Key Points
Emergence of a CD34+CD41low population with a high capacity to generate proplatelet-producing MKs and functional platelet-like elements.
Platelet production is inversely correlated to CYP1B1 expression, a target of the aryl hydrocarbon receptor.
Abstract
The mechanisms regulating megakaryopoiesis and platelet production (thrombopoiesis) are still incompletely understood. Identification of a progenitor with enhanced thrombopoietic capacity would be useful to decipher these mechanisms and to improve our capacity to produce platelets in vitro. Differentiation of peripheral blood CD34+ cells in the presence of bone marrow–human mesenchymal stromal cells (MSCs) enhanced the production of proplatelet-bearing megakaryocytes (MKs) and platelet-like elements. This was accompanied by enrichment in a MK precursor population exhibiting an intermediate level of CD41 positivity while maintaining its expression of CD34. Following sorting and subculture with MSCs, this CD34+CD41low population was able to efficiently generate proplatelet-bearing MKs and platelet-like particles. Similarly, StemRegenin 1 (SR1), an antagonist of the aryl hydrocarbon receptor (AhR) transcription factor known to maintain CD34 expression of progenitor cells, led to an enriched CD34+CD41low fraction and to an increased capacity to generate proplatelet-producing MKs and platelet-like elements ultrastructurally and functionally similar to circulating platelets. The effect of MSCs, like that of SR1, appeared to be mediated by an AhR-dependent mechanism because both culture conditions resulted in repression of its downstream effector CYP1B1. This newly described isolation of a precursor exhibiting strong MK potential could be exploited to study normal and abnormal thrombopoiesis and for in vitro platelet production.
Introduction
Blood platelets play crucial roles in both physiology and pathology, and it is therefore of major importance to understand the mechanisms controlling their production. In adults, platelets are produced by bone marrow megakaryocytes (MKs), which themselves originate from hematopoietic stem and progenitor cells. In vitro production of platelets for transfusion has been the subject of many studies in recent years.1-5 Continuous improvements in the culture conditions make this an attainable goal, like the recent generation of transfusable human red cells.6 Nevertheless, we are still unable to efficiently reproduce the native process that can generate a calculated >1000 platelets per MK.2 One possible strategy to improve in vitro platelet production would be to isolate and amplify MK progenitors with an increased capacity to mature to the proplatelet stage.
Classically, human MK are differentiated in culture from CD34+ cells, a population containing hematopoietic stem cells and a mixture of progenitor cells with various potentials. Since the availability of thrombopoietin (TPO), numerous protocols have been devised to refine MK differentiation.7-9 As a result, improvements have been reported in our ability to expand the number of input CD34+ cells and to differentiate MKs, as evidenced by their increased size and ploidy, the expression of platelet-specific markers (CD41 and CD42), and their capacity to produce proplatelets. Despite this progress, the percentage of MKs reaching the proplatelet stage remains low, and platelet production is well below that of MKs differentiated in situ in the bone marrow.
An obvious strategy to improve this process is to reproduce as closely as possible the conditions of the bone marrow. All the steps leading to the production of fully mature MKs are controlled by a succession of microenvironments constituted of cellular interaction, cytokines, and extracellular matrix, which are for the most part provided by stromal cells within the bone marrow.10 Bone marrow–derived mesenchymal stromal cells (MSCs) could therefore provide a favorable milieu to improve the proliferation and differentiation of MKs from hematopoietic progenitors. Their role in hematopoietic stem or progenitor cell maintenance is well documented, and there have also been reports that MSCs could favor MK maturation.11 In this work, we found that coculture of peripheral blood CD34+ cells with bone marrow–derived human MSCs significantly improved the production of proplatelet-bearing MKs and platelet-like elements. More importantly, culture with MSCs resulted in an enrichment of a CD34+CD41low population, which, on cell sorting, exhibited a high capacity to mature to the proplatelet stage. A similar enrichment of CD34+CD41low cells with enhanced megakaryocytic potential was observed in CD34+ cells cultured with StemRegenin 1 (SR1), a recently developed high affinity aryl hydrocarbon receptor (AhR) antagonist known to maintain CD34 expression of progenitor cells. Coculture with MSCs and exposure to SR1 both led to repression of the AhR pathway, and their positive effect on MK differentiation was prevented by an AhR agonist, indicating that enrichment of CD34+CD41low megakaryocytic precursors proceeds through a similar mechanism.
Materials and methods
MK differentiation in culture
CD34+ cells were seeded in 24-well plates at a density of 4 × 104/mL in StemSpan Serum-Free Expansion Medium (SFEM) medium supplemented with 20 ng/mL human low-density lipoprotein and CC220 (1×), a cocktail of cytokines containing stem cell factor, TPO, interleukin-6 (IL-6), and IL-9 (all from Stemcell Technologies, Vancouver, BC, Canada). Cells were cultured in the presence or absence of a confluent layer of MSCs.12 On day 7, the cells in suspension were harvested, washed, and cocultured at 5 × 104/mL on a new layer of confluent MSCs in StemSpan SFEM medium containing 50 ng/mL TPO for an additional 7 days.
In a second protocol, CD34+ cells were seeded as above with or without addition of 1 µM SR1 (Cellagen Technology, San Diego, CA).
The cultures were incubated at 37°C under normoxic conditions and 5% CO2. In some experiments, cultures were performed in the presence of the AhR agonist 6-formylindolo(3,2-b)carbazole (FICZ) (Enzo Life Sciences, Villeurbane, France) added at 0.2 µM.
Cell sorting
The cells recovered on day 10 were incubated with a mixture of Alexa-488–conjugated anti-CD41 (ALMA.17) and phycoerythrin (PE)-Cy7–conjugated anti-CD34 monoclonal antibodies (mAbs; BD Biosciences) for 30 minutes at 4°C. They were incubated for 30 minutes in phosphate-buffered saline containing 7-aminoactinomycin D (2.5 µg/mL) to select viable cells. The sorting gates were based on fluorescence − 1 controls, and megakaryocytic precursors were sorted at 500 cells/s according to their CD34/CD41 expression using a fluorescence-activated cell sorter (FACS) Aria II flow cytometer (Becton Dickinson, Mountain View, CA). The sorted CD34+41low and CD34−CD41+ cells were then counted and seeded at 4 × 104/mL in 48-well plates in StemSpan medium containing TPO with or without MSCs or SR1 or for 7 days.
Analysis of MK maturation
Cells were analyzed by flow cytometry (Gallios, Beckman Coulter) after labeling with anti–CD34-PE-Cy7 (Beckman Coulter, Villepinte, France), anti–CD41-Alexa-488 (ALMA.17), anti–CD42c-PE (RAM.1), and anti–CD42d-Alexa-647 (V.1) mAbs for 30 minutes at 4°C. The cells were then washed and resuspended in phosphate-buffered saline containing 7-aminoactinomycin D (2.5 μg/mL). The acquired data were analyzed with Kaluza software.
Statistics
Statistical significance was determined by means of the Student t test or 1-way analysis of variance (ANOVA) followed by a Bonferroni posttest. Data were analyzed using Graphpad Prism 5 software.
The supplemental Methods, available on the Blood Web site, provide all technical details.
Results
Coculture of CD34+ cells with MSCs increases the production of proplatelet-bearing MKs and platelet-like elements
We evaluated whether MSCs could provide a favorable milieu for the emergence of a MK precursor. CD34+ cells were cocultured with MSCs in a 2-step protocol. CD34+ cells were first expanded for 7 days in the presence of CC220, an optimized mix of stem cell factor, TPO, IL-6, and IL-9, and then differentiated for a further 7 days in the presence of TPO alone (Figure 1A). Culture with a preformed monolayer of MSCs did not significantly modify cell proliferation during the first 10 days of culture (Figure 1B) but increased the production of proplatelet-bearing MKs counted at day 14 (Figure 1C). Whereas 9.5 ± 1% of the MKs exhibited proplatelets under control conditions, this proportion rose to 22.1 ± 1.6% in the presence of MSCs (Figure 1D). This was accompanied by an increased production of platelet-like elements in the culture media. Whereas under control conditions, 2.9 ± 0.5 × 105 platelet-sized particles were counted per well at day 14, their number increased about threefold to 8.4 ± 1.9 × 105 platelets per well in the presence of MSCs (Figure 1E). These corresponded to a yield of ∼10 and 28 platelets per MK seeded at day 7, respectively.
Coculture of CD34+ cells with MSCs promotes proplatelet and platelet production. (A) MK differentiation protocol. Peripheral blood CD34+ cells were cultured in the absence (Ctrl) or presence of a monolayer of MSCs in a serum-free medium containing a CC220 cytokine cocktail from days 0 to 7 and with TPO (50 ng/mL) from days 7 to 14. (B) Level of proliferation. Viable cells were counted on days 7 and 10 of culture using an automatic cell counter, and the fold increase over the input of CD34+ cells at day 0 was calculated (mean ± standard error of the mean [SEM] in 3 experiments; Student t test, P > .05, not significant [n.s.]). (C) Representative differential interference contrast (DIC) microscopy photographs of culture well at day 7. Zoomed images of the boxed region show extensive proplatelet development in the presence of MSCs. Scale bar, 50 μm. (D) Proportion of MK extending proplatelets at day 14 (22.1 ± 1.5% with MSCs vs 9.5 ± 1.1% for the control; mean ± SEM in 3 experiments; Student t test, ***P < .001). (E) Amount of culture-derived platelets. The cell suspension was subjected to multiple pipetting on day 14 of culture, and platelet-like elements were detected and counted by flow cytometry (mean ± SEM in 3 experiments; Student t test, **P < .01).
Coculture of CD34+ cells with MSCs promotes proplatelet and platelet production. (A) MK differentiation protocol. Peripheral blood CD34+ cells were cultured in the absence (Ctrl) or presence of a monolayer of MSCs in a serum-free medium containing a CC220 cytokine cocktail from days 0 to 7 and with TPO (50 ng/mL) from days 7 to 14. (B) Level of proliferation. Viable cells were counted on days 7 and 10 of culture using an automatic cell counter, and the fold increase over the input of CD34+ cells at day 0 was calculated (mean ± standard error of the mean [SEM] in 3 experiments; Student t test, P > .05, not significant [n.s.]). (C) Representative differential interference contrast (DIC) microscopy photographs of culture well at day 7. Zoomed images of the boxed region show extensive proplatelet development in the presence of MSCs. Scale bar, 50 μm. (D) Proportion of MK extending proplatelets at day 14 (22.1 ± 1.5% with MSCs vs 9.5 ± 1.1% for the control; mean ± SEM in 3 experiments; Student t test, ***P < .001). (E) Amount of culture-derived platelets. The cell suspension was subjected to multiple pipetting on day 14 of culture, and platelet-like elements were detected and counted by flow cytometry (mean ± SEM in 3 experiments; Student t test, **P < .01).
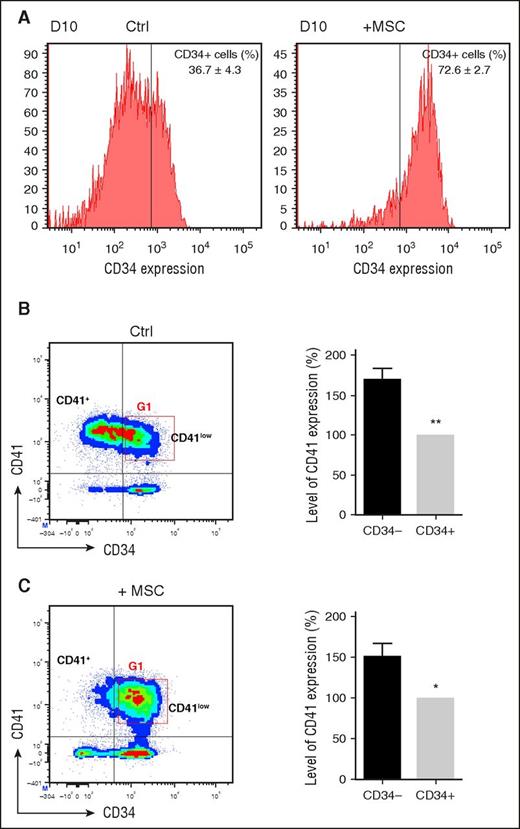
Coculture with MSCs promotes maintenance of a CD34+CD41low population
We next questioned whether the MSC-dependent increase in proplatelets and platelets could be due to selection of a specific progenitor population. We first evaluated expression of the CD34 hematopoietic progenitor marker at different time points of culture. Although before day 10, CD34 expression was similar under both conditions (control and in the presence of MSCs), at day 10, after passage to a TPO-containing medium, a significant loss of CD34 expression was observed in control cultures (36.7 ± 4.3% CD34+ cells), whereas in MSC-treated cultures, CD34 expression was retained at the cell surface (72.6 ± 2.7% CD34+ cells; Figure 2A).
Identification of a CD34+CD41low population. (A) CD34 expression and % of CD41+ cells at day 10. CD34+ cells were cultured as in Figure 1A, and analyses were performed on day 10. (B) (Left) Expression of CD34 and CD41 markers at day 10 in control culture conditions. Representative flow density plot identifies 2 subpopulations based on CD41 expression, CD34−CD41+ and CD34+CD41low. Gate G1 was later used in cell sorting experiments (Figure 3). (Right) Level of CD41 expression at day 10. The level of expression of CD41 in the CD34+CD41+ quadrant as measured by FL2 fluorescence intensity was adjusted at 100% (gray bar). In CD34−CD41+ (black bar), CD41 expression was increased by 1.7 ± 0.1-fold (mean ± SEM in 8 experiments; paired Student t test, **P < .01), defining a CD34−CD41+ population by comparison with the CD34+CD41+ population defining CD34+CD41low cells. (C) (Left) Expression of CD34 and CD41 markers at day 10 in MSC-treated cultures. (Right) Level of CD41 expression at day 10.
Identification of a CD34+CD41low population. (A) CD34 expression and % of CD41+ cells at day 10. CD34+ cells were cultured as in Figure 1A, and analyses were performed on day 10. (B) (Left) Expression of CD34 and CD41 markers at day 10 in control culture conditions. Representative flow density plot identifies 2 subpopulations based on CD41 expression, CD34−CD41+ and CD34+CD41low. Gate G1 was later used in cell sorting experiments (Figure 3). (Right) Level of CD41 expression at day 10. The level of expression of CD41 in the CD34+CD41+ quadrant as measured by FL2 fluorescence intensity was adjusted at 100% (gray bar). In CD34−CD41+ (black bar), CD41 expression was increased by 1.7 ± 0.1-fold (mean ± SEM in 8 experiments; paired Student t test, **P < .01), defining a CD34−CD41+ population by comparison with the CD34+CD41+ population defining CD34+CD41low cells. (C) (Left) Expression of CD34 and CD41 markers at day 10 in MSC-treated cultures. (Right) Level of CD41 expression at day 10.
We then analyzed the appearance of the CD41 megakaryocytic marker combined with that of CD34. After 7 days of culture with the CC220 cytokine mix, a similar high proportion (60% and 69%, respectively) of CD34+ cells had acquired the CD41 marker in control and MSC-treated cultures (data not shown). In control cultures at day 10, the percentage of CD41-positive cells increased to 86.7 ± 5.5%, and 63.2 ± 3.1% of the cells became negative for CD34 (Figure 2B, left). Analysis of the CD34− and CD34+ regions revealed a 1.7-fold increase in the level of CD41 expression in CD34− cells (Figure 2B, right). This allowed us to delineate 2 populations (CD34−CD41+ and CD34+CD41low) that represented 57.2 ± 5.1% and 29.5 ± 5.9% of the total population, respectively (Figure 2B). By comparison, in MSC-treated cultures at day 10 (Figure 2C), a much larger proportion of the CD34+CD41low population (50.7 ± 4.5%) was identified, whereas CD34−CD41+ represented a minority of the cells (12.9 ± 3.7% of the cells; Figure 2C).
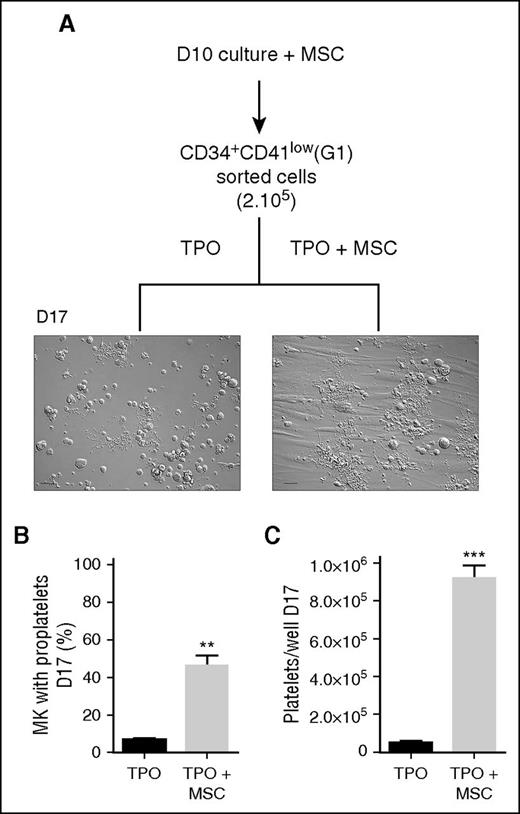
CD34+CD41low cells have a high capacity to produce proplatelets and platelet-like particles
To evaluate the MK progenitor potential of the CD34+CD41low population, these cells were sorted by flow cytometry from an MSC-treated culture (sorting window corresponding to gate 1 in Figure 2B) and differentiated for 7 days in a TPO-containing medium (Figure 3A). Under this condition, a high proportion of the cells reached the proplatelet stage (46 ± 5%) when grown in the presence of MSCs (Figure 3B). Much lower frequencies were observed when CD34+CD41low cells were cultured in the absence of MSC (6 ± 0.8%). The increased proplatelet yield was accompanied by an 18-fold enhanced production of platelet-like elements in CD34+CD41low cells cultured with MSCs compared without MSCs (9.1 ± 0.7 × 105 vs 0.5 ± 0.4 × 105 platelets per well, respectively; Figure 3C). These results indicated that the CD34+CD41low population enriched in the presence of MSCs has a strong potential to produce proplatelet-bearing MKs and to release platelet-like elements.
CD34+CD41low population from MSC coculture has enhanced proplatelet and platelet-producing capacity. (A) Flow sorting and culture protocol of CD34+CD41low cells. CD34+ cells cultured for 10 days in the presence of MSCs were sorted according to their CD34+CD41low phenotype (G1) using a FACS Aria II flow cytometer and then cultured for 7 days in a medium containing TPO with or without MSCs. Representative DIC microscopy photographs of culture wells at day 7 showed extensive proplatelet development in the presence of MSCs. Scale bar, 50 μm. (B) Proportion of MK-extending proplatelets at day 7 (6.6 ± 0.8% for TPO vs 46.4 ± 5.2% for TPO + MSCs; mean ± SEM in 3 experiments; Student t test, **P < .01). (C) Amount of culture-derived platelets at day 7 (0.5 ± 0.04 × 105 in the control vs 9.1 ± 0.7 × 105 with MSCs; mean ± SEM in 3-4 experiments; Student t test, ***P < .001).
CD34+CD41low population from MSC coculture has enhanced proplatelet and platelet-producing capacity. (A) Flow sorting and culture protocol of CD34+CD41low cells. CD34+ cells cultured for 10 days in the presence of MSCs were sorted according to their CD34+CD41low phenotype (G1) using a FACS Aria II flow cytometer and then cultured for 7 days in a medium containing TPO with or without MSCs. Representative DIC microscopy photographs of culture wells at day 7 showed extensive proplatelet development in the presence of MSCs. Scale bar, 50 μm. (B) Proportion of MK-extending proplatelets at day 7 (6.6 ± 0.8% for TPO vs 46.4 ± 5.2% for TPO + MSCs; mean ± SEM in 3 experiments; Student t test, **P < .01). (C) Amount of culture-derived platelets at day 7 (0.5 ± 0.04 × 105 in the control vs 9.1 ± 0.7 × 105 with MSCs; mean ± SEM in 3-4 experiments; Student t test, ***P < .001).
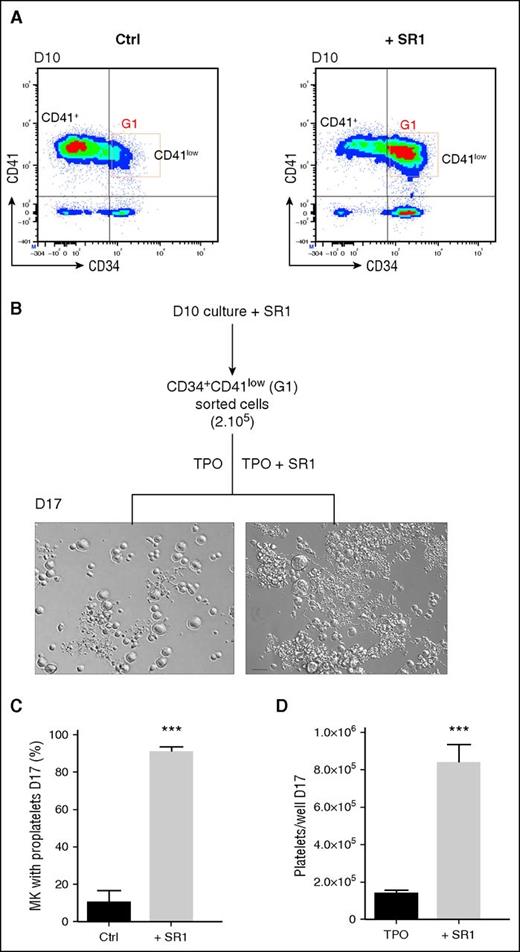
AhR antagonist SR1 maintains CD34 expression and increases proplatelet and platelet formation
The above-described experiments suggested that the improved MK maturation could be linked to sustained CD34 expression. Maintenance of CD34 expression has been reported in response to antagonists of the AhR such as the high affinity SR1.13 In agreement with this, we observed that CD34 expression was retained in cells cultured according to the 2-stage protocol in the presence of SR1 (Figure 4A). Whereas only 40.7% of the cells in control cultures expressed CD34 at day 10 after passage in TPO media, this population represented 71.6% of the total cells after SR1 treatment (Figure 4B). Furthermore, exposure to SR1 increased the proportion of proplatelet-bearing MKs at day 14 (11.5 ± 4.5% in the control vs for 34.6 ± 2.1% with SR1; Figure 4C-D) and augmented the yield in platelet-like particles (2.7 ± 0.7 × 105 per well in the control vs 9.8 ± 2.8 × 105 per well with SR1; Figure 4E). At this stage, transmission electron microscopy (TEM) examination did not reveal signs of MK damage and apoptosis, and caspase-3 activation was restricted to 24% of the cells (data not shown).
Preservation of CD34 expression and increased production of proplatelets and platelet-like elements in the presence of SR1. (A) Peripheral blood CD34+ cells were cultured in the absence (Ctrl) or presence of SR1 (1 µM) in a serum-free medium containing a CC220 cytokine cocktail from days 0 to 7 and with TPO (50 ng/mL) from days 7 to 14. (B) Proportion of CD34+ cells. The proportion of CD34+ cells vs total cells was determined on the indicated days by flow cytometry. Experiments were performed ≥3 times (mean ± SEM; 1-way ANOVA and a Bonferroni posttest, P > .05, not significant [n.s.]; and ***P < .001). (C) Representative DIC microscopy photographs of culture well at day 7. Zoomed images of the boxed region show extensive proplatelet development in the presence of SR1. Scale bar, 50 μm. (D) Proportion of MK extending proplatelets at day 14 (11.5 ± 4.5% in the control vs 34.6 ± 2.1% with SR1; mean ± SEM in 3 experiments; Student t test, **P < .01). (E) Amount of culture-derived platelets. The cell suspension was subjected to multiple pipetting on day 14 of culture, and platelet-like elements were detected and counted by flow cytometry (2.7 ± 0.7 × 105 in the control vs 9.8 ± 2.8 × 105 with SR1; mean ± SEM in 5 experiments; Student t test, *P < .05).
Preservation of CD34 expression and increased production of proplatelets and platelet-like elements in the presence of SR1. (A) Peripheral blood CD34+ cells were cultured in the absence (Ctrl) or presence of SR1 (1 µM) in a serum-free medium containing a CC220 cytokine cocktail from days 0 to 7 and with TPO (50 ng/mL) from days 7 to 14. (B) Proportion of CD34+ cells. The proportion of CD34+ cells vs total cells was determined on the indicated days by flow cytometry. Experiments were performed ≥3 times (mean ± SEM; 1-way ANOVA and a Bonferroni posttest, P > .05, not significant [n.s.]; and ***P < .001). (C) Representative DIC microscopy photographs of culture well at day 7. Zoomed images of the boxed region show extensive proplatelet development in the presence of SR1. Scale bar, 50 μm. (D) Proportion of MK extending proplatelets at day 14 (11.5 ± 4.5% in the control vs 34.6 ± 2.1% with SR1; mean ± SEM in 3 experiments; Student t test, **P < .01). (E) Amount of culture-derived platelets. The cell suspension was subjected to multiple pipetting on day 14 of culture, and platelet-like elements were detected and counted by flow cytometry (2.7 ± 0.7 × 105 in the control vs 9.8 ± 2.8 × 105 with SR1; mean ± SEM in 5 experiments; Student t test, *P < .05).
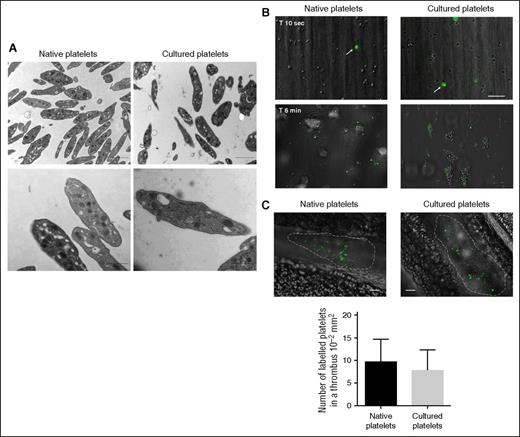
Ultrastructural and functional characterization of culture-derived platelets
On isolation by differential centrifugation, the in vitro–produced platelets exhibited characteristics that were similar to native resting platelets in terms of ultrastructure. They displayed a discoid shape, had a normal distribution of the open canalicular system, and contained α and δ granules (Figure 5A). These platelets were anucleate and harbored a well-defined microtubular marginal band revealed in confocal microscopy with a tubulin β1-specific antibody (supplemental Figure 1C). Their size was, however, slightly larger than that of circulating human platelets (3.9 ± 0.1 vs 2.8 ± 0.1 μm, n > 30; ***P < .0001). The functionality of culture-derived platelets was first assessed in a flow chamber system. RAM.1-488–labeled human blood or culture-derived platelets mixed to hirudinated blood (ratio 1/100) were perfused over immobilized type I collagen at 300 seconds−1. Cultured and native platelets similarly adhered to the matrix after 10 seconds and became incorporated into the developing thrombi (Figure 5B). In addition, cultured platelets adhered and spread on a fibrinogen matrix and expressed active integrin αIIbβ3 in response to thrombin, although to a slightly lower level than native platelets (supplemental Figure 1C). In vivo functionality of culture-derived platelets was also documented in a laser-induced arteriolar injury model showing incorporation into the growing thrombus with 7.8 ± 4.5 platelets per thrombus compared with 9.7 ± 4.9 platelets per thrombus for native platelets (Figure 5C). The somewhat lower efficacy of cultured platelets might be due to the presence of still undeveloped cellular fragments.
Ultrastructure, in vitro, and in vivo functionality of platelet-like elements produced in the presence of SR1. (A) Platelet ultrastructure. (Upper) Representative microscopic fields of resting platelet populations examined by TEM. Bar, 2 µm. (Lower) Higher-magnification TEMs of native and culture-derived platelets. Bar, 1 µm. Culture-derived platelets exhibited similar ultrastructural characteristics to native platelets. (B) Flow-dependent thrombus formation. RAM.1-488–labeled native or culture-derived platelets mixed to hirudin-anticoagulated blood at a 1:100 ratio were perfused over immobilized type I collagen at 300 seconds−1 and observed in real time by DIC. (Upper) Individual labeled platelets (arrows) adhering to the matrix after 10 seconds of perfusion. (Lower) Platelet thrombi formed after 6 minutes of perfusion showing incorporation of labeled native and cultured platelets. Scale bars, 20 µm. (C) Laser-induced thrombosis model. Representative images of thrombi 2 minutes after laser injury of a mesenteric artery showing incorporation of RAM.1-488–labeled native or culture-derived platelets injected immediately after lesion. Scale bars, 20 µm. Bar graph, number of labeled platelets per thrombus (mean ± SEM of 4 experiments).
Ultrastructure, in vitro, and in vivo functionality of platelet-like elements produced in the presence of SR1. (A) Platelet ultrastructure. (Upper) Representative microscopic fields of resting platelet populations examined by TEM. Bar, 2 µm. (Lower) Higher-magnification TEMs of native and culture-derived platelets. Bar, 1 µm. Culture-derived platelets exhibited similar ultrastructural characteristics to native platelets. (B) Flow-dependent thrombus formation. RAM.1-488–labeled native or culture-derived platelets mixed to hirudin-anticoagulated blood at a 1:100 ratio were perfused over immobilized type I collagen at 300 seconds−1 and observed in real time by DIC. (Upper) Individual labeled platelets (arrows) adhering to the matrix after 10 seconds of perfusion. (Lower) Platelet thrombi formed after 6 minutes of perfusion showing incorporation of labeled native and cultured platelets. Scale bars, 20 µm. (C) Laser-induced thrombosis model. Representative images of thrombi 2 minutes after laser injury of a mesenteric artery showing incorporation of RAM.1-488–labeled native or culture-derived platelets injected immediately after lesion. Scale bars, 20 µm. Bar graph, number of labeled platelets per thrombus (mean ± SEM of 4 experiments).
SR1 promotes the expansion of a CD34+CD41low population
The responses obtained with SR1 resembled those observed with MSCs, which prompted us to evaluate the CD34/CD41 phenotype of the cells produced in presence of SR1. Similar to cultures in the presence of MSCs, a population with the CD34+CD41low profile was clearly predominant by day 10 of culture, representing 56.1 ± 3.2% of the total cells compared with 29.5 ± 5.9% in control cultures (Figure 6A). As shown in supplemental Figure 2, similar CD34+CD42 low and CD34−CD42+ profiles were observed when analyzing CD42b expression.
SR1-derived CD34+CD41low population has an increased proplatelet and platelet-producing capacity. (A) Evolution of CD34 and CD41 expression. Representative flow density plots of CD34 and CD41 expression at day 10. CD34+ cells were cultured as in Figure 4A, and analyses were performed on day 10. (B) Flow sorting and culture protocol of CD34+CD41low cells. CD34+ cells cultured for 10 days in the presence of SR1 were sorted according to their CD34+CD41low phenotype (G1) using a FACS Aria II flow cytometer and then cultured for 7 days in a medium containing TPO with or without SR1. Representative DIC microscopy photographs of culture wells at day 7 showing extensive proplatelet development in the presence of SR1. Scale bar, 50 μm. (C) Proportion of MK extending proplatelets at day 7 (10.0 ± 6.6% for the control vs 91.0 ± 2.4% with SR1; mean ± SEM in 5 experiments; Student t test, ***P < .001). (D) Amount of culture-derived platelets at day 7 (1.3 ± 0.2 × 105 platelets per well in the control vs 8.3 ± 0.9 × 105 with SR1; mean ± SEM in 3-4 experiments; Student t test, ***P < .001).
SR1-derived CD34+CD41low population has an increased proplatelet and platelet-producing capacity. (A) Evolution of CD34 and CD41 expression. Representative flow density plots of CD34 and CD41 expression at day 10. CD34+ cells were cultured as in Figure 4A, and analyses were performed on day 10. (B) Flow sorting and culture protocol of CD34+CD41low cells. CD34+ cells cultured for 10 days in the presence of SR1 were sorted according to their CD34+CD41low phenotype (G1) using a FACS Aria II flow cytometer and then cultured for 7 days in a medium containing TPO with or without SR1. Representative DIC microscopy photographs of culture wells at day 7 showing extensive proplatelet development in the presence of SR1. Scale bar, 50 μm. (C) Proportion of MK extending proplatelets at day 7 (10.0 ± 6.6% for the control vs 91.0 ± 2.4% with SR1; mean ± SEM in 5 experiments; Student t test, ***P < .001). (D) Amount of culture-derived platelets at day 7 (1.3 ± 0.2 × 105 platelets per well in the control vs 8.3 ± 0.9 × 105 with SR1; mean ± SEM in 3-4 experiments; Student t test, ***P < .001).
Following sorting at day 10, CD34−CD41+ and CD34+CD41low cells exhibited a similar size and ultrastructure (supplemental Figure 3). Upon culture for 4 days in TPO-containing medium, significant increases in size and degree of maturation were observed for CD34+CD41low-derived cells (supplemental Figure 3A-B) that were accompanied by an increased ploidy (supplemental Figure 4), upregulation of NF-E2 and FOG1, and downregulation of c-MYB transcripts (supplemental Figure 5). At day 10+4, almost all the CD34+CD41low sorted cells expressed high levels of CD42, whereas in CD34−CD41+ sorted cells, a wider range of low as well as high levels of CD42 expression were observed (supplemental Figure 2), suggesting that SR1 via AhR blockade slows maturation and/or conditions cells, which in the presence of TPO alone will then mature as a whole to produce fully mature MKs.
After 7 days, these cells sustained production of an unprecedented high proportion of proplatelet-producing MKs (91.0 ± 2.4%) when further grown in the presence of SR1 (Figure 6B-C). Much lower frequencies were observed when these same cells were cultured in the absence of SR1 (10.0 ± 6.6%; Figure 6C). The increased proplatelet yield led to a 6.8-fold enhanced production of platelet-like elements in CD34+CD41low cells cultured with SR1 compared with and without SR1 (1.3 ± 0.2 × 105 vs 8.4 ± 0.9 × 105 platelets per well, respectively; Figure 6D).
AhR pathway is involved in MSC-triggered MK maturation
In view of the similar responses to MSC and SR1 treatments, we investigated whether the effects of MSCs might be mediated by a pathway downstream of the AhR. Transcription of the CYP1B1 gene, a member of the cytochrome P450 superfamily, is induced by agonists of the AhR and repressed by antagonists such as SR1. CYP1B1 mRNA levels were decreased by >100-fold in CD34+ cells cultured for 10 days in the presence of SR1 compared with control cultures (Figure 7A). Remarkably, culture of CD34+ cells with MSCs similarly resulted in a >90% decrease in CYP1B1 expression. Furthermore, this decrease was reversed by adding the AhR agonist FICZ13 to the coculture, indicating that repression of CYPB1 by MSCs occurred downstream of the AhR.
Role of the AhR pathway in the enhanced platelet production with MSCs. (A) Inhibition of CYP1B1 expression in CD34 cells cultured with MSC and its prevention with an AhR agonist. CD34+ cells were cultured as in Figure 1 for 10 days under control conditions or in the presence of SR1 (1 µM), MSCs, or MSCs + the AhR agonist FICZ (0.2 µM). mRNA was extracted, and the level of CYP1B1 transcript, a downstream effector of the AhR, was quantified by real-time polymerase chain reaction. The level of transcript, normalized in reference to control cultures (dimethyl sulfoxide samples that were normalized such that their median value for CYP1B1 mRNA level was 1), represented a ratio of 0.04 ± 0.04, 0.09 ± 0.04, and 6.5 ± 1 in SR1, MSC, and MSC + FICZ conditions, respectively (mean ± SEM in 3 experiments; 2-way ANOVA and a Bonferroni posttest, **P < .01, ***P < .001). (B) An AhR agonist prevents the positive effect of MSCs on proplatelet and platelet formation. CD34+ cells were cultured for 14 days under control conditions or with MSCs or with MSCs + FICZ (0.2 µM). Incubation with FICZ reversed the MSC-dependent increase in proplatelet formation (left: representative DIC microscopy photographs of culture wells) and platelet release (right: 8.3 ± 1.9 × 105 platelets per well for MSCs vs 0.7 ± 0.5 × 105 platelets per well for MSCs + FICZ; mean ± SEM in 3 experiments; 2-way ANOVA and a Bonferroni posttest, *P < .1; and P > .5, not significant [n.s.]).
Role of the AhR pathway in the enhanced platelet production with MSCs. (A) Inhibition of CYP1B1 expression in CD34 cells cultured with MSC and its prevention with an AhR agonist. CD34+ cells were cultured as in Figure 1 for 10 days under control conditions or in the presence of SR1 (1 µM), MSCs, or MSCs + the AhR agonist FICZ (0.2 µM). mRNA was extracted, and the level of CYP1B1 transcript, a downstream effector of the AhR, was quantified by real-time polymerase chain reaction. The level of transcript, normalized in reference to control cultures (dimethyl sulfoxide samples that were normalized such that their median value for CYP1B1 mRNA level was 1), represented a ratio of 0.04 ± 0.04, 0.09 ± 0.04, and 6.5 ± 1 in SR1, MSC, and MSC + FICZ conditions, respectively (mean ± SEM in 3 experiments; 2-way ANOVA and a Bonferroni posttest, **P < .01, ***P < .001). (B) An AhR agonist prevents the positive effect of MSCs on proplatelet and platelet formation. CD34+ cells were cultured for 14 days under control conditions or with MSCs or with MSCs + FICZ (0.2 µM). Incubation with FICZ reversed the MSC-dependent increase in proplatelet formation (left: representative DIC microscopy photographs of culture wells) and platelet release (right: 8.3 ± 1.9 × 105 platelets per well for MSCs vs 0.7 ± 0.5 × 105 platelets per well for MSCs + FICZ; mean ± SEM in 3 experiments; 2-way ANOVA and a Bonferroni posttest, *P < .1; and P > .5, not significant [n.s.]).
We next evaluated whether MK maturation induced by MSCs was dependent on the AhR. For that, CD34+ cells were cultured for 14 days according to the 2-step protocol in the presence of MSCs with or without addition of FICZ (Figure 7B). Addition of FICZ prevented the increase in platelet production triggered by the coculture with MSCs. FICZ added without MSCs also decreased proplatelet formation (data not shown), suggesting that a fine tuning of AhR is required to promote proplatelet formation. These results support that MSCs, similar to SR1, promoted MK maturation and platelet production by acting on the AhR pathway.
Discussion
In this study, we report the identification and generation of a discrete population of adult hematopoietic progenitors primed for MK differentiation that can efficiently mature to proplatelet-bearing MKs. This population, identified by means of its CD34+CD41low signature, was enriched when adult CD34+ cells were cultured in the presence of an MSC monolayer or an antagonist of the AhR. We found that culture with MSCs or SR1, in addition to promoting the appearance of this MK progenitor, greatly improved the yield of proplatelet-producing MKs and the release of platelet-like elements.
Several features of the CD34+CD41low population identified here in the human system, such as the small size and low ploidy of the cells (supplemental Figure 2) and their high capacity to fully mature into MKs able to extend proplatelets, appear to correspond to the definition of a platelet-biased progenitor. Its distinctive phenotype combines a CD34+ progenitor signature with expression of intermediate levels of the CD41 megakaryocytic marker. CD41-positive cells have been described among human CD34+ cells isolated directly from bone marrow or after culture under MK-promoting conditions.14,15 However, these populations did not fully recapitulate the present CD34+CD41low phenotype because they were highly polyploid and unable to proliferate.15 A population, also, with a decreased intensity of CD41 expression, called CD34+CD41dim cells, has been previously observed after coculture of bone marrow–derived CD34+ cells on human MSCs without TPO, but no evidence was provided for a distinct CD41 subpopulation.16 Cells with a CD34+CD41+ phenotype representing a very minor population were recently reported in cultures derived from peripheral blood but were not characterized further.17 A CD31+CD34+CD41+ megakaryoblastic population resembling the cells described here was also observed in reprogrammed induced pluripotent stem cells cultured in a 3-step serum-free system.4 Altogether, the literature and the present work strongly favor the existence of platelet-biased progenitors among cells of human origin. An MK-oriented progenitor has been better documented in the mouse using diverse combinations of markers. Progenitors primed for the megakaryocytic lineage were identified through their c-Kithigh phenotype.18 Cells with a stem cell signature (signaling lymphocyte activation molecule–like) and positive for von Willebrand factor with a propensity to reconstitute platelets have likewise been identified.19 Also in the mouse, CD41+ hematopoietic stem cells were found to exhibit myeloerythroid and MK gene priming.20 Finally, a unipotent megakaryocytic progenitor was recently reported in a subpopulation of CD41+CD42+LSK bone marrow cells.21 Although difficult to translate to the human species due to the use of very different sets of markers, these data strongly support the existence of a unipotent MK progenitor distinct from the bipotent megakaryocyte/erythrocyte progenitor and suggest that the CD34+CD41low population could represent or include such a progenitor.
The role of stromal cells in hematopoietic stem or progenitor cell maintenance is well documented, and there have also been reports that MSCs could favor MK maturation.22 Here, we observed that bone marrow–derived human MSCs could support the emergence of a MK-oriented population characterized by its CD34+CD41low expression. Preservation of CD34 expression alongside acquisition of MK-oriented CD41 expression is a key signature provided by the MSC coculture that is even better exhibited in cells grown in the presence of SR1. Further indication that MSC coculture phenocopied SR1 treatment was the fact that the same MK maturation capacity was observed when (1) sorted CD34+CD41low cells derived from MSC cultures were differentiated in the presence of SR1 and (2) sorted CD34+CD41low cells from SR1 cultures were grown in the presence of MSCs (data not shown). Because SR1 phenocopied the effects of MSCs, we hypothesized that the mechanism involved interference with the AhR signaling pathway. This is strongly supported by the repression of the target gene CYP1B1 in MK cocultured with MSCs and by the opposing effect of the FICZ AhR agonist. One hypothesis is that MSCs could provide an AhR ligand or affect its signaling pathway. To determine whether the supportive effects of MSCs were mediated by secreted factors, cultures were performed in the presence of conditioned medium derived from MSCs (supplemental Figure 6). Analysis of CD34 and CD41 expression at day 10 and quantification of platelet production at day 14 showed no change with the conditioned medium compared with the control, suggesting that that interaction with the stroma might be required to support the emergence of CD34+CD41low cells and to favor platelet production.
SR1 was even more efficient than MSCs in promoting the appearance of the MK-oriented CD34+CD41low population in our culture system. SR1 was originally described to increase the number of CD34+ cells (two- to threefold during 7 days of culture) and to promote the retention of CD34 expression.13 Under our experimental conditions, SR1 did not increase the number of CD34+ cells but efficiently maintained CD34 expression up to day 10 of culture. The effect of SR1 on CD34+ cell expansion has been attributed to its capacity to antagonize the AhR and to repress the induction of CYP1B1 mRNA.13 Prior work has followed the role of the AhR throughout hematopoietic expansion and differentiation of human induced pluripotent stem cells. These authors observed that AhR activation of hematopoietic progenitor cells drives an expansion of these cells and also of megakaryocyte- and erythroid-progenitor cells, indicating a positive role of the receptor at the progenitor stage.23 Interestingly, however, they also observed that repression of the AhR in these expanding progenitor cells favored megakaryocyte specification, an observation that appears in line with the conclusions of the present work. Here, we further showed that blockade of the AhR significantly improved the yield of more mature MKs susceptible to extend proplatelets. On the contrary, treatment with the AhR agonist FICZ prevented full MK maturation and platelet release. These effects of SR1 and FICZ were observed under conditions where they, respectively, blocked or enhanced AhR downstream expression of the well-known CYP1B1 target gene. Other studies have evaluated the in vivo role of the AhR and reported decreased platelet counts in AhR knockout (KO) mice,24,25 but agonists of the AhR have been found to decrease platelet counts.26 In addition to platelets, altered red and leukocyte cell counts have also been observed in AhR KO mice.27 Therefore, AhR likely impacts many hematopoietic stages and lineages and answers on its specific role in megakaryopoiesis might require a tissue-restricted KO approach.
Culture with MSC or SR1 promoted the appearance of a MK progenitor but was also able to greatly improve the yield in proplatelet-producing MKs and the release of platelet-like elements. Indeed, their absence during the second step of culture where only TPO was present significantly decreased the efficiency of proplatelet formation. This capacity to sustain the last stages of MK maturation has been observed in some cases for MSCs16 but not with SR1. Implication of the AhR pathway is again suggested by the effect of the AhR antagonist. Observation that this antagonist favors MK engagement and MK maturation is compatible with the fact that the AhR is present all along the differentiation pathway (data not shown). Possible responses targeted by this pathway to accomplish full maturation of MKs to the proplatelet stage include development of the demarcation membrane system and mobilization of the actomyosin and microtubule cytsoskeletons, but the exact mechanisms remain to be defined.
In conclusion, the present work provides improved conditions for the efficient generation of mature MK and platelets in vitro and new directions to better understand the mechanisms of thrombopoiesis. The addition of a small molecule SR1 to a well-defined 2-step protocol for the culture of CD34+ cells could provide a scalable source of MK precursors. We showed that an unprecedented proportion (90%) of these cells can reach the proplatelet stage in the presence of TPO, which seemed inversely correlated to CYP1B1 expression. Alternatively, this MK precursor population could also be isolated from cocultures of CD34+ cells with human MSCs, and we hypothesized that a SR1-like molecule could be secreted or exposed by these stromal cells (supplemental Figure 6). Subject to improvements in our capacity to induce platelet release, this should bring us closer to the yields required for transfusion purposes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank S. Vacher for performing quantitative reverse transcriptase-polymerase chain reaction experiments (Unité de Pharmacogénomique, Institut Curie), J. Dulong for providing mesemchymal stromal cells (INSERM U917, Rennes, France), and J. M. Mulvihil for reviewing the English in the manuscript.
Authorship
Contribution: C.S., C.G., and F.L. conceived and designed the experiments; L.M. and C.S. performed the experiments; A.E. performed electron microscopy; P.M. and N.R. performed functional analysis in vivo and in vitro; I.B. performed quantitative transcriptase-polymerase chain reaction; C.S., I.B., and L.M. analyzed the data; C.S., I.B., N.B., K.T., C.G., and F.L. discussed the techniques and results; C.G. and F.L. supervised the work; and C.S., C.G., and F.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Catherine Strassel, UMR_S949, EFS-Alsace, 10 rue Spielmann, F-67065 Strasbourg Cedex, France; e-mail: catherine.strassel@efs.sante.fr.
References
Author notes
C.G. and F.L. contributed equally to this work.

![Figure 1. Coculture of CD34+ cells with MSCs promotes proplatelet and platelet production. (A) MK differentiation protocol. Peripheral blood CD34+ cells were cultured in the absence (Ctrl) or presence of a monolayer of MSCs in a serum-free medium containing a CC220 cytokine cocktail from days 0 to 7 and with TPO (50 ng/mL) from days 7 to 14. (B) Level of proliferation. Viable cells were counted on days 7 and 10 of culture using an automatic cell counter, and the fold increase over the input of CD34+ cells at day 0 was calculated (mean ± standard error of the mean [SEM] in 3 experiments; Student t test, P > .05, not significant [n.s.]). (C) Representative differential interference contrast (DIC) microscopy photographs of culture well at day 7. Zoomed images of the boxed region show extensive proplatelet development in the presence of MSCs. Scale bar, 50 μm. (D) Proportion of MK extending proplatelets at day 14 (22.1 ± 1.5% with MSCs vs 9.5 ± 1.1% for the control; mean ± SEM in 3 experiments; Student t test, ***P < .001). (E) Amount of culture-derived platelets. The cell suspension was subjected to multiple pipetting on day 14 of culture, and platelet-like elements were detected and counted by flow cytometry (mean ± SEM in 3 experiments; Student t test, **P < .01).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/18/10.1182_blood-2015-09-670208/4/m_2231f1.jpeg?Expires=1768490462&Signature=WhK~pz-c9WqJmguPa8oJHs7twO1LR4O4U0dI-qspZnjViT19SV2B9FJBsyB9lfvhfmesSizA9j8F9EMn1cegAi8yf-fyeSk7TtBX33Jv8bM~nhLSvV1Q9NreHut0lRIb8X0lbWItO2YUphO6Gv2D2ZtoaglTYhoNqUbdJUI-uqf67KO5kdlzk~M9vIeqMq9B7BbVltzc8rWSVdq50XbrXeWzLevz8nllHaw~Rg7cFZCaYmRJZJt~oYhwwGX-N6asaI0~Ww38vkmDPfc7nHOMcHE5uHdCPwlEE~bprXFrP45Chk6kE1BD7PXCkgajwpm2zhX1PPqxcspITKrluMKQ5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Preservation of CD34 expression and increased production of proplatelets and platelet-like elements in the presence of SR1. (A) Peripheral blood CD34+ cells were cultured in the absence (Ctrl) or presence of SR1 (1 µM) in a serum-free medium containing a CC220 cytokine cocktail from days 0 to 7 and with TPO (50 ng/mL) from days 7 to 14. (B) Proportion of CD34+ cells. The proportion of CD34+ cells vs total cells was determined on the indicated days by flow cytometry. Experiments were performed ≥3 times (mean ± SEM; 1-way ANOVA and a Bonferroni posttest, P > .05, not significant [n.s.]; and ***P < .001). (C) Representative DIC microscopy photographs of culture well at day 7. Zoomed images of the boxed region show extensive proplatelet development in the presence of SR1. Scale bar, 50 μm. (D) Proportion of MK extending proplatelets at day 14 (11.5 ± 4.5% in the control vs 34.6 ± 2.1% with SR1; mean ± SEM in 3 experiments; Student t test, **P < .01). (E) Amount of culture-derived platelets. The cell suspension was subjected to multiple pipetting on day 14 of culture, and platelet-like elements were detected and counted by flow cytometry (2.7 ± 0.7 × 105 in the control vs 9.8 ± 2.8 × 105 with SR1; mean ± SEM in 5 experiments; Student t test, *P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/18/10.1182_blood-2015-09-670208/4/m_2231f4.jpeg?Expires=1768490462&Signature=kgdd4iBWMjcZbeZtvsgOw~3s3AgrqViDAUVlNzCpIn33KRn58MWJZvvdqTcuLQmEQdJtVFcUndFNkfxZAXqo9vrOUlhd5ArPjhQ8rkn4YQQPT95WPJX-fBIXFwPh3zCwixmXfwYLdHQtWhkHy9T0cDtdI8q4aIsm~jKbdnCkyuNYkqdPH6WAfY2OncLh4Nr-BA36bKXrV27hOO6QMmqzqzDJ04monBO2VXsft5quZNLQbrkUBHAvF0zJV7hBC4yxZx2~F-W6~E59sYWEvNWilUlGP0Rgd4DmGr3LRCntHPNu6jJ6bxnEeFN0LZvCGlouBQS0c3IYI1cSC62pd4ImIQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Role of the AhR pathway in the enhanced platelet production with MSCs. (A) Inhibition of CYP1B1 expression in CD34 cells cultured with MSC and its prevention with an AhR agonist. CD34+ cells were cultured as in Figure 1 for 10 days under control conditions or in the presence of SR1 (1 µM), MSCs, or MSCs + the AhR agonist FICZ (0.2 µM). mRNA was extracted, and the level of CYP1B1 transcript, a downstream effector of the AhR, was quantified by real-time polymerase chain reaction. The level of transcript, normalized in reference to control cultures (dimethyl sulfoxide samples that were normalized such that their median value for CYP1B1 mRNA level was 1), represented a ratio of 0.04 ± 0.04, 0.09 ± 0.04, and 6.5 ± 1 in SR1, MSC, and MSC + FICZ conditions, respectively (mean ± SEM in 3 experiments; 2-way ANOVA and a Bonferroni posttest, **P < .01, ***P < .001). (B) An AhR agonist prevents the positive effect of MSCs on proplatelet and platelet formation. CD34+ cells were cultured for 14 days under control conditions or with MSCs or with MSCs + FICZ (0.2 µM). Incubation with FICZ reversed the MSC-dependent increase in proplatelet formation (left: representative DIC microscopy photographs of culture wells) and platelet release (right: 8.3 ± 1.9 × 105 platelets per well for MSCs vs 0.7 ± 0.5 × 105 platelets per well for MSCs + FICZ; mean ± SEM in 3 experiments; 2-way ANOVA and a Bonferroni posttest, *P < .1; and P > .5, not significant [n.s.]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/18/10.1182_blood-2015-09-670208/4/m_2231f7.jpeg?Expires=1768490462&Signature=q3k3TpXiYUwl6JZsJh2xPm6WDfx9eA9Nc0qMnKF3NoDRece0U0Z8CN8HEA8X0ylWb0iMdhFuXJD8ISDnBA87-RU4w007hj3UUqP0xEYmvxcdGTjWGOlsm29kX7aTPPx87E-ZQqphFr7bAA8U~7mqO2E6cgPOu5~AHRlNzjXQ3p~9IuQ2B0RTCvRYqZK0ZT2DFOGPIAzBEo1O7C1IuBtmfMXYNwwzFx5x8nh~9vOCiCMpeHyAnKiz2we7HlhpyDjReFD2Ev5mCc-hJWrozpI2AL-6CtGC9DibAbsCPCzEMSo1Ipl~A6Zho9GtHXoTfkAwM66udKqG3zz7AzLk-xhMxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal