasmablasts and long-lived PCs.10-12 Specifically, BCMA is increased on the cell membrane of malignant PCs compared with normal PCs.7,8,13 BCMA mRNA and protein is undetectable on normal human tissues except for PCs9 and plasmacytoid dendritic cells (pDCs),8 which promote tumor cell growth, survival, and drug resistance.14 Although BCMA is expressed at significantly lower levels on pDCs vs PCs from the same patient or normal donors, it is markedly increased on pDCs from MM patients vs normal donors, as in PCs.8 These results further indicate that BCMA, as a more selective MM antigen than SLAMF7 and CD38, may be a key membrane receptor on both MM cells and tumor-promoting BM accessory cells. To date, however, the functional role of BCMA on MM growth in the BM milieu is not defined.
A proliferation inducing ligand (APRIL), 1 of 2 identified ligands for BCMA, is a more PC-specific ligand than B-cell activating factor (BAFF) due to its stronger binding affinity to receptors on PCs, but not other B- and T-lineage cells.15-17 It is elevated in the circulation of MM patients vs normal donors.18,19 Besides binding to BCMA, APRIL also binds to the receptor transmembrane activator and calcium modulator (TACI), which is also overexpressed on malignant PCs from patients vs normal donors.20 Despite its association with survival of MM cells on the BM microenvironment,20,21 TACI expression on MM cell lines and patient MM cells is reported to be variable and lower compared with BCMA.7,22,23 APRIL is primarily secreted by myeloid cells and sustained during abnormal myelopoiesis in MM-infiltrated BM.24,25 In ex vivo cultures, osteoclasts (OCs), which stimulate MM growth and bone lesions during disease progression, produce more APRIL than CD14+ unstimulated monocytes.23,26-28 In addition to receptor-mediated effects and in contrast to BAFF, APRIL can promote the survival of malignant PCs directly through heparan sulfate proteoglycans, ie, CD138, which regulate growth factor signaling, cytoskeleton organization, cell adhesion, and migration.20,21,25,29-31 APRIL can rescue interleukin 6 (IL-6)–dependent MM cell lines from apoptosis following IL-6 deprivation,18,22,32 and stimulate MM cell growth via cyclin D-dependent G1/S cell cycle progression.33 Taken together, these results suggest a potential treatment strategy using a blocking anti-APRIL mAb to prevent BCMA activation in MM.
Using genetic and therapeutic approaches, we here define multiple functional sequelae and molecular targets triggered by BCMA overexpression and APRIL stimulation in MM cells. We show that BCMA overexpression or paracrine APRIL directly promote MM cell growth in vivo and concomitantly induce immune inhibitory signaling cascades in MM cells, providing the framework for development of anti-APRIL34 immunotherapeutics in MM.
Methods
Lentiviral BCMA small hairpin RNA transduction
BCMA small hairpin RNA (shBCMA) and control pLKO.1 (shCnt) lentivirus (RNAi Consortium at Dana-Farber Cancer Institute)35 were transduced into indicated cell lines. Lentiviruses expressing shBCMA targeting different RNA sequences of the BCMA gene were also used to confirm effects of BCMA downregulation in MM cells. Three days after transduction, CellTiter-Glo Luminescent Cell Viability (Promega, Madison, WI), Caspase-Glo 3/7 (Promega), and colony formation on methylcellulose (R&D Systems, Minneapolis, MN)36 assays were performed.
Real-time quantitative reverse transcription-polymerase chain reaction and TaqMan gene expression array
RNAs from indicated samples were extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA) and subject to real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and TaqMan array (Thermo Fisher Scientific). TaqMan array human IL10 pathway was used to simultaneously assay 40 IL-10–related genes with 4 internal controls (glyceraldehyde-3-phosphate dehydrogenase [GAPDH], 18S, HPRT1, and GUSB) (Thermo Fisher Scientific, Waltham, MA). Gene changes were reconfirmed by real-time qRT-PCR using TaqMan gene expression assay primer sets from Applied Biosystems (Thermo Fisher Scientific) and the Applied Biosystems 7300 Real-Time PCR System, with analysis using 7300 System SDS v1.4 Software. Gene expression was normalized using GAPDH and 18S.
Generation of BCMA-overexpressing MM and B-lymphoma cells
BCMA was overexpressed in RPMI8226 (MM) and Daudi (B lymphoma) cells using a pLoC-based lentiviral transfection followed by Blasticidin S (10 µg/mL; Life Technologies, Grand Island, NY)–induced positive selection TurboGFP vector (Thermo Scientific) containing the target sequence (BC058291) or empty vector, according to the manufacturer's specifications (Thermo Scientific). These gene-engineered permanent cell lines were generated following >2-month selection for BCMA overexpression and validated by real-time qRT-PCR, immunoblotting, and flow cytometry.
Nuclear factor-κB activation studies
MM cells were serum starved (1% fetal calf serum/RPMI 1640) and stimulated with APRIL for indicated time points (200 ng/mL; BioNovion) (kindly provided by Prof Jan Paul Medema), with or without 01 Ab (BioNovion).34 Whole cell lysates were made for immunoblotting using specific mAbs (Cell Signaling) to determine changes in nuclear factor (NF)-κB canonical and noncanonical pathways. Nuclear protein was extracted to determine NF-κB DNA binding activity using TransAM NF-κB p65, p52, and p50 enzyme-linked immunosorbent assay (ELISA) Kit (Active Motif, Carlsbad, CA). Data from 3 separate experiments are presented as mean percent control ± standard deviation.
SCID-hu model of human MM
All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee (Dana Farber Cancer Institute).36 Human fetal bone grafts were implanted subcutaneously into SCID-beige mice (SCID-hu mice; Taconic Farms) housed in our Animal Research Facility. Two days following the injection of 3 × 106 INA6 MM cells directly into the human bone implant, mice were treated with 01A (BioNovion; 20 mg/kg) or control immunoglobulin (Ig)G intraperitoneally for 5 days/week for 4 weeks. In this and prior studies,34 we used 20 mg/kg anti-APRIL IgG1 monoclonal antibody daily, which achieves ∼250 μg/mL maximum concentration in the blood and a maximum dose level of 50 to 75 μg/mL in the BM. Previous experiments examining mice treated for 3 weeks to 3 months with the 01A antibody did not demonstrate a phenotype in spleen or lymph nodes,34 also consistent with the absence of an overt spontaneous phenotype in the APRIL−/− mouse.37 Mouse sera were serially measured for shuIL-6R by ELISA (R&D Systems) as a surrogate marker for tumor growth. 01A does not bind to shIL-6R.
Statistical analysis
In vitro experiments were done in triplicate and repeated >2 times. A representative experiment (mean ± standard error) was selected for figures, except when otherwise indicated. Statistical significance of differences (set at P < .05) observed in 01A-treated compared with control cultures was determined using 1-way analysis of variance (ANOVA), with Bonferroni post hoc comparison (for >3 groups) or a 2-tailed unpaired Student t test (for 2 groups), with all the analyses using PrismVersion 4.0 (GraphPad Software, La Jolla, CA).
Results
BCMA promotes growth and survival of MM cells
Elevated BCMA in patient MM cells vs normal PCs suggests that BCMA may trigger prosurvival pathways in malignant PCs. Indeed, BCMA downregulation following shBCMA lentiviral infection in dexamethasone-resistant MM1R cells significantly decreases cell viability and induces caspase 3/7 activity, associated with decreased colony formation (Figure 1A; supplemental Figure 1A-B, available on the Blood Web site). Similar results were obtained when H929 and RPMI8226 (R) MM cells were used (Figure 1B; supplemental Figure 1C). H929 and R cells express higher and lower BCMA, respectively, compared with MM1R cells.8
BCMA promotes MM cell proliferation and survival. (A) MM1R cells were infected with lentiviruses expressing shBCMA or control shRNA (shCnt) followed by these assays: CellTiter-Glo for viability, Caspase-Glo 3/7 for apoptosis, and methylcellulose-based colony formation. (B) BCMA mRNA levels were measured by real time qRT-PCR (upper) and viable cell number were determined by trypan blue (lower) in BCMA-overexpressing RPMI8226 (R-BCMA) and empty control vector-expressing RPMI8226 (R-empty) cells which were infected with shBCMA or shCnt lentivirus. Cell growth and survival were determined by (C) DNA synthesis and (D) CellTiter-Glo in paired (C) R-BCMA/R-empty MM and (E) Daudi-BCMA/Daudi-empty B lymphoma cells. (D) Colony formation is determined in R-BCMA vs R-empty cells. (F) Protein lysates were examined by immunoblotting using indicated (F) Abs and (G) Kinex KAM-880 antibody microarray using Kinexus Bioinformatics. (G) Most significant 29 protein hits in R-BCMA relative to R-empty cells are presented. (H) Nuclear extracts were assayed for p65, p50, and p52 DNA binding induction to determine NF-κB activity. *P < .01, **P < .001 Shown are means and standard error of the means of ≥3 independent experiments.
BCMA promotes MM cell proliferation and survival. (A) MM1R cells were infected with lentiviruses expressing shBCMA or control shRNA (shCnt) followed by these assays: CellTiter-Glo for viability, Caspase-Glo 3/7 for apoptosis, and methylcellulose-based colony formation. (B) BCMA mRNA levels were measured by real time qRT-PCR (upper) and viable cell number were determined by trypan blue (lower) in BCMA-overexpressing RPMI8226 (R-BCMA) and empty control vector-expressing RPMI8226 (R-empty) cells which were infected with shBCMA or shCnt lentivirus. Cell growth and survival were determined by (C) DNA synthesis and (D) CellTiter-Glo in paired (C) R-BCMA/R-empty MM and (E) Daudi-BCMA/Daudi-empty B lymphoma cells. (D) Colony formation is determined in R-BCMA vs R-empty cells. (F) Protein lysates were examined by immunoblotting using indicated (F) Abs and (G) Kinex KAM-880 antibody microarray using Kinexus Bioinformatics. (G) Most significant 29 protein hits in R-BCMA relative to R-empty cells are presented. (H) Nuclear extracts were assayed for p65, p50, and p52 DNA binding induction to determine NF-κB activity. *P < .01, **P < .001 Shown are means and standard error of the means of ≥3 independent experiments.
In contrast, BCMA-overexpressing R-BCMA and Daudi-BCMA cells were generated following lentiviral transduction and selection for cells expressing greater than sixfold higher BCMA at mRNA and protein levels (Figure 1B,F; supplemental Figure 1D). Both p53mut tumor cell lines harbor p53 mutations,38,39 associated with adverse prognosis. Using either DNA synthesis or viability assays, R-BCMA and Daudi-BCMA cells show significantly increased growth and survival vs control vector-infected R-empty and Daudi-empty cells (Figure 1C,E). Importantly, R-BCMA cells form significantly more colonies than R-empty cells in a 3-week methylcellulose culture (Figure 1D).
Whole cell lysates were then made and subjected to immunoblotting to determine whether signaling cascades are altered in R-BCMA vs R-empty cells. R-BCMA cells have enhanced phosphorylation of protein kinase B (AKT), MEK1/2, and p65, as well as antiapoptotic protein MCL1, when compared with R-empty cells (Figure 1F). Cell lysates were further analyzed using Kinex KAM-880 antibody microarray40 to simultaneously assay multiple proteins and phosphorylated proteins in 3 independent R-BCMA vs 3 R-empty cell clones. The top hits that showed significantly increased phosphorylation or expression levels in R-BCMA vs R-empty cells included receptor tyrosine kinases such as VGFR2/KDR and FGFR3, as well as intracellular signaling proteins PKCγ, p38MAPK, pERK1/2, MEK1, MEK2, MEK5, cdc25B, AKT1, activated cdc42-associated kinase 1 (ACK1), Hsp27, and NEK2 (Figure 1G). Changes in NF-κB signaling were specifically examined using nuclear extracts to assay DNA binding activity of p65, p50, and p52. R-BCMA cells show enhanced DNA binding activity of all 3 proteins compared with R-empty cells (Figure 1H), indicating direct upregulation of both canonical and noncanonical NF-κB pathways by BCMA overexpression.
BCMA overexpression enhances MM growth in vivo and upregulates genes associated with osteoclast activation, adhesion, angiogenesis/metastasis, and immunosuppression
To address whether overexpressed BCMA accelerates MM cell growth and survival in vivo, R-BCMA or R-empty cells were subcutaneously injected into the flank of SCID mice. All mice receiving R-BCMA cells developed tumors at an earlier time point than those receiving R-empty cells (n = 6 each group), and R-BCMA tumors grew significantly more rapidly than R-empty tumors (P < .002 at day 25; P < .001 at day 32; Figure 2A). Using immunohistochemistry, R-BCMA–derived tumors displayed significantly elevated CD31 (PECAM1) staining and microvessel density (MVD) compared with R-empty–derived tumors (Figure 2B), indicating enhanced angiogenic processes.
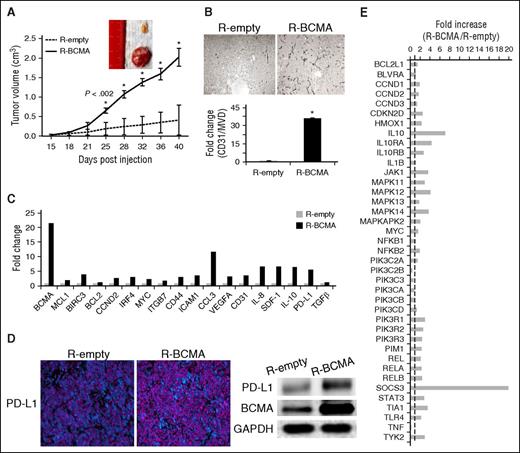
BCMA overexpression enhances MM tumor growth in vivo via upregulation of multiple MM-related genes and immunosuppressive molecules. (A) Mice (n = 6) were injected subcutaneous with equal numbers of R-BCMA (solid line) or R-empty (control vector, dotted line) cells, and tumor development was monitored. *P < .002. Tumors were removed at day 40. (B) Immunohistochemistry for tumor sections with similar volume from additional mice were used for CD31/MVD assessment. Original magnification, ×200. (C) Fold increase in 19 mRNA levels in R-BCMA vs R-empty cells was determined by real-time qRT-PCR. GAPDH and 18S were used as internal controls. (D) Programmed death ligand 1 (PD-L1) increase in R-BCMA vs R-empty cells was further confirmed with immunofluorescence staining (left; PD-L1, pink and DAPI, blue) and immunoblotting (right). (E) RNA of R-BCMA– and R-empty–derived tumors was further assayed for IL-10 pathway-associated genes using TaqMan array human IL10 pathway. Transcripts were normalized by geomean of 4 internal controls, and fold increases in R-BCMA vs R-empty cells are shown.
BCMA overexpression enhances MM tumor growth in vivo via upregulation of multiple MM-related genes and immunosuppressive molecules. (A) Mice (n = 6) were injected subcutaneous with equal numbers of R-BCMA (solid line) or R-empty (control vector, dotted line) cells, and tumor development was monitored. *P < .002. Tumors were removed at day 40. (B) Immunohistochemistry for tumor sections with similar volume from additional mice were used for CD31/MVD assessment. Original magnification, ×200. (C) Fold increase in 19 mRNA levels in R-BCMA vs R-empty cells was determined by real-time qRT-PCR. GAPDH and 18S were used as internal controls. (D) Programmed death ligand 1 (PD-L1) increase in R-BCMA vs R-empty cells was further confirmed with immunofluorescence staining (left; PD-L1, pink and DAPI, blue) and immunoblotting (right). (E) RNA of R-BCMA– and R-empty–derived tumors was further assayed for IL-10 pathway-associated genes using TaqMan array human IL10 pathway. Transcripts were normalized by geomean of 4 internal controls, and fold increases in R-BCMA vs R-empty cells are shown.
RNA was also made from tumors to determine expression of genes enhanced by BCMA overexpression. Using real-time qRT-PCR, >20-fold increased BCMA is seen in RNA derived from R-BCMA vs R-empty tumors, confirming BCMA overexpression in R-BCMA vs R-empty tumors (Figure 2C). At least 18 genes are significantly enhanced in R-BCMA– vs R-empty–derived tumors (Figure 2C). These targets include genes critical in survival (MCL1, BIRC3, BCL2), growth (CCND2, myc), adhesion (CD44, ICAM1), osteoclast activation (CCL3, CCL4), and angiogenesis/metastasis (VEGFA, CD31, IL-8, SDF-1), as well as immunosuppression (VEGFA, IL-10, PD-L1, TGFβ). Increased PD-L1 protein associated with upregulated BCMA is further validated using immunofluorescence staining and immunoblottings (Figure 2D).
Sixty hours following lentiviral transduction with shBCMA or shCnt when cell viability was still unaffected by shBCMA, RNA was extracted from MM1R and H929 MM cells. Expression of multiple genes was decreased in BCMA-downregulated MM1R and H929 MM cells (supplemental Figure 2), validating them as downstream targets of BCMA activation and further demonstrating that BCMA-induced biology was reproducible in all MM cell lines tested. TaqMan Array Human IL10 Pathway analysis showed prominent upregulation of >30 IL-10–related genes in R-BCMA- vs R-empty tumors (Figure 2E), further confirming that BCMA-induced IL-10 is functional in MM cells. Thus, BCMA overexpression plays a central role in MM pathogenesis in vivo via upregulation of crucial genes previously linked to MM pathogenesis. Concurrently, BCMA overexpression was associated with induction of key immunosuppressive molecules.
Paracrine APRIL stimulates MM cell growth and survival, which is blocked by 01A Ab
We next examined production of APRIL in ex vivo cell cultures of macrophages and OCs obtained by stimulation of CD14+ monocytes from MM patient BM samples with macrophage colony-stimulating factor (M-CSF) and M-CSF/receptor activator of nuclear factor kappa-B ligand, respectively. Consistent with previous reports,20,27 OCs secreted more APRIL than unstimulated CD14+ monocytes, as measured by ELISA41 and real-time qRT-PCR (Figure 3A). Macrophages, implicated in adhesion-dependent drug resistance in MM,42 also produced significant amounts of APRIL (Figure 3A), as reported in mucosa-associated lymphoid tissue B lymphoma.43
01A blocks proliferation and viability of MM cells induced by paracrine APRIL. (A) Supernatant was collected from CD14+ cells purified from MM patients (1-5) BM aspirates cultured with media (−, open column), M-CSF (M) (gray column), or M with receptor activator of nuclear factor kappa-B ligand (M+R, black column). APRIL secretion is measured by specific ELISA. APRIL mRNA was also confirmed by real-time qRT-PCR in 1 representative sample (left). (B) Daudi-BCMA/Daudi-empty cells, (C) IL-6–dependent INA6, IL-6–independent MM1S, and (D) RPMI8226, as well as (E) CD138+ cells from a relapsed MM patient, were cultured for 3 days with APRIL in the presence or absence of anti-APRIL 01A mAb (10 μg/mL), followed by (B,E) [3H]thymidine uptake and (C-D) luminescence-based viability assays. Two additional patient MM cells were subject to viability and caspase 3/7 activity (F; newly diagnosed) and annexin V/PI (G; relapsed) flow cytometry.
01A blocks proliferation and viability of MM cells induced by paracrine APRIL. (A) Supernatant was collected from CD14+ cells purified from MM patients (1-5) BM aspirates cultured with media (−, open column), M-CSF (M) (gray column), or M with receptor activator of nuclear factor kappa-B ligand (M+R, black column). APRIL secretion is measured by specific ELISA. APRIL mRNA was also confirmed by real-time qRT-PCR in 1 representative sample (left). (B) Daudi-BCMA/Daudi-empty cells, (C) IL-6–dependent INA6, IL-6–independent MM1S, and (D) RPMI8226, as well as (E) CD138+ cells from a relapsed MM patient, were cultured for 3 days with APRIL in the presence or absence of anti-APRIL 01A mAb (10 μg/mL), followed by (B,E) [3H]thymidine uptake and (C-D) luminescence-based viability assays. Two additional patient MM cells were subject to viability and caspase 3/7 activity (F; newly diagnosed) and annexin V/PI (G; relapsed) flow cytometry.
APRIL-induced proliferation and survival were next assayed in lymphoma and MM cells, in the presence or absence of an antagonistic anti-APRIL Ab 01A (01A).34 APRIL further enhances Daudi-BCMA cell proliferation, which was completely blocked by 01A (Figure 3B). 01A also abolished proliferation induced by BCMA overexpression (Figure 3B). 01A prevented baseline and APRIL-induced survival of MM cells in a BCMA-dependent manner (Figure 3C-D; supplemental Figure 3). 01A induced more cytotoxicity against IL-6–dependent INA6 cells expressing higher levels of BCMA with shorter latency and stronger potency than against MM1S and RPMI8226 cells, expressing lower levels of BCMA. Because TACI was barely detected in INA6 compared with MM1S or RPMI8226 cells, these results also indicated that 01A-induced cytotoxicity primarily depended on BCMA levels. 01A was also confirmed to be active against patient MM cells (n = 3; Figure 3E-G). Importantly, 01A displayed cytotoxicity against MM cells cocultured with APRIL-secreting OCs (Figures 3D, 6A-B, and 7B-C).
APRIL-induced signaling through BCMA involving multiple pathways can be blocked with 01A
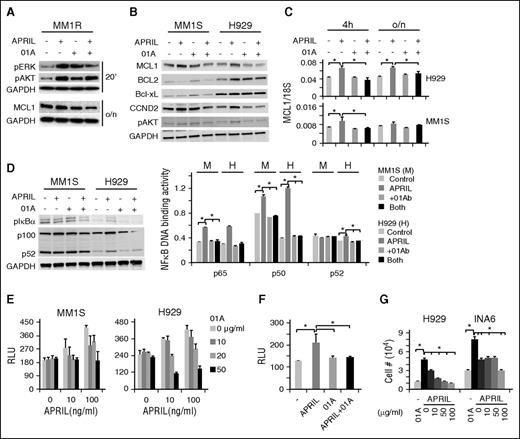
Next, cell lysates were made to examine specific inhibition by 01A of APRIL-induced growth and survival signaling cascades in multiple MM cell lines. APRIL was shown to significantly induce pERK1/2 and pAKT, followed by increased CCDN2, MCL1, and BCL2 in MM cells (Figure 4A-C; supplemental Figure 4A-B). The intensity of APRIL induced protein expression and phosphorylation is highest in H929 cells, followed by MM1R and MM1S, again showing an association with BCMA expression levels. Conversely, 01A specifically inhibits APRIL-induced signaling pathways and molecules critical for proliferation and survival. As expected, APRIL induced NF-κB activation and subsequent DNA binding activity, which was completely blocked by 01A (Figure 4D; supplemental Figure 4C). Both canonical and noncanonical NF-κB pathways are induced to a greater extent in H929 than in MM1S cells. Because H929 cells express the highest levels of BCMA and lack TACI, whereas MM1S cells express lower levels of BCMA and express TACI, these experiments confirm that APRIL-induced effects predominantly depend on BCMA levels in MM cell lines.
01A inhibits various APRIL-induced signaling cascades and further blocks APRIL-induced adhesion and migration in MM cells. (A) MM1R cells were serum starved, pretreated with 01A, and stimulated with APRIL (100 ng/mL). Cell lysates were made and subjected to immunoblotting with specific Abs. MM1S and H929 cells were also treated with indicated reagents for 4 hours. (B,D) Cell lysates and (C) mRNA were assayed by immunoblotting and real-time qRT-PCR, respectively. (D) NF-κB DNA binding activity assays were performed in cell lysates (right). (E) MM1S and H929 cells, (F) as well as CD138+ patient MM cells, were subjected to adhesion assays on BMSC-coated plates, in the presence or absence of 01A and APRIL. (G) H929 and INA6 MM cells were incubated with 01A in APRIL-induced migration assays.
01A inhibits various APRIL-induced signaling cascades and further blocks APRIL-induced adhesion and migration in MM cells. (A) MM1R cells were serum starved, pretreated with 01A, and stimulated with APRIL (100 ng/mL). Cell lysates were made and subjected to immunoblotting with specific Abs. MM1S and H929 cells were also treated with indicated reagents for 4 hours. (B,D) Cell lysates and (C) mRNA were assayed by immunoblotting and real-time qRT-PCR, respectively. (D) NF-κB DNA binding activity assays were performed in cell lysates (right). (E) MM1S and H929 cells, (F) as well as CD138+ patient MM cells, were subjected to adhesion assays on BMSC-coated plates, in the presence or absence of 01A and APRIL. (G) H929 and INA6 MM cells were incubated with 01A in APRIL-induced migration assays.
01A inhibits APRIL-induced adhesion and migration via blockade of APRIL-induced signaling and downstream targets
Because BCMA overexpression upregulated adhesion and migration-related molecules, we next tested whether APRIL induces MM cell adhesion and migration and whether this could be modulated by 01A. Indeed, APRIL-induced adhesion of MM cell lines and patient MM cells to BM stromal cells in a dose-dependent manner, which was blocked by 01A (Figure 4E-F; supplemental Figure 5A). In transmigration assays,44 APRIL further enhanced MM cell migration, which was inhibited by 01A in a dose-dependent fashion (Figure 4G).
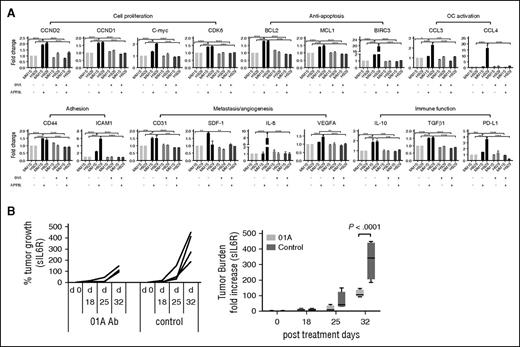
Genes regulating the cell cycle (CCND2, CCND1, C-myc, CDK6) and apoptosis (BCL2, MCL1, BIRC3) were induced by APRIL in MM cells (Figure 5A; supplemental Figure 4D). APRIL further induces genes associated with adhesion (CD44, ICAM1) and angiogenesis/metastasis (CD31, SDF-1, IL-8, VEGFA), as well as immunosuppression (IL-10, TGFβ, PD-L1), whereas 01A abrogates their induction (Figure 5A). As expected, APRIL induced adhesion, migration, and cytokine secretion, (ie, CCL3, CCL4, IL-8, RANTES, MDC/CCL22) dependent on BCMA expression (supplemental Figure 5B-C). These characteristics largely overlapped with those described for BCMA overexpression and confirmed the critical role for the APRIL–BCMA pathway in MM survival, proliferation, adhesion, migration, and immunosuppression.
01A significantly decreases many APRIL-induced target genes and blocks MM cell growth in a SCID-hu mouse model of human MM. (A) MM1S and H929 MM cells were incubated overnight with (+) or without (−) APRIL in the presence (+) or absence (−) of 01A. Levels of downstream target genes were assayed using real-time qRT-PCR, normalized to 18S or GAPDH internal controls. Fold changes to control media are shown. Two-way ANOVA test (Tukey’s multiple comparison) was done for all genes except BIRC3 and IL-8 (1-way ANOVA). Fold changes are significantly higher on APRIL induction in H929 vs MM1S cells for BIRC3 (24-fold vs 2-fold) and IL-8 (8-fold vs 1.3-fold). *P < .05; **P < .01; ***P < .001; ***P < .0001. (B) INA-6 MM cells were injected into the implanted human bone chips in SCID-hu mice. Two days later, mice were treated (5 days/week) with 01A (20 mg/kg, n = 4) or isotype IgG control (n = 4); tumor growth was then monitored by measuring shuIL-6R in mouse serum samples weekly. Two-way ANOVA test (Tukey’s multiple comparison) determined P < .0001 at day 32.
01A significantly decreases many APRIL-induced target genes and blocks MM cell growth in a SCID-hu mouse model of human MM. (A) MM1S and H929 MM cells were incubated overnight with (+) or without (−) APRIL in the presence (+) or absence (−) of 01A. Levels of downstream target genes were assayed using real-time qRT-PCR, normalized to 18S or GAPDH internal controls. Fold changes to control media are shown. Two-way ANOVA test (Tukey’s multiple comparison) was done for all genes except BIRC3 and IL-8 (1-way ANOVA). Fold changes are significantly higher on APRIL induction in H929 vs MM1S cells for BIRC3 (24-fold vs 2-fold) and IL-8 (8-fold vs 1.3-fold). *P < .05; **P < .01; ***P < .001; ***P < .0001. (B) INA-6 MM cells were injected into the implanted human bone chips in SCID-hu mice. Two days later, mice were treated (5 days/week) with 01A (20 mg/kg, n = 4) or isotype IgG control (n = 4); tumor growth was then monitored by measuring shuIL-6R in mouse serum samples weekly. Two-way ANOVA test (Tukey’s multiple comparison) determined P < .0001 at day 32.
01A inhibits in vivo MM growth in the SCID-hu model of human MM
We next test whether 01A as a single agent has anti-MM activity in the SCID-hu model. IL-6R–secreting INA6 MM cells were injected into implanted bone chips in SCID-hu mice. Tumor burden, as monitored by soluble IL-6R in murine blood, was significantly decreased following 4 weeks of 01A treatment compared with vehicle control treatment (P < .0001 at day 32, n = 4 each group; Figure 5B). These results indicate that 01A is cytotoxic against MM cells in the BM microenvironment in vivo.
01A significantly blocks MM cell proliferation induced by BM accessory cells
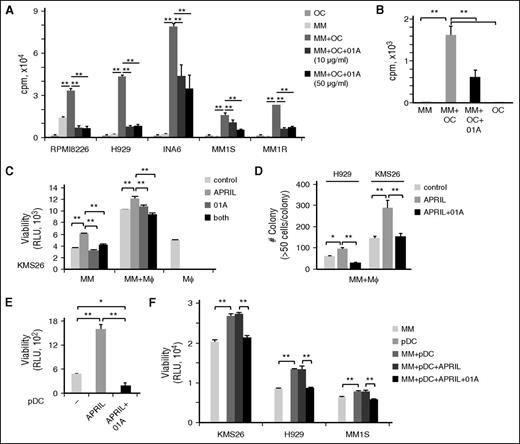
The anti-MM effects of 01A were next examined in MM cells in the context of APRIL-secreting and tumor-promoting BM accessory cells. We assessed antiproliferative and cytotoxic activity of 01A against MM cells in cultures with OCs for 4 days. 01A significantly blocks growth of MM cell lines (n = 5) and patient MM cells (n = 3) induced by OCs (Figure 6A-B). 01A also showed similar activity against MM cells cocultured with macrophages (Figure 6C-D).
01A inhibits MM cell proliferation and survival induced by osteoclasts, macrophages, and pDCs. (A) MM cell lines (n = 5) and (B) patient MM cells (n = 3) were cultured with osteoclasts (OCs), with or without 01A, followed by DNA synthesis assays. MM cells were cultured with macrophages (Mϕ), in the presence or absence of 01A (20 μg/mL) with or without APRIL, for (C) 1 or (D) 21 days, followed by (C) viability and (D) colony formation assays. Macrophages do not form colonies under these culture conditions. (E) pDCs were purified from patient BM aspirates (n = 3) and incubated with APRIL in the presence of 01A for 2 days. (F) MM cells were cultured with pDCs in the presence of APRIL and 01A for 3 days.
01A inhibits MM cell proliferation and survival induced by osteoclasts, macrophages, and pDCs. (A) MM cell lines (n = 5) and (B) patient MM cells (n = 3) were cultured with osteoclasts (OCs), with or without 01A, followed by DNA synthesis assays. MM cells were cultured with macrophages (Mϕ), in the presence or absence of 01A (20 μg/mL) with or without APRIL, for (C) 1 or (D) 21 days, followed by (C) viability and (D) colony formation assays. Macrophages do not form colonies under these culture conditions. (E) pDCs were purified from patient BM aspirates (n = 3) and incubated with APRIL in the presence of 01A for 2 days. (F) MM cells were cultured with pDCs in the presence of APRIL and 01A for 3 days.
Furthermore, APRIL significantly enhanced viability of pDC, isolated from MM patient samples (n = 2); conversely, 01A inhibits APRIL-induced survival of pDCs (Figure 6E). Thus, although pDCs express relatively lower levels of BCMA compared with PC, the receptor appeared to be functional. Cultures of 3 MM cell lines with pDCs significantly increase their viability. Importantly, 01A maintained its time-dependent cytotoxic activity against MM cells even in pDC cocultures (Figure 6F; supplemental Figure 6). These results show that 01A induces cytotoxicity against MM cells even in the presence of tumor-promoting BM accessory cells.
OC-induced MM cell proliferation is partially blocked by 01A, an effect that is further enhanced by lenalidomide or bortezomib
We further examined the antiproliferative activity of 01A in cocultures of MM cells with OCs, alone and in combination with other anti-MM agents. In a dose-dependent manner, 01A significantly blocked APRIL-induced protection against lenalidomide and dexamethasone (Figure 7A; supplemental Figure 7). 01A significantly inhibited OC-induced proliferation of MM cells and restored sensitivity to len (Figure 7B). The antibody also increased bortezomib-induced cytotoxicity in MM cells and is cytotoxic against bortezomib-resistant ANBL6VR cells cultured with OCs (Figure 7C). These results suggest that 01A can enhance MM cell sensitivity to multiple anti-MM agents, in resistant MM cells.
01A selectively blocks APRIL-induced protection in MM cells, alone and in cocultures with OCs, an effect that is further enhanced by lenalidomide or bortezomib. (A) U266 cells were incubated with APRIL or IL-6 in the presence or absence of lenalidomide, with or without 01A (10 μg/mL), and assayed by [3H]thymidine uptake at day 3. MM cell lines were cultured for 4 days in 2% fetal calf serum growth media with OCs in the presence of 01A with either (B) lenalidomide or (C) bortezomib. *P < .01.
01A selectively blocks APRIL-induced protection in MM cells, alone and in cocultures with OCs, an effect that is further enhanced by lenalidomide or bortezomib. (A) U266 cells were incubated with APRIL or IL-6 in the presence or absence of lenalidomide, with or without 01A (10 μg/mL), and assayed by [3H]thymidine uptake at day 3. MM cell lines were cultured for 4 days in 2% fetal calf serum growth media with OCs in the presence of 01A with either (B) lenalidomide or (C) bortezomib. *P < .01.
Discussion
We identify for the first time multiple molecular mechanisms by which BCMA promotes MM progression in vivo. We demonstrate critical paracrine stimulation of BCMA by APRIL produced in the MM BM microenvironment, providing the therapeutic rationale for disrupting constitutively active APRIL/BCMA signaling cascade with novel targeted immunotherapy.
We show that activation of the APRIL/BCMA pathway, either by BCMA overexpression or by soluble APRIL, significantly increases MM cell proliferation and survival both in vitro and in vivo via induction of essential signaling cascades (AKT, MAPK, NF-κB) and gene expression associated with MM cell cycle progression (ie, cdc25B, ACK1), inhibition of apoptosis (ie, MCL1, BIRC3), angiogenesis (ie, VGFR2, FGFR3, PKC), and drug resistance (ie, Hsp27,45,46 NEK247 ). Among antiapoptotic proteins of the BCL-2 family, MCL1 and BCL2, but not Bcl-xL, are consistently induced by APRIL/BCMA activation in MM cells, as in a previous report.18 MCL1 is indispensable for the maintenance of PCs in vivo, because deletion of this gene in PCs rapidly eliminates this population in mice.48 MCL1, but not BCL2 or Bcl-xL, was identified as an important and selective myeloma survival gene by RNAi lethality screening of the druggable genome.49 Our current results identify the role of upstream BCMA in maintaining high expression of MCL1 in MM cells in vivo.
We also showed that inhibition of apoptosis family member BIRC3 is significantly induced following APRIL/BCMA activation in MM cells. Fold changes in BIRC3 after APRIL/BCMA activation or inhibition in MM cells are even greater than those in MCL1, regardless of genetic status and responses to drugs. BIRC3 regulates caspases and apoptosis in MM cells, acting as a critical component in MM cells addicted to the NF-κB pathway.50-52 Specific BIRC3 inhibitors, including SMAC/DIABLO protein, are being developed as a new cancer therapeutic strategy, and several small-molecule SMAC mimetics are also in clinical trials.53,54 Furthermore, ACK1 has recently been implicated in cell spreading and migration, besides cell growth and survival, in multiple carcinomas.55-57 It transduces extracellular signals to cytosolic and nuclear effectors via phosphorylated AKT1, as well as confers metastatic properties to drive cancer progression.55 Hsp27, downstream to p38 MEK and implicated in dexamethasone and bortezomib resistance in MM cells,45,46 can further promote migration and angiogenesis by NF-κB–dependent upregulation of VEGF gene transcription and secretion of VGFR2/KDR in endothelial cells.58 Finally, NEK2 regulates centrosome disjunction/splitting and is highly correlated with drug resistance, rapid relapse, and poor outcome in MM and many other cancers.47,59 All these data indicate that activity of the APRIL/BCMA pathway plays a central role in MM pathogenesis.
Indeed, BCMA overexpression in RPMI8226 MM and Daudi B lymphoma cells, both harboring various p53 mutations, augments cell proliferation in a time-dependent manner. Importantly, enforced BCMA overexpression induces early tumor onset and accelerates growth of RPMI8226 MM xenografts in mice. These BCMA-overexpressing tumors significantly display higher CD31/MVD and VEGF, in addition to various proteins associated with proliferation and survival. Other angiogenesis/metastasis factors including IL-8 and stromal cell–derived factor 1 are also significantly upregulated, together with adhesion molecules (CD44, ICAM1) and OC activators (CCL3, CCL4), thereby providing a positive feedback loop whereby MM cells further enhance OC function during disease progression. We showed that OCs and macrophages, which support MM cell growth via adhesion and cytokine secretion,42,60 are major sources of APRIL in an ex vivo culture system. Importantly, APRIL stimulation, like BCMA overexpression, induces both canonical and noncanonical NF-κB pathways, further increasing angiogenesis and metastasis factors, adhesion, and migration molecules, as well as growth and survival genes. These results further support a recent study showing that APRIL, but not BAFF, is correlated with expression of VEGF and its receptors, MVD and CD138, and with progression of human MM.19 Furthermore, in a xenogeneic model where MM cells were subcutaneously injected into SCID mice deficient in APRIL, APRIL by itself was shown to be essential for in vivo MM development.25 Moreover, the lack of APRIL equally impaired U266 (TACIlow/neg) and L363 (TACIhigh) growth in mice, suggesting that APRIL stimulation to MM cells is primarily via BCMA, rather than TACI, which is associated with CD138. The ubiquitous expression of BCMA, but not TACI, among all MM cells,22,23 the in vivo pathogenic role of BCMA shown here, and the clinical activity by TACI-Ig61 all identify and further support targeting APRIL/BCMA signaling in MM.
Because BCMA overexpression by itself is sufficient to augment MM cell proliferation and survival, the majority of MM cell lines (also TACIlow/neg) may bypass APRIL-induced proliferation. Nonetheless, APRIL rescues serum-deprived MM cells and confers resistance to lenalidomide and dexamethasone.18 Our data further show that APRIL binds to BCMA in TACIlow/neg cells (ie, H929, U266, and INA6) and induces protection against lenalidomide-induced apoptosis, indicating that paracrine APRIL promotes MM growth and survival in the BM.25,62 01A, which completely blocks all APRIL-induced target genes in MM cells, significantly inhibits baseline cell proliferation and completely abolishes APRIL-induced U266 (p53mut) cell growth and drug resistance. Further, APRIL induces MM cell adherence to bone marrow stromal cells (BMSCs) and drug resistance, which is abrogated by 01A. Importantly, 01A triggers significant cytotoxicity against all tested MM cells in cultures with OCs, macrophages, and pDCs, as well as enhances activity of lenalidomide or bortezomib, providing the rationale for combination treatments in MM. 01A also prevents MM development in the SCID-hu mouse model. These results support the potential therapeutic value of 01A in MM, especially when BCMA is relatively low and APRIL-induced effects are prominent. Of note, soluble BCMA detected in patient serum samples may compromise the potential efficacy of directly targeting BCMA, and targeting BCMA may be also less effective due to abundant APRIL in the MM BM.
We are the first to demonstrate robust induction of MM-related immune inhibitory molecules including VEGF, PD-L1, IL-10, and TGFβ by APRIL/BCMA activation in MM cells. These critical immune suppressive checkpoint molecules are significantly elevated in patient MM cells vs normal PCs and further support MM cell growth and survival, as well as inhibit various effector cell-mediated anti-MM immune responses.63-72 For example, PD-L1 is induced in MM cells by interferon γ, IL-6, and stromal cells.64-66,73 It increases tumor growth and mediates tumor-induced immune inhibition.64-66,73 IL-10 is a growth factor for human myeloma cells74 and to be significantly overexpressed in osteoclasts compared with other BM cell subpopulations.75 These immune-suppressive factors are further enhanced by either BCMA overexpression or APRIL stimulation in vivo; conversely, PD-L1, IL-10, and expression of 38 IL-10 downstream genes is completely blocked by either BCMA downregulation or 01A. Moreover, additional PD-L1 may be induced via APRIL binding to BCMA in pDCs, further augmenting their abnormal immunosuppressive function in the MM BM. Future experiments can be planned to examine these molecules in the SCID-hu model. These data therefore indicate that APRIL/BCMA activation may allow MM cells to evade the immune system. They further confirm that interactions of MM cells with BM accessory cells (OC, macrophages, pDCs, and BMSCs) inhibit antitumor immunity, as well as increase MM growth, survival, and drug resistance.
In summary, we define the molecular mechanisms whereby APRIL/BCMA activation promotes MM pathogenesis and drug resistance via bidirectional interactions between tumor cells and their supporting BM microenvironment. Importantly, this study identifies the novel role of APRIL/BCMA activation triggering key immunosuppressive molecules, ie, VEGF, PD-L1, and IL-10. Together, our results provide the framework for therapeutically targeting this pathway to improve patient outcome and relieve immune suppression in MM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jan Paul Medema (Center for Experimental Molecular Medicine, Academic Medical Center, Amsterdam, The Netherlands) for valuable materials and Lilian Driessen for performing the analysis of APRIL ELISA. The authors thank Nicholas DaSilva and Lay Hong for excellent technical support. The authors also thank the nursing staff and clinical research coordinators of the LeBow Institute for Myeloma Therapeutics and the Jerome Lipper Multiple Myeloma Center of the Dana-Farber Cancer Institute for support and help.
This work was supported by National Institutes of Health, National Cancer Institute grants RO1050947, R01-CA178264, PO1-CA078378, and the Dana-Farber/Harvard Cancer Center Specialized Program in Research Excellence SPORE in Multiple Myeloma P50CA100707. K.C.A. is an American Cancer Society Clinical Research Professor. Fellowship support provided by BioNovion.
Authorship
Contribution: Y.-T.T., C.A., and G.A. conceptualized research and formed the hypothesis of this paper; Y.-T.T., C.A., G.A., A.C., M.Y.Z. designed, performed experiments, and analyzed data; C.A., G.A., M.Y.Z., X.F., M.C., K.W., and L.Q. performed the in vitro research and collected data; C.A., M.M., G.A., and M.C. designed, performed, and analyzed animal work; H.v.E., and A.v.E. provided reagents and analytic tools; P.R., N.M., and K.C.A. provided MM patient samples; Y.-T.T. wrote the manuscript; and Y.-T.T., A.v.E., H.v.E., and K.C.A. critically evaluated and edited the manuscript.
Conflict-of-interest disclosure: H.v.E. and A.v.E. are employees of Aduro Biotech Europe (formerly known as BioNovion). P.R. serves on advisory boards to Millennium, Celgene, Novartis, Johnson & Johnson, and Bristol-Myers Squibb. N.M. serves on advisory boards to Millennium, Celgene, and Novartis. K.C.A. serves on advisory boards Celgene, Millennium, BMS, and Gilead and holds equity ownership in Oncopep and Acetylon. All other authors declare no competing financial interests.
Correspondence: Yu-Tzu Tai, Department of Medical Oncology, Dana-Farber Cancer Institute, M551, 450 Brookline Ave, Boston, MA 02215; e-mail: yu-tzu_tai@dfci.harvard.edu.


![Figure 3. 01A blocks proliferation and viability of MM cells induced by paracrine APRIL. (A) Supernatant was collected from CD14+ cells purified from MM patients (1-5) BM aspirates cultured with media (−, open column), M-CSF (M) (gray column), or M with receptor activator of nuclear factor kappa-B ligand (M+R, black column). APRIL secretion is measured by specific ELISA. APRIL mRNA was also confirmed by real-time qRT-PCR in 1 representative sample (left). (B) Daudi-BCMA/Daudi-empty cells, (C) IL-6–dependent INA6, IL-6–independent MM1S, and (D) RPMI8226, as well as (E) CD138+ cells from a relapsed MM patient, were cultured for 3 days with APRIL in the presence or absence of anti-APRIL 01A mAb (10 μg/mL), followed by (B,E) [3H]thymidine uptake and (C-D) luminescence-based viability assays. Two additional patient MM cells were subject to viability and caspase 3/7 activity (F; newly diagnosed) and annexin V/PI (G; relapsed) flow cytometry.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/25/10.1182_blood-2016-01-691162/4/m_3225f3.jpeg?Expires=1769085838&Signature=P1l3S0t30ie8tN3do1luuGJdRcfjCv4xZvEcQXTXh54Jtu2xTLqx8aJ0NMkUXrrQQooMdspLVaHdxNEOwA1mnUPrqpJzTi2WI6F~Z~u99U~VD5EbsdPcc~RNOif6DdNNc6pqCK5yfT~R~0Rcj2dYOs~cY9VVBSQIwaOs97XBxKexpqCbZ8atZp5Zq7kvE17aZcYNGFHZfiqK3JgweqIHukFERw8rAve5msxV865dCrHo1bkvU2o3xL2EqdmxVd0Kfe8kcpS-fg6yc8vFis64OhqUUs6LrkAAxnjAqB1aNP~Yc16tWLFoeiM5hx1SIgq26ZiqsXlWCIwx7CgzB3HxUQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. 01A selectively blocks APRIL-induced protection in MM cells, alone and in cocultures with OCs, an effect that is further enhanced by lenalidomide or bortezomib. (A) U266 cells were incubated with APRIL or IL-6 in the presence or absence of lenalidomide, with or without 01A (10 μg/mL), and assayed by [3H]thymidine uptake at day 3. MM cell lines were cultured for 4 days in 2% fetal calf serum growth media with OCs in the presence of 01A with either (B) lenalidomide or (C) bortezomib. *P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/25/10.1182_blood-2016-01-691162/4/m_3225f7.jpeg?Expires=1769085838&Signature=U3JPkAs1OYbu184KQjnKYrhwNXwDlDSactJ1Em7c8rCPbHL-DwsmjrXWk-GwYA8uLTiRcZFVp5bUKGVEE1bN4gYpFk2-djUCIfRdnVA-eMiocBvtQRDftRRphmv2RdRCK7GAsARU4YkYFOI9lpCKA8nOJjf6cjUuTHtaCwJrEmB-P3bDNJUc~rjXBiLdBxzalqboItqBHkbTkGt5HlcwMJ0ldHupWZdUHgTlr2Bq0qZ06cjT-9IzPK1lqKvxFl3AeZJInKIUrEMlwBvWrFDAgnecnhLXjHncNCJfaMCChmDH4j7hPHgyQ3f-NXnqzmA1UeY2o39MTil84RcH4bOK6A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)