In this issue of Blood, Knittel et al report on the expression of MYD88L265P in B lymphocytes of mice prone to neoplasms that resemble human diffuse large B-cell lymphoma (DLBCL).1
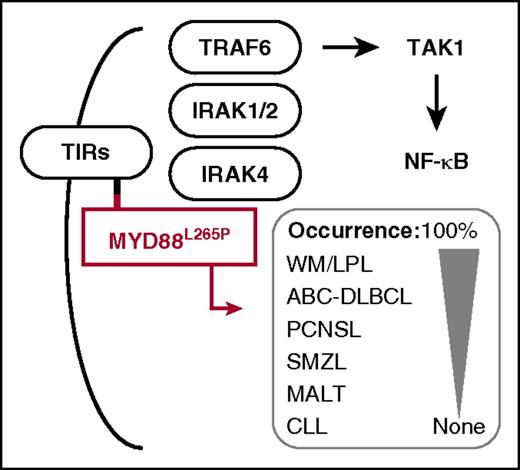
MYD88L265P harbors a leucine-to-proline exchange in the hydrophobic core of its Toll/interleukin-1 receptor (TIR) domain (small red square), thus facilitating homotypic interaction with the TIR domain (small black square) of receptors from the TIR superfamily (TIRs). Upon recruitment of MYD88 to TIRs, MYD88 recruits interleukin 1 receptor-associated kinase 4 (IRAK4), which phosphorylates IRAK1 and -2 and promotes recruitment of tumor necrosis factor receptor-associated factor 6 (TRAF6). This results in ubiquitylation and activation of nuclear receptor subfamily 2 group C member 2, better known as TGF-β-activated kinase 1 (TAK1) and, ultimately, in activation of canonical NF-κB signaling. Subsequent to the discovery of the MYD88L265P allele in ABC-DLBCL (an accomplishment of the Staudt Laboratory at the US National Cancer Institute4 ), the same mutation was demonstrated by investigators in Waldenström macroglobulinemia (WM),5 including its precursor condition immunoglobulin M (IgM) monoclonal gammopathy of undetermined significance and the related disease, IgM-associated light chain amyloidosis,6 in primary central nervous system lymphoma (PCNSL), in splenic marginal zone lymphoma (SMZL), in mucosa-associated lymphoid tissue (MALT) lymphoma and, rarely, in chronic lymphocytic leukemia (CLL). The mouse model of MYD88L265P-dependent lymphoma developed by Knittel et al may be useful for studies of all of these neoplasms, and particularly WM, in which the L265P substitution occurs with the highest frequency (ie, in nearly all patients).
MYD88L265P harbors a leucine-to-proline exchange in the hydrophobic core of its Toll/interleukin-1 receptor (TIR) domain (small red square), thus facilitating homotypic interaction with the TIR domain (small black square) of receptors from the TIR superfamily (TIRs). Upon recruitment of MYD88 to TIRs, MYD88 recruits interleukin 1 receptor-associated kinase 4 (IRAK4), which phosphorylates IRAK1 and -2 and promotes recruitment of tumor necrosis factor receptor-associated factor 6 (TRAF6). This results in ubiquitylation and activation of nuclear receptor subfamily 2 group C member 2, better known as TGF-β-activated kinase 1 (TAK1) and, ultimately, in activation of canonical NF-κB signaling. Subsequent to the discovery of the MYD88L265P allele in ABC-DLBCL (an accomplishment of the Staudt Laboratory at the US National Cancer Institute4 ), the same mutation was demonstrated by investigators in Waldenström macroglobulinemia (WM),5 including its precursor condition immunoglobulin M (IgM) monoclonal gammopathy of undetermined significance and the related disease, IgM-associated light chain amyloidosis,6 in primary central nervous system lymphoma (PCNSL), in splenic marginal zone lymphoma (SMZL), in mucosa-associated lymphoid tissue (MALT) lymphoma and, rarely, in chronic lymphocytic leukemia (CLL). The mouse model of MYD88L265P-dependent lymphoma developed by Knittel et al may be useful for studies of all of these neoplasms, and particularly WM, in which the L265P substitution occurs with the highest frequency (ie, in nearly all patients).
The frequent detection of a highly recurrent, oncogenic, gain-of-function mutation that substitutes a leucine (L) residue at position 265 of the adapter protein myeloid differentiation primary response gene 88 (MYD88) with a proline (P) residue has implicated the mutant MYD88L265P allele in the natural history and clinical outcome of an important subset of diffuse DLBCL. However, a genetically engineered mouse model (GEMM) for in-depth mechanistic studies on the role of MYD88L265P in the biology and genetics of DLBCL has been lacking. In their article, Knittel and his associates1 remedy this shortcoming by recapitulating the MYD88L265P mutation in B lymphocytes of transgenic mice that go on to develop DLBCL-like neoplasms.
DLBCL, the most common non-Hodgkin lymphoma in the United States, has 2 major subtypes, as defined by distinct gene expression programs that correspond to different putative cells of origin: a germinal center B cell in the case of GCB-DLBCL and a plasmablast (activated B cell) in the case of ABC-DLBCL.2 Combination chemotherapy results in ∼80% 3-year survival for patients with GCB-DLBCL but achieves only 45% survival for patients with ABC-DLBCL. Thus, ABC-DLBCL patients have an unmet medical need that warrants additional research efforts and new therapeutic options.
A well-established hallmark of ABC-DLBCL is the constitutive activation of the classical NF-κB pathway. In ∼40% of patients, this is accomplished by somatic (acquired) mutations in MYD88, an adaptor protein of crucial importance for cellular signal transduction pathways that govern pattern recognition, inflammation, innate and adaptive immune responses and, importantly, malignant cell transformation. MYD88 links upstream members of the Toll-like receptor and interleukin-1 receptor superfamily (see figure) with downstream effector hubs that regulate, in addition to NF-κB, Janus kinase signal transducer and activator of transcription (JAK-STAT), mitogen-activated protein kinases (MAPKs), and type-I interferon (IFN) binding to the IFN-α/β receptor signaling in ABC-DLBCL and other cells.
Evidence indicates that the L265P substitution is the most common and most oncogenic representative of a variety of mutations that occur in MYD88 in ABC-DLBCL. Moreover, the L265P exchange is of predictive value for these patients because it is associated with extranodal tumor dissemination and poor clinical outcome.3 Subsequent to its discovery in ABC-DLBCL,4 the MYD88L265P mutation has also been found in the great majority of patients with Waldenström macroglobulinemia (nearly 100%)5,6 and a fraction of patients with primary central nervous system lymphoma (∼35%), splenic marginal zone lymphoma (∼15%), gastric mucosa-associated lymphoid tissue lymphoma (∼9%), and chronic lymphocytic leukemia (∼3%). These findings demonstrated that MYD88L265P is by no means specific for ABC-DLBCL; instead, it is broadly involved in the natural history of a large subset of mature B-lineage neoplasms.
Fully appreciating that lymphoma modeling in transgenic mice enables fundamental and translational studies that are difficult to pursue in humans, Knittel et al1 used an elegant genetic engineering tool to express the orthologous mouse allele of human MYD88L265P, Myd88L252P, in laboratory mice. This was accomplished by Cre recombinase-induced activation of an MYD88L252P knockin gene inserted into the germline Myd88 locus using homologous recombination (gene targeting) in embryonic stem cells. The investigators took advantage of three different Cre drivers (AID, CD19, and CD22) to express MYD88L252P in different B-cell populations. Regardless, transgenic mice from all experimental groups developed lymphoma that shared important features with human ABC-DLBCL. Practical limitations relating to tumor incidence (low) and tumor onset (long) were readily overcome by co-expression of the Myc oncogene. Thus, Knittel et al achieved, for the first time, a GEMM of MYD88L265P-driven ABC-DLBCL.
Another advance reported by Knittel et al1 concerns the collaboration of MYD88L265P and deregulated expression of B-cell leukemia 2 (BCL2) in lymphoma development. BCL2 is a survival-enhancing oncoprotein that is frequently overexpressed in ABC-DLBCL by virtue of gene amplification (focal copy number gains) at 18q. The investigators cleverly recapitulated this aspect of the ABC-DLBCL genetic network by combining the MYD88L252P allele with a newly developed inducible BCL2 allele in double-transgenic mice that developed tumors resembling human ABC-DLBCL with full penetrance (100% tumor incidence). Knittel et al propose to use these tumors as a heretofore unavailable model system of actionable BCL2 addiction that lends itself to preclinical co-trials of the BCL2 inhibitors venetoclax (ABT-199) and navitoclax (ABT-263)7 and the Bruton tyrosine kinase (BTK) inhibitor ibrutinib, which synergizes with BCL2 inhibition in killing ABC-DLBCL cells.8
In summary, Knittel et al1 produced a mouse model of MYD88L265P-driven ABC-DLBCL that should facilitate efforts to design and test new approaches to treat this difficult-to-cure lymphoma. These may include small-molecule IRAK4 and TAK1 inhibitors currently in the preclinical drug pipeline or combination therapies that target BCL2, MAPK, or JAK-STAT in addition to MYD88-NFκB signaling. Be this as it may, the newly developed MYD88L252P transgene will also be of value for developing GEMMs for human B-cell tumors (eg, Waldenström macroglobulinemia) for which such models are still lacking.
Conflict-of-interest disclosure: The author declares no competing financial interests.