Key Points
The overall response rate following 4 induction cycles of VTD prior to ASCT is higher than that of 4 cycles of VCD.
Abstract
The Intergroupe Francophone du Myélome conducted a randomized trial to compare bortezomib-thalidomide-dexamethasone (VTD) with bortezomib-cyclophosphamide-dexamethasone (VCD) as induction before high-dose therapy and autologous stem cell transplantation (ASCT) in patients with newly diagnosed multiple myeloma. Overall, a total of 340 patients were centrally randomly assigned to receive VTD or VCD. After 4 cycles, on an intent-to-treat basis, 66.3% of the patients in the VTD arm achieved at least a very good partial response (primary end point) vs 56.2% in the VCD arm (P = .05). In addition, the overall response rate was significantly higher in the VTD arm (92.3% vs 83.4% in the VCD arm; P = .01). Hematologic toxicity was higher in the VCD arm, with significantly increased rates of grade 3 and 4 anemia, thrombocytopenia, and neutropenia. On the other hand, the rate of peripheral neuropathy (PN) was significantly higher in the VTD arm. With the exception of hematologic adverse events and PN, other grade 3 or 4 toxicities were rare, with no significant differences between the VTD and VCD arms. Our data support the preferential use of VTD rather than VCD in preparation for ASCT. This trial was registered at www.clinicaltrials.gov as #NCT01564537 and at EudraCT as #2013-003174-27.
Introduction
Induction therapy followed by autologous stem cell transplantation (ASCT) is the standard of care for patients with symptomatic multiple myeloma (MM) who are younger than 66 years of age.1-3 A recent integrated analysis of data from phase 3 studies in transplant-eligible patients with previously untreated MM showed that bortezomib-based induction resulted in significant improvements in response and progression-free survival (PFS) and overall survival (OS) vs non–bortezomib-based induction.4 Based on response rates, depth of response, and PFS as surrogate markers for outcome, 3-drug combinations including bortezomib and dexamethasone plus either an immunomodulatory drug (IMiD) (thalidomide or lenalidomide), cyclophosphamide, or doxorubicin (PAD) are currently the standard of care prior to ASCT.1-5 The triplet combinations bortezomib-thalidomide-dexamethasone (VTD) and bortezomib-cyclophosphamide-dexamethasone (VCD) have demonstrated high response rates in prospective phase 26-8 and phase 39-12 clinical trials, they are two of the most commonly used induction regimens prior to ASCT and are both recommended in the international guidelines.1-3 To date, no comparative data from prospective randomized trials of the safety and efficacy of VTD vs VCD are available. This provided the rationale for the phase 3 investigation of VTD vs VCD prior to ASCT in patients with de novo MM in this randomized multicenter study (NCT01564537).
Methods
Patients
Eligible patients were 65 years of age or younger and had untreated symptomatic MM with measurable paraprotein in the serum (>1 g/dL) or urine (>0.2 g/24 hours). Key inclusion criteria were Eastern Cooperative Oncology Group performance status ≤2 and adequate renal function. Key exclusion criteria comprised confirmed amyloidosis, HIV positivity, history of other malignancy (other than basal cell carcinoma and carcinoma of the cervix in situ), uncontrolled diabetes, and grade ≥2 peripheral neuropathy (National Cancer Institute Common Toxicity Criteria Version 4.0). All patients provided written informed consent. The study was approved by the relevant national health authority agency and the French national ethics committee and was conducted in accordance with the International Conference on Harmonization of Good Clinical Practice Guidelines and the principles of the Declaration of Helsinki.
Study design
This open-label phase 3 randomized trial was conducted at 56 centers of the Intergroupe Francophone du Myélome (IFM) from November 2013 to March 2015. The data cutoff date for this report was August 1, 2015. Patients were centrally randomized to receive 4 cycles of VTD or VCD. Patients were stratified by baseline International Staging System (1–2 vs 3) and cytogenetics (deletion 17p and /or t(4;14), presence vs absence) by fluorescence in situ hybridization analysis (centralized analysis, H.A.-L., Toulouse). VTD treatment consisted of four 3-week cycles of 1.3 mg/m2 bortezomib administered subcutaneously (SC) on days 1, 4, 8, and 11; 40 mg dexamethasone on days 1–4 and 9–12; plus 100 mg/day thalidomide administered orally. Therapy with VCD was composed of four 3-week cycles of SC bortezomib and dexamethasone at the same doses and schedules as for the VTD regimen plus 500 mg/m2 cyclophosphamide administered orally on days 1, 8, and 15. Recommended concomitant medications included bisphosphonates, antibiotics, and antiviral prophylaxis in accordance with local practice. Enoxaparin was systematically used in the VTD arm. Stem cells were mobilized with 3 g/m2 cyclophosphamide plus 10 mg/kg granulocyte-macrophage colony-stimulating factor after cycle 3 and before cycle 4. The target yield was 2 × 106 CD34+ cells/kg. Following induction therapy, all patients had to proceed to ASCT. The use of a conditioning regimen was left at the discretion of each center, as was the decision to conduct a single or tandem ASCT and the application of consolidation and/or maintenance therapy. Therefore, PFS and OS were not evaluated in this protocol.
Assessment
The primary end point was postinduction very good partial response (VGPR) rate. Secondary end points were complete response (CR) and overall response rates (ORR; ie, ≥ partial response [PR]), safety of the induction regimen, and stem cell harvest. Blood and 24-hour urine samples were taken at baseline and after cycle 4 (centralized analysis, T.D., Nantes). Response was evaluated centrally according to International Myeloma Working Group Uniform Criteria.13 Adverse events (AEs) were graded by National Cancer Institute Common Toxicity Criteria Version 4.0. All patients who received 1 dose of therapy were included in the intent-to-treat (ITT) and safety analyses. Al patients who completed the 4 cycles of treatment were included in the per-protocol (PP) analysis.
Statistical analysis
Considering the VGPR rate obtained with the VTD regimen in newly diagnosed patients, 340 patients were to be enrolled. This provided 80% power (2-sided test with type I error of .05) to detect a 15% difference in the postinduction VGPR rate, assuming a VGPR rate of 45% with VCD. Comparisons of response rates, including the primary end point (postinduction VGPR rate) were performed using a χ2 test, and differences in VGPR rates were expressed as proportions with corresponding 95% confidence intervals (CIs).
Results
Patient characteristics and disposition
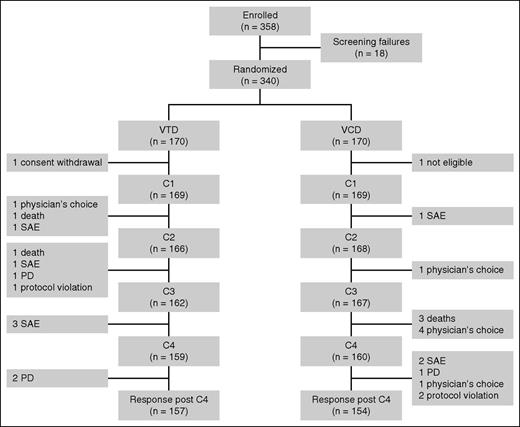
A total of 358 patients provided written informed consent, and 18 of these were withdrawn from the analysis because of violation of inclusion criteria. Overall, a total of 340 patients were randomly assigned to receive VTD (170 patients) or VCD (170 patients). One patient in the VTD arm withdrew consent before the administration of the first dose of cycle 1, and 1 patient in the VCD arm was found to fulfill an exclusion criterion after randomization. Overall, the ITT population consisted of 338 patients, 169 in each arm (Figure 1). Baseline characteristics are summarized in Table 1. No significant difference was observed between the 2 groups. Eighteen percent of the patients had adverse cytogenetics at diagnosis.
Trial profile. SAE, severe adverse event; PD, progressive disease; C, cycle.
Patient characteristics
| . | VTD (n = 169) . | VCD (n = 169) . |
|---|---|---|
| Male/female | 103/66 | 108/61 |
| Median age (range) | 59 (34-65) | 60 (26-65) |
| β2-microglobulin, mg/L (range) | 3.6 (2.1-8.9) | 3.8 (2.0-9.3) |
| Albumin, g/L (range) | 36.9 (28.2-41.7) | 35.7 (27.9-41.3) |
| International Staging System | ||
| ISS1 | 38 (22%) | 43 (25%) |
| ISS2 | 94 (56%) | 90 (53%) |
| ISS3 | 37 (22%) | 36 (21%) |
| No t(4;14), no17p | 137 (81%) | 140 (83%) |
| t(4;14) and/or 17p | 32 (19%) | 29 (17%) |
| Creatinine level, mmol/L, median (range) | 75 (38-170) | 79 (20-163) |
| Hemoglobin level, g/dL, median (range) | 11.2 (6.6-16.6) | 11 (8-16.6) |
| Calcium level, mmol/L, median (range) | 2.34 (2.05-3.5) | 2.37 (2.0-3.61) |
| Platelet count, ×109/L, median (range) | 253 (110-350) | 229 (105-380) |
| . | VTD (n = 169) . | VCD (n = 169) . |
|---|---|---|
| Male/female | 103/66 | 108/61 |
| Median age (range) | 59 (34-65) | 60 (26-65) |
| β2-microglobulin, mg/L (range) | 3.6 (2.1-8.9) | 3.8 (2.0-9.3) |
| Albumin, g/L (range) | 36.9 (28.2-41.7) | 35.7 (27.9-41.3) |
| International Staging System | ||
| ISS1 | 38 (22%) | 43 (25%) |
| ISS2 | 94 (56%) | 90 (53%) |
| ISS3 | 37 (22%) | 36 (21%) |
| No t(4;14), no17p | 137 (81%) | 140 (83%) |
| t(4;14) and/or 17p | 32 (19%) | 29 (17%) |
| Creatinine level, mmol/L, median (range) | 75 (38-170) | 79 (20-163) |
| Hemoglobin level, g/dL, median (range) | 11.2 (6.6-16.6) | 11 (8-16.6) |
| Calcium level, mmol/L, median (range) | 2.34 (2.05-3.5) | 2.37 (2.0-3.61) |
| Platelet count, ×109/L, median (range) | 253 (110-350) | 229 (105-380) |
Response to induction
On an ITT basis, 66.3% of the patients in the VTD arm achieved at least a VGPR vs 56.2% in the VCD arm (P = .05; Table 2). The difference in VGPR rate (primary end point) between the VTD and VCD arms was 10.1% (95% CI, 1% to 18%). No significant difference was observed in terms of CR rate between the 2 arms, but the ORR was significantly higher in the VTD arm, 92.3% vs 83.4% in the VCD arm (P = .01; difference 8.9%; 95% CI, 2% to 16%). Two percent of the patients progressed on treatment in each arm of the study.
Response to induction
| . | VTD (n = 169) . | VCD (n = 169) . | P value . |
|---|---|---|---|
| Intent to treat | |||
| ≥CR | 13.0% | 8.9% | .22 |
| ≥VGPR | 66.3% | 56.2% | .05 |
| ≥PR | 92.3% | 83.4% | .01 |
| Per protocol | n = 157 | n = 154 | |
| ≥CR | 14.0% | 9.1% | .17 |
| ≥VGPR | 70.7% | 60.4% | .05 |
| ≥PR | 98.7% | 90.3% | .001 |
| . | VTD (n = 169) . | VCD (n = 169) . | P value . |
|---|---|---|---|
| Intent to treat | |||
| ≥CR | 13.0% | 8.9% | .22 |
| ≥VGPR | 66.3% | 56.2% | .05 |
| ≥PR | 92.3% | 83.4% | .01 |
| Per protocol | n = 157 | n = 154 | |
| ≥CR | 14.0% | 9.1% | .17 |
| ≥VGPR | 70.7% | 60.4% | .05 |
| ≥PR | 98.7% | 90.3% | .001 |
According to the PP analysis, the VGPR rate was also significantly higher in the VTD arm (70.7% vs 60.4%; P = .05; difference 10.3%; 95% CI, 2% to 21%). No significant difference was observed in terms of CR rate between the 2 arms, but the ORR was significantly higher in the VTD arm (98.7% vs 90.3% in the VCD arm; P = .001; difference 8.4%; 95% CI, 3.4% to 13.5%).
The differences observed in terms of response between VTD and VCD were not related to imbalances in the cumulative doses of each drug used in the 2 triplet combinations, as the dose intensity of bortezomib was 94.9% in the VTD arm and 96.4% in the VCD arm and the dose intensity of dexamethasone was 92.4% in the VTD arm and 96.1% in the VCD arm. Furthermore, the dose intensity of thalidomide was 89.9% in the VTD arm and 94.5% for cyclophosphamide for VCD (Table 3).
Dose intensity
| . | VTD (n = 169) . | VCD (n = 169) . |
|---|---|---|
| Dexamethasone | ||
| 100% of the planned dose | 76.3% | 84.6% |
| Dose reduction | 14.2% | 10.9% |
| Discontinuation | 9.5% | 8.6% |
| Dose intensity | 92.4% | 96.1% |
| Bortezomib | ||
| 100% of the planned dose | 76.9% | 78.1% |
| Dose reduction | 16.0% | 13.6% |
| Discontinuation | 7.1% | 8.3% |
| Dose intensity | 94.9% | 96.4% |
| Thalidomide/cyclophosphamide | ||
| 100% of the planned dose | 62.7% | 71.3% |
| Dose reduction | 21.3% | 16.6% |
| Discontinuation | 16% | 12.1% |
| Dose intensity | 89.9% | 94.5% |
| . | VTD (n = 169) . | VCD (n = 169) . |
|---|---|---|
| Dexamethasone | ||
| 100% of the planned dose | 76.3% | 84.6% |
| Dose reduction | 14.2% | 10.9% |
| Discontinuation | 9.5% | 8.6% |
| Dose intensity | 92.4% | 96.1% |
| Bortezomib | ||
| 100% of the planned dose | 76.9% | 78.1% |
| Dose reduction | 16.0% | 13.6% |
| Discontinuation | 7.1% | 8.3% |
| Dose intensity | 94.9% | 96.4% |
| Thalidomide/cyclophosphamide | ||
| 100% of the planned dose | 62.7% | 71.3% |
| Dose reduction | 21.3% | 16.6% |
| Discontinuation | 16% | 12.1% |
| Dose intensity | 89.9% | 94.5% |
Safety
The safety population included all 338 patients (169 in each arm) who had received at least 1 dose of either bortezomib, dexamethasone, thalidomide, or cyclophosphamide.
As shown in Table 4, the proportion of patients with at least 1 AE or AEs ≥ grade 3 was not different between the 2 groups. Hematologic toxicity was higher in the VCD arm, with significantly increased rates of grade 3 and 4 anemia, thrombocytopenia, and neutropenia. This higher rate of neutropenia in the VCD arm was not associated with a higher rate of grade 3 and 4 infections. On the other hand, the rate of peripheral neuropathy (PN) was significantly increased in the VTD arm. With the exception of hematologic AEs and PN, other grade 3 or 4 toxicities were rare, with no significant differences between the VTD and VCD arms.
Safety profile of induction therapy with VTD or VCD
| . | VTD (n = 169) grade 3-4 (%) . | VCD (n = 169) grade 3-4 (%) . | P value . |
|---|---|---|---|
| Any AEs | 63.9 | 68.2 | .40 |
| Anemia | 4.1 | 9.5 | .05 |
| Neutropenia | 18.9 | 33.1 | .003 |
| Infection | 7.7 | 10.1 | .45 |
| Thrombocytopenia | 4.7 | 10.6 | .04 |
| Thrombosis | 1.8 | 1.8 | .99 |
| Cardiac disorders | 1.2 | 0 | .16 |
| Cystitis | 0 | 0.6 | .32 |
| Gastrointestinal symptoms | 5.3 | 3.5 | .42 |
| PN | 7.7 | 2.9 | .05 |
| PN grade 2 - 4 | 21.9 | 12.9 | .008 |
| . | VTD (n = 169) grade 3-4 (%) . | VCD (n = 169) grade 3-4 (%) . | P value . |
|---|---|---|---|
| Any AEs | 63.9 | 68.2 | .40 |
| Anemia | 4.1 | 9.5 | .05 |
| Neutropenia | 18.9 | 33.1 | .003 |
| Infection | 7.7 | 10.1 | .45 |
| Thrombocytopenia | 4.7 | 10.6 | .04 |
| Thrombosis | 1.8 | 1.8 | .99 |
| Cardiac disorders | 1.2 | 0 | .16 |
| Cystitis | 0 | 0.6 | .32 |
| Gastrointestinal symptoms | 5.3 | 3.5 | .42 |
| PN | 7.7 | 2.9 | .05 |
| PN grade 2 - 4 | 21.9 | 12.9 | .008 |
Five patients died during induction therapy (1.5%), 2 in the VTD arm from infection (1) and pulmonary embolism (1) and 3 in the VCD arm from progression to extramedullary myeloma (1) and infections (2).
Stem cell mobilization
A total of 314 patients (93% of the overall population, 159 in the VTD arm, 155 in the VCD arm) underwent stem cell mobilization as stated in the protocol. The median number of CD34+ cells/kg collected was 10.7 × 106 in the VTD arm vs 9.2 × 106 in the VCD arm (P = .05).
Discussion
The goal of induction treatment before ASCT is the achievement of the highest possible response rate while avoiding an impairment of stem cell collection and significant toxicity that may preclude intensive therapy.1-5 The quality of response to the induction treatment prior to ASCT, as well as the response achieved following high-dose melphalan, is important prognostic factors and are predictive of PFS following ASCT.1-5,9,14,15 Therefore it is important to optimize this first sequence of therapy. Three-drug combinations including bortezomib and dexamethasone plus either an IMiD (thalidomide [VTD regimen] or lenalidomide [RVD regimen]), cyclophosphamide (VCD), or doxorubicin (PAD) are recommended in international guidelines,1,3 but until now, very few comparisons of triplet regimens were available. Recently, results of the German MM5 prospective trial, which compared 3 cycles of VCD with 3 cycles of PAD in 504 patients with newly diagnosed MM eligible for ASCT, were reported.12 VCD was found to be noninferior to PAD with respect to ≥ VGPR rates (37.0% vs 34.3%) and less toxic, with a serious AE rate of 24% vs 32.7% (P = .04). Therefore, the authors concluded that VCD was preferable to PAD as induction therapy. This study is, to our knowledge, the only prospective trial comparing 2 induction regimens that consist of 3 drugs. VCD and VTD are 2 of the most commonly used regimens prior to ASCT1-3 but have not been prospectively evaluated yet. In a retrospective pair-mate analysis of 2 consecutive trials, Cavo et al compared the response rates of 3 cycles of VTD with 3 cycles of VCD and showed superiority of the VTD regimen in terms of CR (19% vs 6%), VGPR (64% vs 37%), and PR rates (93% vs 81%), respectively.16
Our study is the first to prospectively compare VTD and VCD prior to ASCT. The VGPR and PR rates were significantly higher with VTD, both in the ITT and PP populations. These differences were not the result of dose attenuation or the use of a “VCD-light” regimen, as the dose of cyclophosphamide that was administered in our trial was 500 mg/m2 on days 1, 8, and 15, which is higher than that reported in the retrospective study by Cavo et al (500 mg/2 on days 1 and 8)16 or in other VCD regimens reported previously.6-8 The dose intensity in both arms was high and was greater than 90% for each drug. The VGPR rate of 56% with VCD described in our trial (ITT) is much higher than the 37% VGPR rate reported by Mai et al in the prospective German trial12 and the 37% VGPR rate that was reported by Cavo et al in the retrospective Italian analysis.16 This could be attributed not only to the number of cycles (4 in our study vs 3 in the Cavo and Mai studies) but also to the higher dose of cyclophosphamide used in our trial. We demonstrate a 10% difference in terms of VGPR rate in favor of VTD, both in the ITT and PP populations. The 66% VGPR rate (ITT) achieved with VTD is consistent with what has previously been reported in large phase 3 trials comparing this regimen with 2-drug induction regimens, such as thalidomide and dexamethasone or bortezomib-dexamethasone, prior to ASCT.9-11
Our results are of major importance, because the quality of response to induction, and especially the achievement of at least a VGPR, clearly correlates with the outcome following ASCT.9,14,15 In our trial, per protocol, no recommendation was provided regarding post-ASCT therapy. Subsequent consolidation and maintenance treatments were at the discretion of the treating physician, and neither PFS nor OS data were collected. This is the main weakness of our trial.
The response data in favor of VTD over VCD also have to be interpreted in light of the analysis of the safety profile of these 2 regimens. In our study, VCD was more frequently associated with severe hematologic toxicity than VTD, which, in contrast, induced severe PN more often than VCD. The rate of grade 3/4 PN was 7.7% in the VTD arm, which is slightly lower than that described in large phase 3 trials of VTD vs 2-drug induction regimens (10% to 17%) in which biweekly bortezomib was provided IV, and not SC, as in the present trial.9-11 Peripheral neuropathy, which can be irreversible and may impair the quality of life of a patient, is an important issue when selecting a specific induction regimen, but the analysis of a large Italian trial that investigated VTD induction followed by ASCT showed that PN induced by this triplet combination may resolve in the majority of the cases or may improve to grade 1 in at least 90% of the patients.17 Overall, in our trial, the rate of severe AEs was identical in both arms, and the number of patients that could not receive the planned schedule of induction was very low. Importantly, >90% of the patients underwent successful stem cell harvest, and VTD induction was associated with a superior stem cell yield as compared with VCD.
The high response rate achieved with VTD strongly confirms the significant and synergistic activity of an IMiD combined with bortezomib and dexamethasone. This has also been observed with lenalidomide in the RVD regimen, first described as induction therapy in de novo patients in 2010.18 Our data can be used to support the use of RVD as part of frontline therapy prior to ASCT. The switch from thalidomide to lenalidomide is likely to improve safety, with a reduction of PN, while maintaining the quality of the response. In the pilot study of frontline RVD, the rate of grade 3 neuropathic pain was only 3%, with no grade 4 events observed.18 Indeed, RVD is widely used in the United States and was also tested in the pilot trial of the IFM, which investigated RVD induction and consolidation in the context of ASCT.19 The triplet RVD regimen was also the backbone of the IFM/DFCI 2009 trial, designed to compare front-line ASCT vs front-line RVD without stem cell transplantation, which was reported at the 2015 annual meeting of the American Society of Hematology.20
In the near future, the cost of triplet combinations used for the treatment of myeloma will become an important issue. The cost of thalidomide and cyclophosphamide is highly variable from one country to the other, but these 2 combinations are considered affordable for the majority of centers performing ASCT all over the world. In this setting, VCD is cheaper than VTD.
Overall, our results show that both VTD and VCD are active induction regimens, producing PR rates >80%, VGPR rates >50%, and progressive disease rates <3%. The respective toxicities of the 2 regimens are manageable, and the quality of stem cell harvest is high. Nevertheless, the 10% difference in terms of both PR and VGPR in favor of VTD strongly suggests that the combination of a proteasome inhibitor plus an IMiD plus dexamethasone is the best option prior to intensive therapy and ASCT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Annex participating centers included the following: University Hospital, Toulouse, France (M.A. and M.R.); University Hospital Caen, France (M.M.); University Hospital, Angers, France (M. Dib); Hospital, Bayonne, France (C. Araujo); Hospital Avignon, France (B. Slama); University Hospital, Besançon, France (J. Fontan); University Hospital Saint-Antoine, Paris, France (L. Garderet); University Hospital, Amiens, France (B. Royer); Hospital, La Roche-sur-Yon, France (M. Tiab); University Hospital, Bordeaux, France (G. Marit); Hospital Bordeaux Nord, France, (O. Fitoussi); Hospital Annecy, France (F. Orsini-Piocelle); University Hospital, Dijon, France (D. Caillot); University Hospital, Nantes, France (P. Moreau and C. Touzeau); University Hospital, Brest, France (J. R. Eveillard); Hospital Chalon-sur-Sâone, France (L. Voillat); Hospital Sud-Francilien, Evry, France (B. Joly); University Hospital, Lille, France (T. Facon and X. Leleu); Hospital Argenteuil, France (D. Chaoui); University Hospital, Rennes, France (M. Escoffre); University Hospital, Nice, France (J.-G. Fuzibet); University Hospital, Clermont-Ferrand, France (C. Chaleteix); University Hospital, Tours, France (L. Benboubker); University Hospital, Nancy, France (C. Hulin); University Hospital, Reims, France (B. Kolb); University Hospital, Lyon, France (L. Karlin); Hospital Blois, France (P. Rodon); University Hospital Saint-Louis, Paris, France (J. P. Fermand); University Hospital, Limoges, France (A. Jaccard); Hospital Le Mans, France (K. Laribi); Hospital Mulhouse, France (C. Eisenmann); Hospital Saint-Brieuc, France (O. Allangha); University Hospital Henri Mondor, France (K. Belhadj); Hospital, Dunkerque, France (M. Wetterwald); University Hospital, Paris Saint-Antoine, France (L. Garderet); University Hospital, Grenoble, France (B. Pégourié); University Hospital, Poitiers, France (M. Puyade); Institut Paoli-Calmette, Marseille, France (A. M. Stoppa); Centre Léon Bérard, Lyon, France (C. Sebban); Percy Hospital, Clamart, France (J. V. Malfuson); Hospital Huguenin, Saint-Cloud, France (S. Glaisner); Hospital Colmar, France (B. Audhuy); Centre Bernard, Le Mans, France (E. Voog); Hospital Lorient, France (O. Luycx); University Hospital, Metz, France (V. Dorvaux); Centre Becquerel, Rouen, France (P. Lenain); Hospital Vannes, France (P. Godmer); University Hospital, Bobigny, France (S. Brechignac); Hospital Chartres, France (M. Maigre); University Hospital Cochin, Paris, France, Orléans Hospital, Orléans, France (M. Alexis); University Hospital, Nîmes, France (P. Bourquard); University Hospital Cochin, Paris, France (D. Bouscary); Hospital Perpignan, France (L. Sanhes); Hospital Pointoise, France (R. Benramdane); University Hospital, Strasbourg, France (C. Fohrer); Hospital Valence, France (B. Anglaret); and University Hospital, Saint-Etienne, France (K. Augel-Meunier).
Authorship
Contribution: P.M. and M.A. designed the study and wrote the manuscript; T.D. centrally analyzed blood and urine electrophoreses; M.A. centrally analyzed cytogenetics; P.M. and M.A. analyzed the data; and all authors served as investigators, contributed patient data, and approved the manuscript.
Conflict-of-interest disclosure: P.M., C.H., T.F., H.A.-L., and M.A. received honoraria from Celgene and Janssen-Cilag.
Correspondence: Philippe Moreau, University Hospital, Place Alexis Ricordeau, 44093 Nantes, France; e-mail: philippe.moreau@chu-nantes.fr.