Compared with rapamycin, mammalian target of rapamycin (mTOR) dual inhibitors have shown improved ability to kill acute lymphocytic leukemia (ALL) cells in preclinical models. In this issue of Blood, Yun et al have identified novel signaling pathways downstream of mTOR that are disrupted by dual inhibitors leading to ALL cell death.1
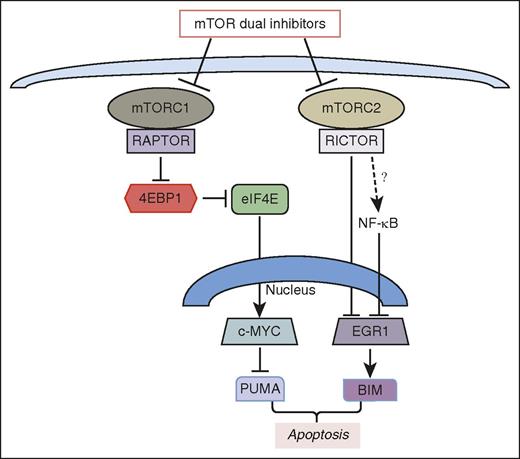
mTOR dual inhibitors induce apoptosis in ALL cells through 2 distinct pathways controlled by the 2 mTOR complexes, mTORC1 and mTORC2. mTORC1 inhibition reduces activity of EIF4E and expression of c-MYC, leading to increased expression of the proapoptotic protein PUMA. mTORC2 inhibition increases expression of EGR1 that activates transcription of the proapoptotic protein BIM. The expression of EGR1 depends in part on NF-κB activity which might be suppressed by mTORC2. The figure has been adapted from Figure 7H in the article by Yun et al that begins on page 2711.
mTOR dual inhibitors induce apoptosis in ALL cells through 2 distinct pathways controlled by the 2 mTOR complexes, mTORC1 and mTORC2. mTORC1 inhibition reduces activity of EIF4E and expression of c-MYC, leading to increased expression of the proapoptotic protein PUMA. mTORC2 inhibition increases expression of EGR1 that activates transcription of the proapoptotic protein BIM. The expression of EGR1 depends in part on NF-κB activity which might be suppressed by mTORC2. The figure has been adapted from Figure 7H in the article by Yun et al that begins on page 2711.
mTOR signaling is elevated in both T-cell and B-cell ALL and correlates with high risk of relapse and resistance to chemotherapy regimens.2 Consequently, small-molecule inhibitors of mTOR have been evaluated extensively in preclinical models of ALL and in human clinical trials. First-generation allosteric mTOR inhibitors, exemplified by rapamycin and its analogs (rapalogs), have some efficacy in mouse models but have yielded mixed results in clinical trials.3 Second-generation adenosine triphosphate–competitive mTOR inhibitors provide more complete inhibition of mTOR enzyme activity in both cellular complexes known as mTOR complexes 1 and 2 (mTORC1 and mTORC2).4 This class of compounds (known as mTOR catalytic inhibitors, mTOR kinase inhibitors, or mTOR dual inhibitors) has shown particular promise in ALL cells where they can induce apoptosis as a single treatment or prime cells for killing by other targeted agents.5,6 However, the mechanisms by which mTOR dual inhibitors promote ALL cell death have not been established. Uncovering these mechanisms will increase understanding of mTOR signaling networks in leukemia cells and might lead to new therapeutic targets.
In this issue, Yun et al identify 2 novel prosurvival pathways (see figure) that are driven by mTOR activity and disrupted by dual inhibitors in ALL cell lines and primary patient specimens. First, they addressed the important question of whether inhibition of mTORC1 and/or mTORC2 is relevant to the prodeath effects of mTOR dual inhibitors. Using short hairpin RNAs to knock down regulatory-associated protein of mTOR (RAPTOR; a required component of mTORC1) or rapamycin-insensitive companion of mTOR (RICTOR; a required component of mTORC2), they show that disruption of either complex reduces ALL cell survival whereas combined knockdown has a more pronounced effect. Next, they focused attention on the mTORC1 substrate eukaryotic initiation factor 4E (EIF4E)-binding protein 1 (4EBP1), a protein that binds reversibly to EIF4E to modulate the efficiency of translation for specific subsets of messenger RNA transcripts. They show that 4EBP1 is required for cell killing by mTOR dual inhibitors and that a constitutively active 4EBP1 mutant or an EIF4E chemical antagonist are sufficient to increase death. These findings are consistent with studies of the mTORC1/4EBP1/EIF4E axis in other cellular systems.7,8 However, the current study reveals a novel mechanism promoting cell death downstream of EIF4E inhibition. Specifically, disrupting EIF4E function suppresses expression of the MYC oncoprotein leading to increased expression of the proapoptotic protein p53-upregulated modulator of apoptosis (PUMA). Thus, mTORC1 signaling to EIF4E promotes ALL cell survival by increasing MYC expression, which suppresses PUMA expression apparently through miRNA networks (see figure). In many other studies, the primary mechanism by which EIF4E suppresses apoptosis is by increased translation of the induced myeloid cell leukemia (MCL-1) prosurvival protein.
These findings also help to explain the greater cytotoxic effect of mTOR dual inhibitors relative to rapamycin. It is well established that mTOR dual inhibitors are much more effective than rapamycin at inhibiting mTORC1-mediated 4EBP1 phosphorylation. This difference results in less disruption of EIF4E activity by rapamycin. In accord, the data presented here show that rapamycin fails to trigger the downregulation of MYC or upregulation of PUMA that are induced by mTOR dual inhibitors.
This study identifies a second mechanism for ALL cell death involving increased expression of the early growth response protein-1 (EGR1) transcription factor that drives transcription of the gene (BCL2L11) encoding BCL2-like protein 11 (BIM), a powerful proapoptotic protein. Interestingly, mTOR dual inhibitors increase EGR1 expression through elevated activity of nuclear factor κ-light-chain enhancer of activated B cells (NF-κB) transcription factors. This result is surprising in light of a broad array of studies showing prosurvival function of NF-κB activity. Here, disrupting NF-κB activation is shown to prevent induction of EGR1 and BIM in ALL cells treated with mTOR dual inhibitors. It will be important to determine how the typical prosurvival program of NF-κB is suppressed. Another key unanswered question is the mechanism by which mTOR inhibition promotes NF-κB activity in this setting.
A surprising finding of this study is that the prosurvival kinase AKR thymoma (AKT) and its substrates the Forkhead box, subgroup O (FOXO) transcription factors are not involved in the mechanism of ALL cell killing by mTOR dual inhibitors. In many cell types, AKT inhibition leads to accumulation of active FOXO in the nucleus and elevated FOXO-dependent transcription of genes encoding proapoptotic proteins including both PUMA and BIM.9,10 By inhibiting AKT phosphorylation by mTORC2, mTOR dual inhibitors decrease AKT activity toward FOXO transcription factors. Nevertheless, this study shows that a constitutively active AKT is unable to prevent apoptosis induced by dual inhibitors. Similarly, depletion of FOXO3a does not rescue the cells. Thus, the mechanism by which mTORC2 disruption (through RICTOR knockdown) promotes ALL cell death remains unresolved. One possibility is that mTORC2 inhibition blocks a pathway that normally suppresses NF-κB activity in these cells. This is suggested by the observation that RICTOR knockdown increases EGR1 expression (see figure) whereas rapamycin treatment does not upregulate EGR1. However, further experiments are needed to validate the connection of mTORC2 to NF-κB. In fact, other groups have reported a positive correlation between mTORC2 and NF-κB activity.
Overall, the study of Yun et al provides significant advances in our understanding of mTOR-mediated survival pathways in ALL cells. The data also identify MYC and EGR1 as downstream prosurvival proteins that potentially could be targeted to promote ALL cell death. It will be interesting to investigate whether these mechanisms also mediate survival downstream of mTORC1 and mTORC2 in other lymphoid malignancies.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal