Key Points
Venetoclax potently induces rapid onset apoptosis of CLL cells in vitro and in vivo, independently of TP53 function.
Objective responses in patients with del(17p) and/or TP53-mutated CLL are as deep as patients with no perturbation of TP53.
Abstract
BCL2 blunts activation of the mitochondrial pathway to apoptosis, and high-level expression is required for chronic lymphocytic leukemia (CLL) survival. Venetoclax (ABT-199) is a small-molecule selective inhibitor of BCL2 currently in clinical trials for CLL and other malignancies. In conjunction with the phase 1 first-in-human clinical trial of venetoclax in patients with relapsed or refractory CLL (M12-175), we investigated the mechanism of action of venetoclax in vivo, explored whether in vitro sensitivity assays or BH3 profiling correlated with in vivo responses in patients, and determined whether loss of TP53 function affected responses in vitro and in vivo. In all samples tested, venetoclax induced death of CLL cells in vitro at concentrations achievable in vivo, with cell death evident within 4 hours. Apoptotic CLL cells were detected in vivo 6 or 24 hours after a single 20-mg or 50-mg dose in some patients. The extent of mitochondrial depolarization by a BIM BH3 peptide in vitro was correlated with percentage reduction of CLL in the blood and bone marrow in vivo, whereas the half lethal concentration derived from standard cytotoxicity assays was not. CLL cell death in vitro and the depth of clinical responses were independent of deletion of chromosome 17p, TP53 mutation, and TP53 function. These data provide direct evidence that venetoclax kills CLL cells in a TP53-independent fashion by inhibition of BCL2 in patients and support further assessment of BH3 profiling as a predictive biomarker for this drug.
Introduction
Resistance to apoptosis is one of the hallmarks of cancer biology.1,2 In hematologic malignancies, and particularly lymphoid cancers, this is most typically via dysregulation of the intrinsic, or mitochondrial, pathway of apoptosis. Activation of this pathway culminates in mitochondrial outer membrane permeabilization and cell death. The threshold at which mitochondrial outer membrane permeabilization is triggered3 is determined by the balance of activity between 3 subfamilies of the BCL2 family of proteins: the mediators (BAX and BAK), which disrupt the mitochondrial membrane; the antiapoptotic proteins (BCL2, BCLxL, BCLw, MCL-1, and A1), which act to constrain BAX and BAK; and the BH3-only proteins (BIM, BID, PUMA, NOXA, HRK, and BAD), which are activated under conditions of cellular stress and function to inhibit the antiapoptotic proteins or to activate BAX and BAK.4-6
Chronic lymphocytic leukemia (CLL) is a disease uniformly characterized by high BCL2 protein expression7 caused in many cases from loss of microRNA-mediated repression of BCL2 gene expression.8 This results in the inappropriate survival of mature B lymphocytes in vivo and is associated with resistance to chemotherapy.9 CLL cells also commonly express high levels of BH3-only proapoptotic proteins such as BIM, which are tonically sequestered by BCL2, causing cells to be primed for cell death and be dependent on BCL2 function.3,10,11 Thus, although the high levels of BCL2 maintain survival, the elevated level of mitochondrial priming can leave CLL cells highly susceptible to the input of additional proapoptotic signals.
The majority of patients with CLL are thus initially highly responsive to treatment with chemotherapy, which commonly acts by inducing apoptosis via TP53. In the relapsed/refractory disease setting, up to half of patients can acquire abnormalities in TP53 through clonal evolution.12 Reduction in TP53 function can occur either through loss of parts of chromosome 17 [del(17p)] or mutation of TP53 or both. These result in reduced cellular ability to sense DNA damage from standard cytotoxic agents and therefore to trigger an appropriate cell death response.13 As TP53 activates the intrinsic apoptotic pathway by inducing several BH3-only proteins,13 agents that directly antagonize BCL2 function downstream of TP53 may circumvent the block to apoptosis associated with loss of TP53 function.
CLL was thus a logical disease for the initial testing of small-molecule BH3 mimetics, drugs that antagonize the activity of BCL2 prosurvival proteins by mimicking the action of the BH3-only proteins. The first potent and highly selective BH3 mimetic antagonist of BCL2, venetoclax (formerly known as ABT-199 or GDC-0199), was described in 2013.14 Early preclinical studies confirmed the hypothesis that targeted inhibition of BCL2 could be associated with substantial killing of malignant lymphoid cells while avoiding the BCLxL-mediated platelet toxicity14,15 seen with less selective drugs such as navitoclax.16 A first-in-human phase 1 study of venetoclax in patients with relapsed or refractory CLL or small lymphocytic lymphoma (M12-175 arm A; #NCT01328626)17 has recently reported the safety and significant clinical activity of BCL2-selective inhibition. An overall response rate of 79% was observed across a range of doses in 116 heavily pretreated patients with relapsed or refractory high-risk CLL, with 20% of patients achieving a complete remission.17
Importantly, the response rate was equivalent in patients with del(17p) and those without this chromosomal abnormality. Utilizing primary CLL samples from subsets of patients treated with venetoclax on the M12-175 trial, we addressed several questions that have arisen from research to date: first, whether venetoclax directly antagonizes BCL2 in mitochondria of CLL cells; second, whether the mechanism of action defined in vitro can be confirmed through demonstration of apoptosis in vivo in patients; third, whether venetoclax kills CLL cells with loss of TP53 function as efficiently as TP53 wild-type cells; and, finally, whether clinical response is correlated with in vitro sensitivity and baseline mitochondrial priming.
Methods
Patients and samples
Written informed consent was obtained from patients with CLL for the collection of clinical data and peripheral blood (PB) and bone marrow (BM) samples. The studies were performed in line with the Declaration of Helsinki and with the approval and monitoring of the Human Research Ethics Committees/Institutional Review Boards of the participating institutions: Royal Melbourne Hospital (2011.044, 2005.008), Peter MacCallum Cancer Centre (HREC/10/PMCC/27), Walter and Eliza Hall Institute (05/04), and Dana Farber/Harvard Cancer Center (#99-224). The majority of samples used in this study came from patients in the M12-175 trial17 enrolled in either Melbourne or Boston where these correlative laboratory studies were independently conducted. The clinical characteristics of patients at study entry, details of treatment with venetoclax monotherapy with doses of 150 to 1200 mg/d, and patient outcomes are described elsewhere.17 Additional samples were collected from concurrent patients with CLL requiring therapy.
CLL in vitro cytotoxicity assays
Freshly collected CLL cells in density gradient-separated mononuclear cell layers from PB and BM of patients enrolled in Melbourne were assayed for in vitro sensitivity using previously published methods.18,19 Briefly, CLL cells resuspended in 10% fetal calf serum/media were incubated with venetoclax (0.0128-1000 nM) for 4 or 24 hours in parallel with dimethyl sulfoxide (DMSO; final concentration 0.01%) diluent controls. In selected experiments, 20 μM of the pan-caspase inhibitor qVD.OPh (MP Biomedicals, Santa Ana, CA) was also added. To assess TP53-dependent cell death,20 CLL cells were incubated with nutlin-3a (78-10 000 nM; Roche Pharmaceuticals, Switzerland) or 0.01% DMSO for 72 hours. Viable CLL cells were enumerated by flow cytometry on a FACSCalibur analyzer (BD Biosciences) with concurrent propidium iodide (PI) exclusion and surface immunophenotyping with anti-CD5-fluorescein-isothiocyanate (FITC) (clone BL1a) and anti-CD19-phycoerythrin (clone J3.119) antibodies (Beckman Coulter, Villepinte, France). CLL viability at a given drug concentration was reported as a percentage of the number of viable CLL cells in the corresponding DMSO-treated wells. Concentration response curves and half lethal concentration (LC50) were derived using GraphPad Prism (GraphPad Software, San Diego, CA). The stated approximate plasma concentrations of venetoclax observed in vivo in the M12-175 trial were calculated by conversion of the reported data17 to molar data using the molecular weight of 868.4392 g/mol.21
BH3 profiling
The BH3 profiling technique, as previously described22 and detailed in the supplemental Materials (available on the Blood Web site), was applied to cells from viably frozen samples collected from patients enrolled in Boston after thawing. Flow cytometry was performed on a BD FACS Fortessa or Fortessa x20. The percentage of CD19+CD5+ CLL cells was confirmed to be >85%. Loss of cytochrome C was used to assess the interactions between BH3 peptides and BCL2 family proteins. The percentage of cytochrome C loss in response to each BH3 peptide was normalized relative to the cytochrome C change with the solvent control DMSO (0% loss) and an isotype staining control (considered as positive control, 100% loss).
Ex vivo annexin V assay
PB samples from M12-175 clinical trial patients in Melbourne with a PB lymphocytosis (>5 × 109/L) were taken immediately prior to the first dose (t = 0), 6 hours, and 24 hours post first dose. The mononuclear layer was isolated using Ficoll, and the cells were incubated with anti-CD5-allophycocyanin, anti-CD19-phycoerythrin, and anti-annexin-V-FITC (Invitrogen). CLL cells expressing phosphatidylserine (PS) (CD5+CD19+AnnexinV+) were enumerated with a fluorescence-activated cell sorter (FACS) Calibur or FACS Fortessa analyzer.
Clinical response to venetoclax
All clinical parameters collected for the purposes of this study were measured as part of the M12-175 clinical trial of venetoclax (#NCT01328626; full clinical protocol is published elsewhere17 ). Briefly, the clinical responses were assessed using International Workshop on Chronic Lymphocytic Leukemia (iwCLL) 2008 criteria.23 Other parameters of early response analyzed for correlation with laboratory tests included the following: percentage decrease at 6 weeks in PB lymphocyte count from screening; percentage reduction at 6 weeks in the sum product of the diameter (SPD) of 6 computed tomography target lesions identified at screening as a measure of lymph node mass; and percentage reduction at or before 24 weeks in BM infiltration by CLL from screening.
Genetic deletion of TP53
TP53-deficient RS4;11 human B-lymphoblast cell lines were generated using the CRISPR/Cas9 system by established methods24 (see supplemental Materials for details). Parental RS4;11 cells (ATCC, Manassas, VA) bearing Cas9, but without TP53 sGuide (sg TP53), were used as control cells in venetoclax and nutlin-3a concentration-response experiments conducted as for CLL cells. Loss of TP53 protein was confirmed by western blot. Similarly, lymph node B220+ B cells from Trp53−/− mice25 were assayed for drug sensitivity in parallel with Trp53+/+ cells, using the same methodology.19
Data analysis and statistics
Flow cytometry data were analyzed using FACS Diva version 6.1.1 (BD Pharmingen). Additional analysis was performed using GraphPad Prism. Multiple groups were compared using standard 1-way analysis of variance (ANOVA). Unpaired and paired groups were compared with a Student t test. Statistical significance was P ≤ .05 unless otherwise stated.
Results
CLL cells die rapidly via apoptosis in vitro after exposure to venetoclax
Consistent with earlier findings,14,15 CLL cells from the PB of 33 patients screened for entry to the clinical trial (M12-175) were highly sensitive to venetoclax in vitro. Following exposure for 24 hours, the mean LC50 was estimated to be 1.9 nM, and the 90% lethal concentration was 105 nM (Figure 1A). Samples from all patients were sensitive in vitro with 18/33 (55%) demonstrating >90% killing after incubation with 1 μM venetoclax, a concentration corresponding approximately to steady-state concentrations of venetoclax observed in patients receiving the recommended phase 2 dose of 400 mg per day.17 Coincubation with the pan-caspase inhibitor qVD.OPh blocked in vitro cytotoxicity by venetoclax (supplemental Figure 1A).
CLL cells from PB and BM are highly sensitive to venetoclax in vitro. (A) Fitted mean concentration-response curve (solid line) ± 95% prediction bands (thin lines) derived from data of 33 individual PB CLL samples from patients screened for the M12-175 trial. Circles and error bars represent the observed means ± standard deviations. CLL cells were incubated for 24 hours with varying concentrations of venetoclax (0.0128-1000 nM) or DMSO, and CD5+CD19+PI− cells were enumerated. Percent CLL viability was determined by normalizing against the number of viable CLL cells in the corresponding DMSO-treated wells (mean viability 93% [range 68% to 99%]). The horizontal lines indicate 50% (gray, solid) and 90% (black, dotted) cell death. The estimated LC50 was 1.9 nM, and the 90% lethal concentration was 105 nM. The blue shaded area indicates the range of steady-state plasma concentrations of venetoclax at a dose of 400 mg daily, ranging between the mean trough (dashed blue line) and peak postdose (solid blue line) concentrations.17 (B) CLL cells die rapidly in vitro. Mean fitted concentration-response curve (solid line) for 7 PB or BM CLL samples from 5 patients incubated for only 4 hours. The dashed green line (300 nM) represents the concentration approximating the observed peak plasma concentration after a single dose of 50 mg venetoclax in the M12-175 trial; the dashed red line (1.3 μM) represents the peak concentration observed after a single dose of 200 mg.17 (C) Correlation of individual LC50 values for paired PB and BM CLL cells from the same patient at screening incubated in parallel for 24 hours (n = 32; P < .0001, R2 = 0.83). (D) Correlation of mitochondrial depolarization as assessed by percentage cytochrome C loss induced by exposure to venetoclax or to BAD BH3 peptide in the BH3 profiling assay in screening PB samples from 13 CLL patients treated on the M12-175 trial (n = 13; P = .0001, R2 = 0.75).
CLL cells from PB and BM are highly sensitive to venetoclax in vitro. (A) Fitted mean concentration-response curve (solid line) ± 95% prediction bands (thin lines) derived from data of 33 individual PB CLL samples from patients screened for the M12-175 trial. Circles and error bars represent the observed means ± standard deviations. CLL cells were incubated for 24 hours with varying concentrations of venetoclax (0.0128-1000 nM) or DMSO, and CD5+CD19+PI− cells were enumerated. Percent CLL viability was determined by normalizing against the number of viable CLL cells in the corresponding DMSO-treated wells (mean viability 93% [range 68% to 99%]). The horizontal lines indicate 50% (gray, solid) and 90% (black, dotted) cell death. The estimated LC50 was 1.9 nM, and the 90% lethal concentration was 105 nM. The blue shaded area indicates the range of steady-state plasma concentrations of venetoclax at a dose of 400 mg daily, ranging between the mean trough (dashed blue line) and peak postdose (solid blue line) concentrations.17 (B) CLL cells die rapidly in vitro. Mean fitted concentration-response curve (solid line) for 7 PB or BM CLL samples from 5 patients incubated for only 4 hours. The dashed green line (300 nM) represents the concentration approximating the observed peak plasma concentration after a single dose of 50 mg venetoclax in the M12-175 trial; the dashed red line (1.3 μM) represents the peak concentration observed after a single dose of 200 mg.17 (C) Correlation of individual LC50 values for paired PB and BM CLL cells from the same patient at screening incubated in parallel for 24 hours (n = 32; P < .0001, R2 = 0.83). (D) Correlation of mitochondrial depolarization as assessed by percentage cytochrome C loss induced by exposure to venetoclax or to BAD BH3 peptide in the BH3 profiling assay in screening PB samples from 13 CLL patients treated on the M12-175 trial (n = 13; P = .0001, R2 = 0.75).
To assess the rapidity of onset of CLL killing, 7 samples from 5 patients (PB and BM) were incubated for just 4 hours in vitro. After this brief exposure, many CLL cells became PI positive. The LC50 for CLL at 4 hours was 820 nM (Figure 1B). Consistent with the observations of tumor lysis syndrome occurring within 12 hours in some patients initiated at 50 to 200 mg,17 significant in vitro CLL killing was observed at this 4-hour time point, at concentrations approximating those achieved in vivo after single doses of 50 mg (300 nM) and 200 mg (1.3 μM). Rapid onset cell death was prevented by addition of qVD.OPh (supplemental Figure 1B), and associated with caspase activation (supplemental Figure 2) when assessed in samples from 3 patients. We found that the intrinsic susceptibility of a patient’s CLL to venetoclax did not differ between freshly collected cells isolated from PB and BM, with in vitro sensitivity of cells from these tissue compartments being highly correlated (R2 = 0.8 and P < .0001) in paired samples from 32 patients (Figure 1C).
BH3 profiling utilizes BH3 peptides to simulate the action of the full-length BH3-only proteins in a cell. In addition to BH3 peptides, low concentrations of BH3 mimetic drugs can also be used in the profile, which allows the drug to rapidly enter the gently permeabilized cell and interact directly with the native BCL2 family members at the level of the mitochondria. We used venetoclax in this fashion and found that the degree of mitochondrial depolarization it induced was similar to the depolarization induced by the BAD BH3 peptide (n = 13; R2 = 0.75, P = .0001). This is consistent with previous evidence that venetoclax induces apoptosis in CLL cells by selective targeting of BCL214 and demonstrates that antagonism occurs at the level of the mitochondria (Figure 1D).
Taken together, these findings suggest that venetoclax rapidly and potently kills CLL cells in vitro within a few hours of exposure by induction of mitochondrial apoptosis, and that this effect is independent of whether the cells are collected from PB or BM.
Rapid in vivo CLL apoptosis after dosing with venetoclax
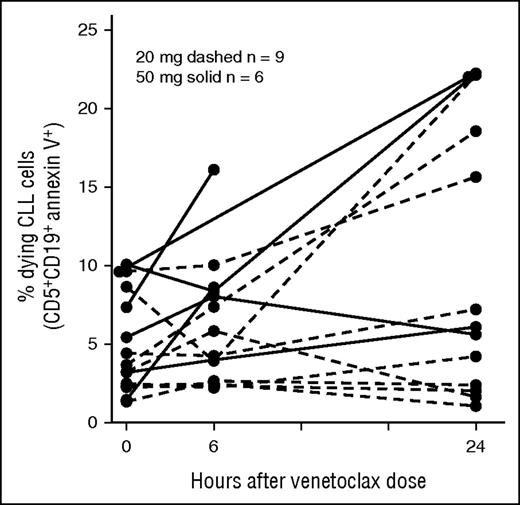
Early phase clinical studies with navitoclax provided proof of concept that BH3 mimetics could induce death of CLL cells in vivo, yielding objective responses in patients.16 Having confirmed that venetoclax induces rapid-onset CLL apoptosis in vitro, we hypothesized that patients receiving venetoclax therapy would also demonstrate rapid-onset apoptosis of CLL cells in vivo. In particular, we sought to demonstrate that the rapid clinical responses to venetoclax were because of induction of apoptosis. We used flow cytometry to detect exposure of PS on the surface of PB CLL cells collected from 15 individuals receiving their first dose of either 20 mg or 50 mg of venetoclax. This experiment was potentially limited by the rapid clearance of apoptotic cells in vivo.26 Nonetheless, increases in the percentage of CLL cells with detectable exposure of PS after a single dose of venetoclax could be demonstrated in some patients at 6 and 24 hours following a single dose of venetoclax, even with doses as low as 20 mg (Figure 2). A significant increase in PS exposure was demonstrated between predose and greatest postdose collections across 15 paired samples (paired Student t test P = .0006). In a limited subset of patients, caspase activity induced by in vivo exposure to venetoclax was analyzed in PB mononuclear cells collected at the same time points. A single 50-mg dose induced an increase in caspase activity by 6 hours, and this was associated with a substantial reduction in PB lymphocytosis within 12 hours in 2 of 3 patients analyzed (supplemental Figure 3). These findings provide in vivo evidence for an on-target mechanism of killing for venetoclax in the treatment of CLL. This rapid induction of apoptosis in vivo after a single dose of venetoclax is likely to explain the very rapid reductions in PB CLL burden observed within 24 hours in many patients.17
CLL cells undergo apoptosis in vivo after the first dose of venetoclax. Increase in the percentage of CLL cells with detectable exposure of PS after a single dose of 20 mg (dashed) or 50 mg (solid) of venetoclax among 15 patients dosed with the drug for the first time. PS exposure was detected by FITC-conjugated annexin V. Paired Student t test P = .0006 for comparison between pre-venetoclax 0 hour collection and peak post-venetoclax collection (at either 6 or 24 hours post first dose).
CLL cells undergo apoptosis in vivo after the first dose of venetoclax. Increase in the percentage of CLL cells with detectable exposure of PS after a single dose of 20 mg (dashed) or 50 mg (solid) of venetoclax among 15 patients dosed with the drug for the first time. PS exposure was detected by FITC-conjugated annexin V. Paired Student t test P = .0006 for comparison between pre-venetoclax 0 hour collection and peak post-venetoclax collection (at either 6 or 24 hours post first dose).
In vitro sensitivity assays as a biomarker for clinical response to venetoclax
The initial clinical findings from the venetoclax monotherapy study in patients with relapsed or refractory CLL indicate overall response rates between 71% and 79% among the most high-risk patients with del(17p) or fludarabine-refractory disease.17 However, ∼20% of heavily pretreated patients did not achieve an objective response. We therefore tested whether the in vitro sensitivity of CLL cells to venetoclax at screening was correlated with, and predictive of, clinical response in the patient when treated with the drug.
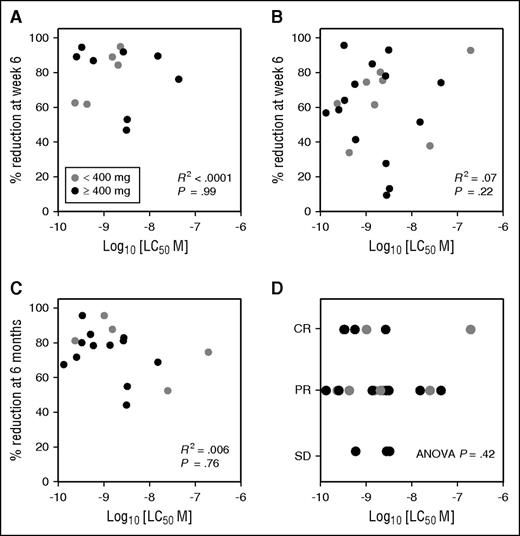
We first evaluated whether in vitro sensitivity as assessed by cell viability testing in 22 patients was predictive of depth of reduction of CLL at the earliest protocol-specified efficacy assessment time point on trial in each of 3 different tissue compartments. In vitro sensitivity of CLL to venetoclax at screening did not correlate with the percentage reduction in either PB lymphocyte count among patients with a baseline lymphocytosis (Figure 3A) or lymph node mass (Figure 3B) at week 6. Similarly, no correlation was observed with the percentage reduction in BM infiltration by CLL at week 24 (Figure 3C). In the trial, patients initially treated in dose cohorts of <400 mg/d had inferior response rates and outcomes to those treated with 400 mg/d or more. Restricting the analyses to patients receiving ≥400 mg/d did not alter the findings of lack of correlation. Finally in vitro sensitivity of CLL to venetoclax at screening did not correlate with the best response by iwCLL criteria (either complete response, partial response, or no response) among the 22 patients (ANOVA P = .42) (Figure 3D). Taken together, these results suggest that the in vitro sensitivity of CLL to venetoclax at screening is not predictive of depth of clinical response to this agent and does not identify the minority of patients who fail to achieve an objective response.
In vitro sensitivity of CLL at screening does not predict for clinical outcomes. (A) Lymphocyte response. For patients with lymphocytosis >5 × 109/L at screening, the percentage reduction in lymphocyte count at week 6 on venetoclax did not correlate with the screening in vitro sensitivity (n = 13; R2 < 0.001). Data points are color coded by cohort dose (≥400 mg in black or <400 mg in gray); lack of correlation is not explained by differences in dose of venetoclax (R2 = 0.001 for patients receiving ≥400 mg/d only). (B) Nodal response. The percentage reduction in the lymph node mass (represented by the SPD of 6 target lesions on CT) at week 6 on venetoclax did not correlate with the screening in vitro sensitivity (n = 22; R2 = 0.07). Data points color coded by cohort dose (≥400 mg in black or <400 mg in gray); lack of correlation is not explained by differences in dose of venetoclax (R2 < 0.01 for patients receiving ≥400 mg/d only). (C) Marrow response. The percentage reduction in BM CLL infiltration at week 24 on venetoclax did not correlate with screening in vitro sensitivity (n = 17; R2 = 0.006). Data points color coded by cohort dose (≥400 mg in black or <400 mg in gray); lack of correlation is not explained by differences in dose of venetoclax (R2 = 0.006 for patients receiving ≥400 mg/d only). (D) Best iwCLL response. Correlation between screening PB CLL LC50 and overall best objective response to venetoclax observed for 22 CLL patients on the M12-175 study as judged by iwCLL criteria (ANOVA P = .42). Data points color coded by cohort dose (≥400 mg in black or <400 mg in gray); lack of correlation is not explained by differences in dose of venetoclax (ANOVA P = .47 for patients receiving ≥400 mg/d only). CR, complete remission or complete remission with incomplete count recovery; PR, partial response; SD, stable disease.
In vitro sensitivity of CLL at screening does not predict for clinical outcomes. (A) Lymphocyte response. For patients with lymphocytosis >5 × 109/L at screening, the percentage reduction in lymphocyte count at week 6 on venetoclax did not correlate with the screening in vitro sensitivity (n = 13; R2 < 0.001). Data points are color coded by cohort dose (≥400 mg in black or <400 mg in gray); lack of correlation is not explained by differences in dose of venetoclax (R2 = 0.001 for patients receiving ≥400 mg/d only). (B) Nodal response. The percentage reduction in the lymph node mass (represented by the SPD of 6 target lesions on CT) at week 6 on venetoclax did not correlate with the screening in vitro sensitivity (n = 22; R2 = 0.07). Data points color coded by cohort dose (≥400 mg in black or <400 mg in gray); lack of correlation is not explained by differences in dose of venetoclax (R2 < 0.01 for patients receiving ≥400 mg/d only). (C) Marrow response. The percentage reduction in BM CLL infiltration at week 24 on venetoclax did not correlate with screening in vitro sensitivity (n = 17; R2 = 0.006). Data points color coded by cohort dose (≥400 mg in black or <400 mg in gray); lack of correlation is not explained by differences in dose of venetoclax (R2 = 0.006 for patients receiving ≥400 mg/d only). (D) Best iwCLL response. Correlation between screening PB CLL LC50 and overall best objective response to venetoclax observed for 22 CLL patients on the M12-175 study as judged by iwCLL criteria (ANOVA P = .42). Data points color coded by cohort dose (≥400 mg in black or <400 mg in gray); lack of correlation is not explained by differences in dose of venetoclax (ANOVA P = .47 for patients receiving ≥400 mg/d only). CR, complete remission or complete remission with incomplete count recovery; PR, partial response; SD, stable disease.
BH3 profiling as a biomarker for clinical response to venetoclax
BH3 profiling has previously been shown to predict clinical response to cytotoxic therapy,27 and decreased mitochondrial priming has been associated with resistance to other treatments in CLL.22 We therefore assessed whether the baseline level of mitochondrial priming in PB CLL cells from 14 patients was predictive of clinical response in the different tissue compartments. Using BIM BH3 peptide as a measure of the overall mitochondrial priming for apoptosis, we found that patients whose CLL cells had a high level of mitochondrial priming at baseline had a greater reduction in circulating lymphocyte count (Figure 4A; P = .05) as well as a greater reduction in CLL disease burden in the BM (Figure 4C; P = .01). There was no clear association between higher levels of baseline mitochondrial priming and deeper lymph node responses (Figure 4B; P = .19). Collectively, these BH3 profiling results suggest that a functional assessment of baseline mitochondrial priming may be a better predictor of compartmental responses to venetoclax than conventional in vitro cell death assays.
Correlation between mitochondrial priming and in vivo response in the PB, lymph node, and BM compartments. (A) Lymphocyte response. Correlation between percentage reduction in the absolute lymphocyte count at 6 weeks in CLL patients on the M12-175 trial and mitochondrial priming, as assessed by percentage cytochrome C loss after exposure of CLL cells (mean viability after thawing 92% [range 80% to 98%]) to BIM BH3 peptide at 0.8 μM (P = .05, R2 = 0.23), in 13 evaluable patients with lymphocytosis at study entry. (B) Nodal response. Correlations between percentage reductions in the SPD of target lymph nodes at 6 weeks and mitochondrial priming (P = .19, R2 = 0.14) in 14 evaluable patients. (C) Marrow response. Correlation between percentage reduction in CLL cell BM infiltrate at first restaging at approximately week 24 and mitochondrial priming (P = .01, R2 = 0.63) in 9 evaluable patients.
Correlation between mitochondrial priming and in vivo response in the PB, lymph node, and BM compartments. (A) Lymphocyte response. Correlation between percentage reduction in the absolute lymphocyte count at 6 weeks in CLL patients on the M12-175 trial and mitochondrial priming, as assessed by percentage cytochrome C loss after exposure of CLL cells (mean viability after thawing 92% [range 80% to 98%]) to BIM BH3 peptide at 0.8 μM (P = .05, R2 = 0.23), in 13 evaluable patients with lymphocytosis at study entry. (B) Nodal response. Correlations between percentage reductions in the SPD of target lymph nodes at 6 weeks and mitochondrial priming (P = .19, R2 = 0.14) in 14 evaluable patients. (C) Marrow response. Correlation between percentage reduction in CLL cell BM infiltrate at first restaging at approximately week 24 and mitochondrial priming (P = .01, R2 = 0.63) in 9 evaluable patients.
CLL apoptosis in response to venetoclax in vitro is independent of TP53 status
TP53 acts to initiate mitochondrial apoptosis by inducing the expression of several BH3-only proteins. Because the BH3-only proteins act downstream of TP53, the BH3 mimetics should be efficacious regardless of whether TP53 is fully functional.5,28 We hypothesized that there would be equivalent in vitro sensitivity between CLL cells from patients with and without loss of TP53 function. Specifically we sought to understand if the presence of a del(17p) or mutation in TP53 affected the in vitro activity of venetoclax against CLL in a wider group of patients.
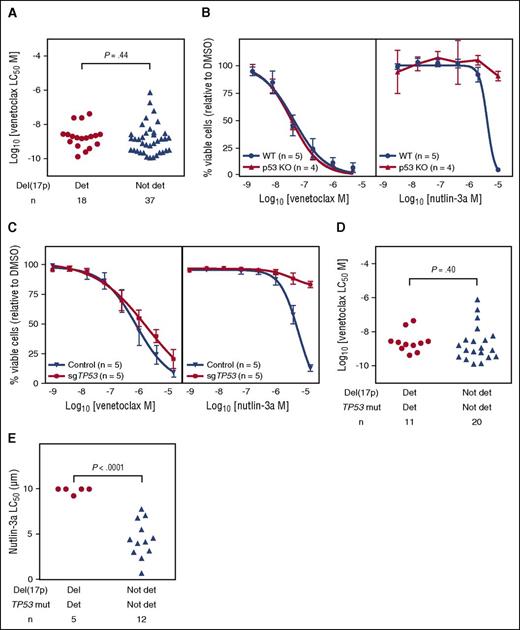
The presence or absence of del(17p) did not affect the pattern of in vitro sensitivity of CLL samples from 55 patients (Figure 5A). As the del(17p) clone may represent only a minor population of cells in a given sample, to further assess whether abnormalities in TP53 function affected venetoclax cytotoxicity we investigated 3 additional lines of evidence. First, we took advantage of the availability of strains of mice genetically lacking TP53 (Trp53−/−) and isogenic wild-type mice. Mature B cells isolated from lymph nodes of Trp53−/− mice were similarly sensitive to venetoclax in vitro as those from wild-type mice (Figure 5B). Second, human RS4;11 B-lymphoblast cell lines lacking TP53 following CRISPR/Cas9-mediated gene mutation displayed similar in vitro sensitivity to parental control cells (Figure 5C). In both the murine B cells and the human cell line, absence of TP53 resulted in relative resistance to nutlin-3a, an agent whose cytotoxicity depends on intact wild-type TP53 function.29 Third, we focused on CLL that harbored both del(17p) and TP53 mutation to enrich for primary samples expected to have dominant populations with TP53 dysfunction. The in vitro sensitivity did not differ between samples from patients whose CLL harbored both del(17p) and TP53 mutation (n = 11) and those with both intact chromosome 17p and wild-type TP53 (n = 20, Student t test P = .4; Figure 5D). We confirmed that TP53 function was diminished in populations with both del(17p) and TP53 mutation using nutlin-3a. Cells from 5 patients whose CLL was both del(17p) and TP53 mutated were significantly more resistant to nutlin-3a at 72 hours when compared with CLL cells carrying neither del(17p) nor TP53 mutation (n = 12, unpaired Student t test P < .0001; Figure 5E).
Venetoclax kills CLL cells, murine lymph node B cells, and RS4;11 human lymphoblast cell lines irrespective of TP53 deletion, mutation, or function. (A) For patient CLL samples, the 24-hour in vitro sensitivity to venetoclax did not differ based on del(17p) status (Student t test P = .44). Deletion 17p was detected by fluorescence in situ hybridization in 18 of 55 samples; the median percentage of del(17p) cells was 44.5% (range 7.5% to 95%) in those samples. (B) For murine lymph node B cells, the concentration-response curves for venetoclax are plotted for isogenic Trp53 wild-type (blue; WT) and Trp53−/− (red; p53KO) mice in the left panel, and for nutlin-3a in the right panel. All cells were incubated for 24 hours. n = 3 independent experiments. p53KO, p53 knockout. (C) For RS4;11 human B-cell lymphoblast cell lines, the concentration-response curves for venetoclax (left panel; 24 hour incubation) are plotted for TP53 wild-type cell lines (blue; Control) bearing a nontargeting sGuide and TP53-deficient cells (red; sgTP53) bearing a TP53targeting sGuide (see “Methods”). Loss of TP53 expression was confirmed by western blot (supplemental Figure 4). The concentration-response curves for nutlin-3a after a 3-day incubation are shown in the right panel. n = 5 independent experiments of pools of cells. (D) For patient CLL samples, the 24-hour in vitro sensitivity to venetoclax of CLL for 31 patients did not differ between samples with both del(17p) and TP53 mutation (n = 11) compared with those with neither del(17p) nor TP53 mutation (n = 20; unpaired Student t test P = .40). Del(17p) was present in a median 52.5% (range 24% to 82%) of CLL cells in the double detected group. Det, detected; mut, mutated. (E) The 72-hour in vitro sensitivity of CLL to nutlin-3a for 17 patients differed between those with both del(17p) and TP53 mutation (n = 5) and those with neither del(17p) nor TP53 mutation (n = 12; unpaired Student t test P < .0001). Del(17p) was present in a median 52.5% (range 26.5% to 58%) of CLL cells in the double detected group.
Venetoclax kills CLL cells, murine lymph node B cells, and RS4;11 human lymphoblast cell lines irrespective of TP53 deletion, mutation, or function. (A) For patient CLL samples, the 24-hour in vitro sensitivity to venetoclax did not differ based on del(17p) status (Student t test P = .44). Deletion 17p was detected by fluorescence in situ hybridization in 18 of 55 samples; the median percentage of del(17p) cells was 44.5% (range 7.5% to 95%) in those samples. (B) For murine lymph node B cells, the concentration-response curves for venetoclax are plotted for isogenic Trp53 wild-type (blue; WT) and Trp53−/− (red; p53KO) mice in the left panel, and for nutlin-3a in the right panel. All cells were incubated for 24 hours. n = 3 independent experiments. p53KO, p53 knockout. (C) For RS4;11 human B-cell lymphoblast cell lines, the concentration-response curves for venetoclax (left panel; 24 hour incubation) are plotted for TP53 wild-type cell lines (blue; Control) bearing a nontargeting sGuide and TP53-deficient cells (red; sgTP53) bearing a TP53targeting sGuide (see “Methods”). Loss of TP53 expression was confirmed by western blot (supplemental Figure 4). The concentration-response curves for nutlin-3a after a 3-day incubation are shown in the right panel. n = 5 independent experiments of pools of cells. (D) For patient CLL samples, the 24-hour in vitro sensitivity to venetoclax of CLL for 31 patients did not differ between samples with both del(17p) and TP53 mutation (n = 11) compared with those with neither del(17p) nor TP53 mutation (n = 20; unpaired Student t test P = .40). Del(17p) was present in a median 52.5% (range 24% to 82%) of CLL cells in the double detected group. Det, detected; mut, mutated. (E) The 72-hour in vitro sensitivity of CLL to nutlin-3a for 17 patients differed between those with both del(17p) and TP53 mutation (n = 5) and those with neither del(17p) nor TP53 mutation (n = 12; unpaired Student t test P < .0001). Del(17p) was present in a median 52.5% (range 26.5% to 58%) of CLL cells in the double detected group.
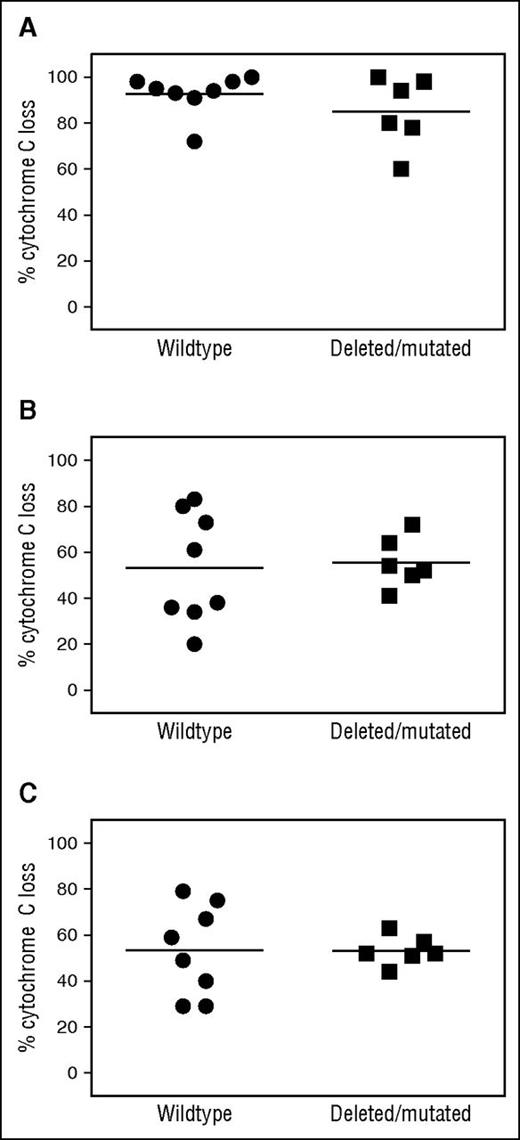
We next assessed whether we could observe differences in the level of baseline mitochondrial priming in patients with intact 17p and wild-type TP53 compared with patients with del(17p) or TP53 mutation. Using BIM BH3 peptide as a measure of the overall level of priming of the cells, we found no difference in baseline priming between CLL with intact 17p and wild-type TP53 (n = 8) compared with CLL with del(17p) or TP53 mutation (Figure 6A; n = 6, P = .55). Using BH3 profiling assays, we also assessed whether the baseline degree of BCL2 dependence differed between patients with or without del(17p) or TP53 mutation. Again, we found no difference in BCL2 dependence in CLL cells at baseline in these 2 groups, measured either with a BAD BH3 peptide (Figure 6B; P = .83) or with venetoclax as a peptide (Figure 6C; P = .99).
BH3 profiling demonstrates equivalent levels of mitochondrial priming and BCL2 dependence in CLL cells with del(17p) or TP53 mutation compared with wild-type CLL. (A) BIM BH3 peptide. Lack of correlation between the del(17p) or TP53 mutation status of CLL patients on the M12-175 trial and mitochondrial priming, as assessed by percent cytochrome C loss after exposure of CLL cells to BIM BH3 peptide at 0.8 μM (P = .55). Horizontal line represents the mean. n = 8 for CLL without del(17p) or a TP53 mutation, and n = 6 for CLL with either del(17p) or TP53 mutation. (B) BAD BH3 peptide. Lack of correlation of del(17p)/TP53 mutation status and functional dependence of CLL cells on BCL2 as assessed by percent cytochrome C loss after exposure of CLL cells to BAD BH3 peptide at 80 μM (P = .83). Horizontal line represents the mean. (C) Venetoclax. Lack of correlation of del(17p)/TP53 mutation status and functional dependence of CLL cells on BCL2 as assessed by percent cytochrome C loss after exposure of CLL cell mitochondria directly to the BH3 mimetic venetoclax 1 μM used analogously to a BH3 peptide in the BH3 profiling assay (P = .99). Horizontal line represents the mean.
BH3 profiling demonstrates equivalent levels of mitochondrial priming and BCL2 dependence in CLL cells with del(17p) or TP53 mutation compared with wild-type CLL. (A) BIM BH3 peptide. Lack of correlation between the del(17p) or TP53 mutation status of CLL patients on the M12-175 trial and mitochondrial priming, as assessed by percent cytochrome C loss after exposure of CLL cells to BIM BH3 peptide at 0.8 μM (P = .55). Horizontal line represents the mean. n = 8 for CLL without del(17p) or a TP53 mutation, and n = 6 for CLL with either del(17p) or TP53 mutation. (B) BAD BH3 peptide. Lack of correlation of del(17p)/TP53 mutation status and functional dependence of CLL cells on BCL2 as assessed by percent cytochrome C loss after exposure of CLL cells to BAD BH3 peptide at 80 μM (P = .83). Horizontal line represents the mean. (C) Venetoclax. Lack of correlation of del(17p)/TP53 mutation status and functional dependence of CLL cells on BCL2 as assessed by percent cytochrome C loss after exposure of CLL cell mitochondria directly to the BH3 mimetic venetoclax 1 μM used analogously to a BH3 peptide in the BH3 profiling assay (P = .99). Horizontal line represents the mean.
TP53 status is not predictive of CLL apoptosis in response to venetoclax in vivo
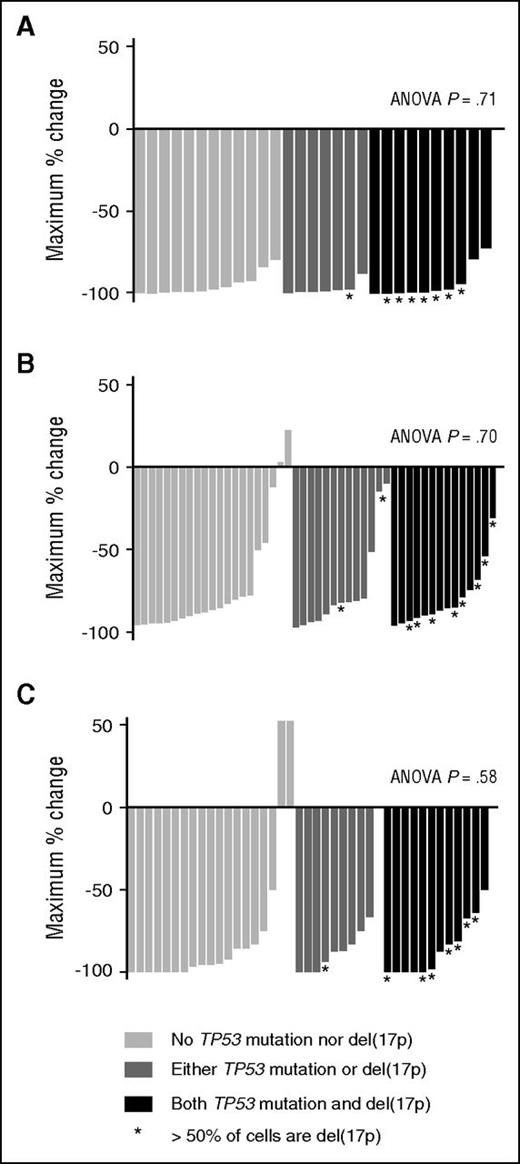
Based on our observations that TP53 dysfunction did not affect sensitivity of CLL cells to venetoclax in vitro (Figure 5) or mitochondrial priming or BCL2 dependence (Figure 6), we hypothesized that del(17p)/TP53 mutation status would not affect CLL cell apoptosis in response to venetoclax in vivo. We tested this by analyzing the depth of compartmental responses in patients for whom both the fluorescence in situ hybridization and TP53 mutant status of CLL was known. The greatest percentage reduction of PB lymphocyte count for 29 patients in M12-175 with a screening lymphocyte count >5 × 109/L did not differ between patients with CLL harboring both del(17p) and TP53 mutation, either del(17p) or TP53 mutation, or neither (Figure 7A; ANOVA P = .71). Similarly, neither the greatest percentage reduction in lymph node mass (Figure 7B; ANOVA P = .70) nor BM infiltration (Figure 7C; ANOVA P = .58) differed between these 3 groups (among 48 and 41 patients, respectively).
TP53 status does not affect clinical parameters of response to venetoclax. (A) PB lymphocyte count. The greatest percentage reduction of PB lymphocyte count for 29 patients in M12-175 with a screening lymphocyte count ≥5 × 109/L was examined, according to the TP53 status of their CLL: neither TP53 mutated nor del(17p) (n = 12); either TP53 mutated or del(17p) (n = 7); or both TP53 mutated and del(17p) (n = 10). There was no difference in depth of reductions: ANOVA P = .71 for comparison among 3 groups. (B) Lymph nodes. The greatest percentage reduction in SPD of 6 target lymph nodes on CT on venetoclax was examined for 48 patients according to the TP53 status of their CLL: neither del(17p) nor TP53 mutated (n = 21); either TP53 mutated or del(17p) (n = 13); or both del(17p) and TP53 mutated (n = 14). There was no difference in outcome between the 3 groups (ANOVA P = .70). (C) BM CLL infiltration. The greatest percentage reduction in BM infiltration by CLL was examined for 41 patients according to the TP53 status of their CLL: neither del(17p) nor TP53 mutated (n = 19); either TP53 mutated or del(17p) (n = 10); or both TP53 mutated and del(17p) (n = 12). The 3 groups demonstrated equal reduction in BM infiltration (ANOVA P = .58).
TP53 status does not affect clinical parameters of response to venetoclax. (A) PB lymphocyte count. The greatest percentage reduction of PB lymphocyte count for 29 patients in M12-175 with a screening lymphocyte count ≥5 × 109/L was examined, according to the TP53 status of their CLL: neither TP53 mutated nor del(17p) (n = 12); either TP53 mutated or del(17p) (n = 7); or both TP53 mutated and del(17p) (n = 10). There was no difference in depth of reductions: ANOVA P = .71 for comparison among 3 groups. (B) Lymph nodes. The greatest percentage reduction in SPD of 6 target lymph nodes on CT on venetoclax was examined for 48 patients according to the TP53 status of their CLL: neither del(17p) nor TP53 mutated (n = 21); either TP53 mutated or del(17p) (n = 13); or both del(17p) and TP53 mutated (n = 14). There was no difference in outcome between the 3 groups (ANOVA P = .70). (C) BM CLL infiltration. The greatest percentage reduction in BM infiltration by CLL was examined for 41 patients according to the TP53 status of their CLL: neither del(17p) nor TP53 mutated (n = 19); either TP53 mutated or del(17p) (n = 10); or both TP53 mutated and del(17p) (n = 12). The 3 groups demonstrated equal reduction in BM infiltration (ANOVA P = .58).
Discussion
Our results provide compelling evidence that selective inhibition of BCL2 at the level of the mitochondria is sufficient to induce apoptosis of CLL cells from patients with high-risk relapsed or refractory disease, both in vitro and in vivo. The cell killing observed in vitro occurs rapidly, is associated with caspase activation, is blocked by caspase inhibitors, and occurs at concentrations of drug achievable in patients. Furthermore, we were able to demonstrate induction of apoptosis in vivo after single doses of only 20 mg or 50 mg venetoclax in some patients. That apoptotic CLL cells in the circulation were only detected in some patients after the first dose most likely reflects the interplay of many factors, including the heterogeneity of sensitivity to the low doses given, interpatient differences in starting lymphocyte count, and pharmacokinetics, as well as the rapid clearance of apoptotic cells. Collectively, these pieces of evidence provide confirmation that the rapid-onset clinical activity of this drug is a function of the mechanism of action previously elucidated in preclinical models.14 Consistent with our findings of near-universal in vitro sensitivity of CLL cells sourced from patients’ PB or BM, almost 100% of patients had major reductions in PB CLL cells, and >95% had some reduction in nodal and marrow disease in the initial clinical trial.17
However, despite this, ∼20% of patients with heavily pretreated relapsed or refractory CLL did not achieve objective clinical responses with venetoclax treatment. Thus, there is a clinical imperative to elucidate clinical and laboratory markers predictive of response to this agent. Our data indicate that screening in vitro sensitivity of CLL to venetoclax is insufficiently discriminatory for clinical use as a predictive biomarker. However, BH3 profiling at screening does appear to correlate with the depth of clinical responses in blood and particularly in the BM. Larger systematic studies including more diverse CLL patient populations are required to determine its merit as a predictive tool for clinical response to this drug. Although not assessed in our study, MCL-1 expression can modulate resistance to BCL2 inhibition and is known to be upregulated in lymph nodes.30 Basal levels of MCL-1 in PB CLL cells did not correlate with treatment outcome with the less specific BCL2 inhibitor navitoclax.16 The contribution of MCL-1 to treatment failure and its potential as a biomarker require further investigation.
TP53 aberration, either mutation or loss through del(17p), is recognized as a major risk factor for death in patients with CLL, with inferior outcomes after standard fludarabine- or alkylator-based chemo-immunotherapy.31,32 Here we demonstrate that despite confirmed loss of TP53 function, normal murine nodal B cells, human B-lymphoblast cell lines, and primary CLL cells are as sensitive in vitro to apoptosis induction by venetoclax as cells with normal TP53 function. Moreover, the baseline level of mitochondrial priming did not differ between CLL samples with or without del(17p) or TP53 mutation, which led us to predict that patients with or without these abnormalities would derive equivalent clinical responses. Indeed, patients with predominant populations of CLL cells lacking TP53 function (those with both TP53 deleterious mutations and del[17p]) were just as likely to achieve similar reductions in CLL burden in all tissue compartments in vivo as those patients with wild-type TP53 in addition to normal chromosome 17p. This is consistent with the clinical observations17,33 that suggest venetoclax will be a particularly useful agent in patients with TP53 aberrant CLL. However, progression-free survival in patients with del(17p) CLL still appears inferior to that observed for patients with non-del(17p) CLL,17 a finding also observed for patients treated with another drug that is highly active against del(17p) CLL, ibrutinib.34-36 Together, the current data indicating equivalent depth of CLL response and the clinical observation of less durable benefit suggest that del(17p) CLL in some patients is associated with factors other than TP53 that determine patient outcome with venetoclax. We speculate that genetically unstable clones with additional molecular aberrations, often accompanied by a complex karyotype, may be responsible for emergent resistance. Given the recognized activity of ibrutinib in patients with del(17p) CLL and recent preclinical data indicating additive benefit,37 partnering of ibrutinib and venetoclax in combination is clinically attractive as a potential strategy to further improve on outcomes observed with either as monotherapy in these high-risk patients.17,35
Overall, the present findings provide in vitro and in vivo proof that venetoclax acts independently of TP53 to rapidly induce apoptosis of CLL, as predicted by its molecular mechanism of action. Further work is required to definitively establish biomarkers for response to this drug, but BH3 profiling is one potential method for identifying patients most likely to respond well.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in the M12-175 trial and their families; the study coordinators and the support staff at the clinical sites; and AbbVie and Genentech venetoclax team members.
This work was supported by AbbVie, in collaboration with Genentech/Roche. Venetoclax (ABT-199/GDC-0199) is being developed through collaboration between AbbVie and Genentech/Roche. M.A.A. was supported by a fellowship from the Webster bequest. Work in the labs of D.C.S.H. and A.W.R. was supported by scholarships, fellowships, and grants from the Australian National Health and Medical Research Council (research fellowships [A.W.R. and D.C.S.H.], program grants 1016647 and 1016701, and Independent Research Institutes Infrastructure Support Scheme grant 9000220); the Leukemia and Lymphoma Society (SCOR grants 7001-13); the Victorian Cancer Agency; the Cancer Council Victoria; the Australian Cancer Research Foundation; and a Victorian State Government Operational Infrastructure Support grant. This work was supported by a grant from the National Cancer Institute, National Institutes of Health (CA129974) (A.L.). M.S.D. is supported by an American Society of Clinical Oncology Career Development Award.
Authorship
Contribution: A.W.R., A.L., M.S.D., and D.C.S.H. devised the experiments; M.A.A., J.D., J.F., L.Y., E.G.S., I.J.M., D.S., and D.W. performed the experiments and generated TP53 data; M.A.A., A.W.R., J.F.S., C.T., J.R.B., and M.S.D. provided clinical assessments of response; S.L.H.E., S.Y.K., M.A.A., A.W.R., J.F.S., J.R.B., and M.S.D. interpreted trial data; M.A.A. wrote the first draft of the manuscript; and all authors contributed to review and analysis of data within the manuscript.
Conflict-of-interest disclosure: A.W.R. and D.C.S.H. received research funding from AbbVie and Genentech and are employees of Walter and Eliza Hall Institute of Medical Research, which receives milestone payments related to venetoclax. M.A.A. (from July 22, 2015) and D.S. and I.J.M. are employees of Walter and Eliza Hall Institute of Medical Research, which receives milestone payments related to venetoclax. J.R.B. and J.F.S. have received consulting fees from Genentech, Pharmacyclics, and Janssen. A.L. is a paid advisor to and his laboratory receives research sponsorship from AbbVie, Astra-Zeneca, and Tetralogic. M.S.D. has received research sponsorship from Pharmacyclics, Infinity, and Genentech and has received consulting fees from Genentech, AbbVie, Infinity, Pharmacyclics, and Janssen. C.T. has received research sponsorship from Janssen and AbbVie and has received consulting fees from Janssen, Roche, Novartis, and AbbVie. S.Y.K. and S.L.H.E. are employees of AbbVie and may hold stock. The remaining authors declare no competing financial interests.
Correspondence: Andrew W. Roberts, Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, VIC 3050, Australia; e-mail: roberts@wehi.edu.au.
References
Author notes
M.A.A. and J.D. contributed equally to this study.
D.C.S.H., M.S.D., A.L., and A.W.R. contributed equally to this study as senior authors.

![Figure 1. CLL cells from PB and BM are highly sensitive to venetoclax in vitro. (A) Fitted mean concentration-response curve (solid line) ± 95% prediction bands (thin lines) derived from data of 33 individual PB CLL samples from patients screened for the M12-175 trial. Circles and error bars represent the observed means ± standard deviations. CLL cells were incubated for 24 hours with varying concentrations of venetoclax (0.0128-1000 nM) or DMSO, and CD5+CD19+PI− cells were enumerated. Percent CLL viability was determined by normalizing against the number of viable CLL cells in the corresponding DMSO-treated wells (mean viability 93% [range 68% to 99%]). The horizontal lines indicate 50% (gray, solid) and 90% (black, dotted) cell death. The estimated LC50 was 1.9 nM, and the 90% lethal concentration was 105 nM. The blue shaded area indicates the range of steady-state plasma concentrations of venetoclax at a dose of 400 mg daily, ranging between the mean trough (dashed blue line) and peak postdose (solid blue line) concentrations.17 (B) CLL cells die rapidly in vitro. Mean fitted concentration-response curve (solid line) for 7 PB or BM CLL samples from 5 patients incubated for only 4 hours. The dashed green line (300 nM) represents the concentration approximating the observed peak plasma concentration after a single dose of 50 mg venetoclax in the M12-175 trial; the dashed red line (1.3 μM) represents the peak concentration observed after a single dose of 200 mg.17 (C) Correlation of individual LC50 values for paired PB and BM CLL cells from the same patient at screening incubated in parallel for 24 hours (n = 32; P < .0001, R2 = 0.83). (D) Correlation of mitochondrial depolarization as assessed by percentage cytochrome C loss induced by exposure to venetoclax or to BAD BH3 peptide in the BH3 profiling assay in screening PB samples from 13 CLL patients treated on the M12-175 trial (n = 13; P = .0001, R2 = 0.75).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/25/10.1182_blood-2016-01-688796/4/m_3215f1.jpeg?Expires=1770375789&Signature=eaI5UBLbwtBO4-kJ4rq0gqmBMfDdAIg32I8T5LdV--CzRqhxSxOaIHnNayA~oSNIEsgpvtDvad0BUyeZ-9iQBnA5QA~XdC3JVxdk1XsRE5Ei7OjXbBXuYR3iYXc7lJZUZTUgHsWpI2K6lvB~xxXCBVBB3bGSjVDgJur39xzDPO9LFkHPYTs5McmUjyywXICjljsNkHyvu3OJa9lVQ0usM2iB88FjBzh4PIr5aZH9ryR8tjUz4Dj6zKNKdh2kMfP~BuN3hudQ7vChKIn9FOZ0O01lCmmRLkUygoZtfy4Ebem93yTbo-WUBMxXjEOD-lEjpgY4Q~L7HOmGsxvzBta6Vw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Correlation between mitochondrial priming and in vivo response in the PB, lymph node, and BM compartments. (A) Lymphocyte response. Correlation between percentage reduction in the absolute lymphocyte count at 6 weeks in CLL patients on the M12-175 trial and mitochondrial priming, as assessed by percentage cytochrome C loss after exposure of CLL cells (mean viability after thawing 92% [range 80% to 98%]) to BIM BH3 peptide at 0.8 μM (P = .05, R2 = 0.23), in 13 evaluable patients with lymphocytosis at study entry. (B) Nodal response. Correlations between percentage reductions in the SPD of target lymph nodes at 6 weeks and mitochondrial priming (P = .19, R2 = 0.14) in 14 evaluable patients. (C) Marrow response. Correlation between percentage reduction in CLL cell BM infiltrate at first restaging at approximately week 24 and mitochondrial priming (P = .01, R2 = 0.63) in 9 evaluable patients.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/25/10.1182_blood-2016-01-688796/4/m_3215f4.jpeg?Expires=1770375789&Signature=4R-TW0JgXFYUueL4nooYUgJP9mLF4ZucnNF2FvVFRDvg2M3s0kWexUQkLora13bUcjsQ~PmUvD5yLYI01Gjrs2DmPV6pBm6scvM0mdAnYtFcDYqj9joXuDEpVMfBIKq5083QKCwXKkEteQzyjWU8BbnAFlJ8OyJKC70M48danaKnW0MBURfn4FXq90xLEusMJuBeDEqNAIZ2YjSj1WvyWLzt1mVdFr4x5h-dqys5b4lK0D0pRYwCfF02xWIM-fKRtH8xqf6pMk6s-evt21DC7iUYmB0UuOlkYaspGJFqsQ4FPcmF0xy0A3fOz~xzLS2iXwrtRTtxxBSpiRBto5proQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)