Key Points
High-dose daunorubicin benefits AML patients with favorable and intermediate cytogenetics and with FLT3-ITD, NPM1, and DNMT3A mutations.
High-dose daunorubicin is required for the favorable impact of the NPM1 mutation in AML.
Abstract
The initial report of the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network Cancer Research Group trial E1900 (#NCT00049517) showed that induction therapy with high-dose (HD) daunorubicin (90 mg/m2) improved overall survival in adults <60 years old with acute myeloid leukemia (AML); however, at initial analysis, the benefit was restricted to younger patients (<50 years) and patients without unfavorable cytogenetics or a FLT3-ITD mutation. Here, we update the results of E1900 after longer follow-up (median, 80.1 months among survivors), focusing on the benefit of HD daunorubicin on common genetic subgroups. Compared with standard-dose daunorubicin (45 mg/m2), HD daunorubicin is associated with a hazard ratio (HR) for death of 0.74 (P = .001). Younger patients (<50 years) benefited from HD daunorubicin (HR, 0.66; P = .002), as did patients with favorable and intermediate cytogenetics (HR, 0.51; P = .03 and HR, 0.68; P = .01, respectively). Patients with unfavorable cytogenetics were shown to benefit from HD daunorubicin on multivariable analysis (adjusted HR, 0.66; P = .04). Patients with FLT3-ITD (24%), DNMT3A (24%), and NPM1 (26%) mutant AML all benefited from HD daunorubicin (HR, 0.61, P = .009; HR, 0.62, P = .02; and HR, 0.50, P = .002; respectively). HD benefit was seen in the subgroup of older patients (50-60 years) with the FLT3-ITD or NPM1 mutation. Additionally, the presence of an NPM1 mutation confers a favorable prognosis only for patients receiving anthracycline dose intensification during induction.
Introduction
The outcome of patients with acute myeloid leukemia (AML) is varied and impacted by patient and disease characteristics and therapy. The combination of anthracycline plus cytarabine has been a common intensive induction regimen for patients with AML for decades.1,2 Unfortunately, there has been little improvement in this treatment since it was developed. Although the addition of gemtuzumab ozogamicin and cladribine to an anthracycline plus cytarabine doublet has recently been shown to improve overall survival (OS) in patients with favorable and unfavorable cytogenetics, respectively,3,4 and high-dose (HD) cytarabine may improve OS in young patients,5 most attempts to improve OS have failed.6-12 Whether anthracycline intensification during induction can improve OS in de novo AML was a question addressed by the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network (ECOG-ACRIN) Cancer Research Group study E1900.13-16
E1900 enrolled patients ≤60 years of age with de novo untreated AML and randomized them to standard dose (SD, 45 mg/m2) or HD (90 mg/m2) daunorubicin for 3 days in combination with cytarabine. Reported in 2009 after a median of 25.2 months of follow-up among survivors, the E1900 trial demonstrated that HD daunorubicin was associated with an increased rate of complete remission (CR) and an OS benefit. Subgroup analyses showed that HD daunorubicin benefited those with age <50 years, those with intermediate cytogenetics, and those with FLT3-ITD–negative AML; however, no benefit was demonstrated in higher-risk subgroups including patients ≥50 years, those with unfavorable cytogenetics, and those with FLT3-ITD mutations.17
Since the report of E1900 in 2009, the prognostic impact of a series of recurrent gene mutations in AML have been defined.18,19 Patel et al18 explored the impact of daunorubicin dose on AML molecular subgroups and reported a benefit of HD daunorubicin in patients with DNMT3A mutations. Here we update the results of E1900 with longer follow-up and demonstrate the benefit of HD daunorubicin in an expanded group of AML patients.
Methods
Patients
Between December 2002 and November 2008, 657 patients between the ages of 17 and 60 years with de novo untreated AML were enrolled on ECOG-ACRIN Cancer Research Group study E1900 (#NCT00049517) as detailed in Fernandez et al.17 Cytogenetic risk assignment was according to Slovak et al.20 Patients were classified as indeterminate risk if they had unknown cytogenetic risk as per Slovak et al or lacked sufficient data for classification. Testing for presence of an FMS-like tyrosine kinase internal tandem duplication (FLT3-ITD) and mixed-lineage leukemia partial tandem duplication (MLL-PTD) was conducted centrally as previously described.17,21-23 Sequencing of NPM1, DNMT3A, IDH1/2, NRAS, CEBPA, TET2, WT1, KIT, RUNX1, ASXL1, PHF6, KRAS, PTEN, and TP53 was later conducted on banked samples as described by Patel et al.18 FLT3-ITD allelic ratio was not available. The median follow-up time from the patients still alive and included in the current analysis, calculated from the time of randomization for induction therapy, was 80.1 months (range, 0.8-120.4 months). In total, 25 (4%) patients were lost to follow-up.
Treatment
Eligible patients were randomly assigned to either an SD (45 mg/m2) or HD (90 mg/m2) of daunorubicin for 3 days in combination with 7 days of continuously infused cytarabine at 100 mg/m2 per day. A second cycle of the same drugs with daunorubicin at 45 mg/m2 per day was administered to patients in either study arm if the nadir bone marrow biopsy demonstrated residual leukemia. Patients who achieved CR proceeded to consolidation with assignment to autologous or allogeneic hematopoietic stem cell transplant (HCT) based on risk of relapse as predicted by cytogenetic risk20 and white blood cell count at presentation. Patients assigned to autologous HCT were randomized to receive or not receive a single dose of gemtuzumab ozogamicin (GO) at 6 mg/m2 after stem cell collection but prior to autologous transplant. Genetic mutations were not used to determine induction or consolidation therapy allocation. Further details of the regimens and eligibility have been previously described.17,23
Statistical methods
OS was measured from induction randomization to death from any cause (censored at last contact) for evaluating the induction effect. For evaluating the GO effect, OS was defined from consolidation randomization to death from any cause (censored at last contact). Event-free survival (EFS) was measured from randomization to completion of protocol induction chemotherapy without documentation of CR, relapse after CR, or death from any cause (censored at last contact). The analysis was performed on the intention-to-treat principle. Time-to-event distributions were estimated using the Kaplan-Meier method and compared using the log-rank test. Hazard ratios (HRs; HD vs SD) for the target events were computed and tested for significance using univariate and multivariable Cox proportional hazards (PH) models. Multivariable Cox PH models were adjusted for sex, age, hemoglobin level, leukocyte count, platelet count, and cytogenetic profile (with age, hemoglobin level, leukocyte count, and platelet count considered continuous variables). When OS was compared between the GO and the no GO groups, stratified (by induction treatment) log-rank tests and stratified Cox PH models were used instead. A secondary analysis on the GO effect (actually received vs not received) was further conducted based on patients who actually received the autologous HCT and the OS compared was measured from the time of autologous HCT. Four-year cumulative relapse rates were computed using the statistical software R, cmprsk package, taking into account death without relapse as a competing event.24 The relapse time was measured from the onset of CR.
To explore the induction treatment effect on subgroups, the above analysis methods were performed with no statistical adjustment for multiple testing. Given the rarity of subsequent allogeneic stem cell transplant on the E1900 enrolled patients (17 randomized to SD daunorubicin, 19 randomized to HD daunorubicin), no further analysis was conducted to address the possible confounding effect of subsequent allogeneic transplant on induction treatment effect. All reported P values are 2 sided.
Results
Overall survival
In total, 657 patients were enrolled in E1900 and were followed for OS. The characteristics of patients at study entry have been previously described.17 For all patients, the HR for death in the HD group, compared with the SD group, was 0.74 (95% confidence interval [CI], 0.61-0.89; Wald P = .001; Table 1). To confirm this benefit, a multivariable Cox PH model was used to examine the impact of HD daunorubicin with adjustment for sex, age, cytogenetics, hemoglobin level, leukocyte count, and platelet count. With adjustment, the HR for death in the HD group remained at 0.74 (95% CI, 0.61-0.89; Wald P = .001). The median OS was 16.6 vs 25.4 months in the SD and HD groups, respectively (log-rank P = .001; Table 1).
Median OS and HRs for death by treatment (HD vs SD), according to subgroup
| Subgroup . | Standard dose (45 mg/m2 per day) . | High dose (90 mg/m2 per day) . | Fisher’s exact P . | Log-rank P . | Univariate . | Multivariable* . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CR rate . | Median OS (months) (no. of events/total) . | 4-year OS . | CR rate . | Median OS (months) (no. of events/total) . | 4-year OS . | HR (95% CI) . | Wald P . | No. of events/total . | HR (95% CI) . | Wald P . | |||
| All patients | 59% | 16.6 (246/330) | 31% | 71% | 25.4 (207/327) | 39% | .001 | .001 | 0.74 (0.61, 0.89) | 0.001 | 450/653 | 0.74 (0.61, 0.89) | .001 |
| Age | |||||||||||||
| <50 years | 61% | 20.7 (133/188) | 35% | 73% | 44.7 (96/172) | 48% | .02 | .002 | 0.66 (0.50, 0.85) | 0.002 | 228/359 | 0.67 (0.52, 0.88) | .004 |
| ≥50 years | 56% | 12.6 (113/142) | 25% | 68% | 17.6 (111/155) | 28% | .03 | .12 | 0.81 (0.62, 1.06) | 0.12 | 222/294 | 0.82 (0.63, 1.07) | .14 |
| Cytogenetic | |||||||||||||
| Favorable | 84% | 39.4 (24/38) | 46% | 80% | NR (19/51) | 64% | .78 | .02 | 0.51 (0.28, 0.93) | 0.03 | 43/89 | 0.44 (0.24, 0.82) | .01 |
| Intermediate | 56% | 20.1 (101/141) | 35% | 77% | 33.5 (71/127) | 45% | .0003 | .01 | 0.68 (0.50, 0.92) | 0.01 | 172/267 | 0.75 (0.55,1.03) | .08 |
| Indeterminate | 62% | 14.3 (66/91) | 29% | 67% | 21.3 (62/85) | 29% | .53 | .47 | 0.88 (0.62, 1.24) | 0.47 | 127/175 | 0.89 (0.63, 1.27) | .53 |
| Unfavorable | 44% | 10.2 (54/59) | 14% | 57% | 10.6 (54/63) | 19% | .20 | .22 | 0.79 (0.54, 1.16) | 0.23 | 108/122 | 0.66 (0.44, 0.98) | .04 |
| Gene mutation | |||||||||||||
| NPM1 | 60% | 16.9 (50/65) | 29% | 89% | 75.9 (31/65) | 52% | .0002 | .002 | 0.50 (0.32, 0.78) | 0.002 | 81/130 | 0.51 (0.32, 0.81) | .005 |
| FLT3-ITD | 48% | 10.1 (74/83) | 17% | 70% | 15.2 (44/64) | 28% | .008 | .009 | 0.61 (0.42, 0.89) | 0.009 | 117/146 | 0.50 (0.32, 0.77) | .002 |
| DNMT3A | 61% | 14.1 (55/61) | 13% | 79% | 16.5 (40/58) | 33% | .03 | .02 | 0.62 (0.41, 0.94) | 0.02 | 95/118 | 0.67 (0.42, 1.05) | .08 |
| MLL-PTD | 56% | 16.2 (16/16) | 6% | 60% | 20.6 (10/15) | 33% | 1.00 | .056 | 0.46 (0.21, 1.04) | 0.06 | 26/30 | 0.60 (0.24, 1.54) | .29 |
| Subgroup . | Standard dose (45 mg/m2 per day) . | High dose (90 mg/m2 per day) . | Fisher’s exact P . | Log-rank P . | Univariate . | Multivariable* . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CR rate . | Median OS (months) (no. of events/total) . | 4-year OS . | CR rate . | Median OS (months) (no. of events/total) . | 4-year OS . | HR (95% CI) . | Wald P . | No. of events/total . | HR (95% CI) . | Wald P . | |||
| All patients | 59% | 16.6 (246/330) | 31% | 71% | 25.4 (207/327) | 39% | .001 | .001 | 0.74 (0.61, 0.89) | 0.001 | 450/653 | 0.74 (0.61, 0.89) | .001 |
| Age | |||||||||||||
| <50 years | 61% | 20.7 (133/188) | 35% | 73% | 44.7 (96/172) | 48% | .02 | .002 | 0.66 (0.50, 0.85) | 0.002 | 228/359 | 0.67 (0.52, 0.88) | .004 |
| ≥50 years | 56% | 12.6 (113/142) | 25% | 68% | 17.6 (111/155) | 28% | .03 | .12 | 0.81 (0.62, 1.06) | 0.12 | 222/294 | 0.82 (0.63, 1.07) | .14 |
| Cytogenetic | |||||||||||||
| Favorable | 84% | 39.4 (24/38) | 46% | 80% | NR (19/51) | 64% | .78 | .02 | 0.51 (0.28, 0.93) | 0.03 | 43/89 | 0.44 (0.24, 0.82) | .01 |
| Intermediate | 56% | 20.1 (101/141) | 35% | 77% | 33.5 (71/127) | 45% | .0003 | .01 | 0.68 (0.50, 0.92) | 0.01 | 172/267 | 0.75 (0.55,1.03) | .08 |
| Indeterminate | 62% | 14.3 (66/91) | 29% | 67% | 21.3 (62/85) | 29% | .53 | .47 | 0.88 (0.62, 1.24) | 0.47 | 127/175 | 0.89 (0.63, 1.27) | .53 |
| Unfavorable | 44% | 10.2 (54/59) | 14% | 57% | 10.6 (54/63) | 19% | .20 | .22 | 0.79 (0.54, 1.16) | 0.23 | 108/122 | 0.66 (0.44, 0.98) | .04 |
| Gene mutation | |||||||||||||
| NPM1 | 60% | 16.9 (50/65) | 29% | 89% | 75.9 (31/65) | 52% | .0002 | .002 | 0.50 (0.32, 0.78) | 0.002 | 81/130 | 0.51 (0.32, 0.81) | .005 |
| FLT3-ITD | 48% | 10.1 (74/83) | 17% | 70% | 15.2 (44/64) | 28% | .008 | .009 | 0.61 (0.42, 0.89) | 0.009 | 117/146 | 0.50 (0.32, 0.77) | .002 |
| DNMT3A | 61% | 14.1 (55/61) | 13% | 79% | 16.5 (40/58) | 33% | .03 | .02 | 0.62 (0.41, 0.94) | 0.02 | 95/118 | 0.67 (0.42, 1.05) | .08 |
| MLL-PTD | 56% | 16.2 (16/16) | 6% | 60% | 20.6 (10/15) | 33% | 1.00 | .056 | 0.46 (0.21, 1.04) | 0.06 | 26/30 | 0.60 (0.24, 1.54) | .29 |
NR, not reached.
Adjusted for sex, age, hemoglobin level, leukocyte count, platelet count, and cytogenetics.
Effect of age: HD daunorubicin benefits age <50
The median age of all patients was 48 years (range, 17-60 years) with 45.2% ≥50 years. For patients <50 years, the HR for death in the HD group was 0.66 (95% CI, 0.50-0.85; Wald P = .002; Table 1), with a median OS of 44.7 vs 20.7 months in the SD group (log-rank P = .002; supplemental Figure 1 available on the Blood Web site). In contrast, for patients aged ≥50 years, a significant benefit of HD daunorubicin was not confirmed (HR, 0.81; 95% CI, 0.62-1.06; Wald P = .12; supplemental Figure 1). Results were similar on multivariable analysis (Table 1).
Effect of cytogenetic profile: HD daunorubicin benefits patients with favorable and intermediate cytogenetic risk
Pretreatment cytogenetics showed 13.6% having favorable, 40.9% having intermediate, 18.6% having unfavorable, and 26.9% having indeterminate risk. On univariate analysis, HD daunorubicin benefited patients with either favorable or intermediate cytogenetics (supplemental Figure 2). In the favorable-risk group, the rate of CR was not different, but superior OS was seen in patients assigned to HD daunorubicin: median OS not reached compared with 39.4 months in the SD group (HR for death, 0.51; 95% CI, 0.28-0.93; Wald P = .03; Table 1). In the intermediate-risk group, HD daunorubicin led to both an improved CR rate and increased OS: median OS was 33.5 months for the HD group vs 20.1 months for SD (HR for death, 0.68; 95% CI, 0.50-0.92; Wald P = .01). On univariate analysis, neither an improvement in CR rate nor a survival benefit could be confirmed in the indeterminate (HR, 0.88; 95% CI, 0.62-1.24; Wald P = .47) and unfavorable-risk group (HR, 0.79; 95% CI, 0.54-1.16; Wald P = .23).
Adjusted analysis confirmed benefit in the favorable-risk group (HR, 0.44; 95% CI, 0.24-0.82; P = .01) and a trended benefit in the intermediate-risk groups (HR, 0.75; 95% CI, 0.55-1.03; P = .08). Additionally, we now demonstrated a benefit in patients in the unfavorable-risk group (HR, 0.66; 95% CI, 0.44-0.98; Wald P = .04; Table 1). In summary, with longer follow-up, the benefit of HD daunorubicin is shown to extend to patients with favorable cytogenetics, in addition to intermediate cytogenetic risk on univariate analysis, with adjusted analysis supporting these conclusions. A benefit for patients with unfavorable cytogenetic risk is suggested after controlling for other confounding factors.
Impact of HD daunorubicin on patients with FLT3-ITD, NPM1, or DNMT3A mutant AML, including older patients
We examined the benefit of HD daunorubicin in AML subgroups defined by gene mutations. We focused on previously examined groups (FLT3-ITD or MLL-PTD) and the 2 other most commonly mutant genes in our cohort: NPM1 (26%) and DNMT3A (24%). Details of analysis of gene data have been reported previously; the number of patients with other gene mutations together with the HR for death based on the updated survival data are shown in supplemental Figure 3.17,18
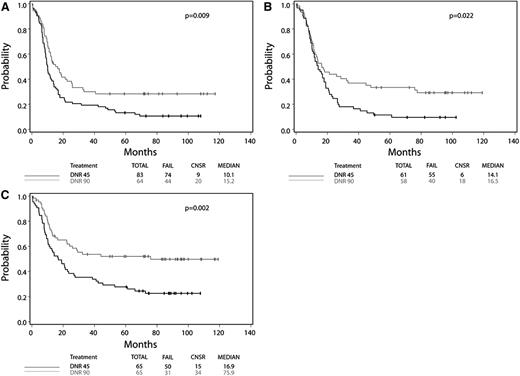
Patients with FLT3-ITD mutant AML benefited similarly from HD daunorubicin as patients with FLT3-ITD wild-type AML (FLT3-ITD mutant: HR, 0.61; 95% CI, 0.42-0.89; Wald P = .009; FLT3-ITD wild type: HR, 0.74; 95% CI, 0.59-0.92; Wald P = .008; Wald P for interaction = .35). Among patients with FLT3-ITD mutant AML, those assigned to HD daunorubicin had a median OS of 15.2 months compared with 10.1 months in the SD group. Additionally 4-year OS was 28% in the HD group compared with 17% in the SD group, suggesting that an additional 10% of FLT3-ITD mutant patients can be cured with HD daunorubicin (Figure 1A; Table 1).
Kaplan-Meier curves of FLT3-ITD, DNMT3A, and NPM1 mutant AML, by treatment assignment. Data for overall survival from the intention-to-treat analysis are shown for patients with (A) FLT3-ITD, (B) DNMT3A, and (C) NPM1 mutant AML according to assignment to SD daunorubicin (45 mg/m2 per day) or HD daunorubicin (90 mg/m2 per day).
Kaplan-Meier curves of FLT3-ITD, DNMT3A, and NPM1 mutant AML, by treatment assignment. Data for overall survival from the intention-to-treat analysis are shown for patients with (A) FLT3-ITD, (B) DNMT3A, and (C) NPM1 mutant AML according to assignment to SD daunorubicin (45 mg/m2 per day) or HD daunorubicin (90 mg/m2 per day).
Patients with DNMT3A mutant AML also benefited from HD daunorubicin, with the magnitude of benefit similar to patients with wild-type DNMT3A (DNMT3A mutant: HR, 0.62; 95% CI, 0.41-0.94; Wald P = .02; DNMT3A wild type: HR, 0.66; 95% CI, 0.52-0.85; Wald P = .001; Wald P for interaction = .79). Among DNMT3A mutant patients, median OS was longer in those receiving HD therapy compared with SD therapy (16.5 vs 14.1 months), with the proportion of patients alive at 4 years higher in the HD group (33%) compared with the SD group (13%; Figure 1B).
Finally, although patients with and without NPM1 mutations benefited from HD therapy, those with an NPM1 mutation derived more benefit (NPM1 wild type: HR, 0.70; 95% CI, 0.55-0.89; P = .003; NPM1 mutant: HR, 0.50; 95% CI, 0.32-0.78; Wald P = .002; Wald P for interaction = .17). Incredibly, patients with NPM1 mutant AML exposed to HD daunorubicin derived a 59-month increase in median OS (16.9- and 75.9-month median OS, respectively, for NPM1 mutant patients receiving SD and HD therapy) and an increase in 4-year OS from 29% to 52% (Figure 1C).
The rate of CR was also improved with HD therapy in patients with NPM1, DNMT3A, or FLT3-ITD mutant AML (Table 1). In sum, HD daunorubicin was shown in univariate analysis to benefit patients with mutant NPM1, DNMT3A, or FLT3-ITD; benefit was similar on adjusted analysis (Table 1). Only 31 patients had the MLL-PTD mutation, and no significant CR or OS benefit was observed.
We also examined the benefit of HD daunorubicin for NPM1, DNMT3A, FLT3-ITD, and MLL-PTD mutant AML separately within the younger (<50 years) and older (≥50-60 years) age strata. Patients under age 50 with NPM1 or DNMT3A mutant AML benefited from HD daunorubicin (univariate and adjusted analysis); benefit was demonstrated for younger patients with FLT3-ITD and MLL-PTD on adjusted analysis (top section, supplemental Table 1). In contrast, patients ≥50 years with NPM1 or FLT3-ITD mutant AML benefited from HD daunorubicin, but the benefit was nonsignificant for older patients with DNMT3A or MLL-PTD mutations (middle section, supplemental Table 1).
Additionally, when restricting to patients with intermediate cytogenetics, the benefit of HD daunorubicin for patients with NPM1, FLT3-ITD, and DNMT3A mutant AML was confirmed in the univariate analysis. The multivariable analysis supports the dose effect on NPM1 and a trended dose effect on DNMT3A (bottom section, supplemental Table 1).
Based on previous reports demonstrating that the prognostic impact of NPM1 and FLT3-ITD is not independent,18,25,26 we examined the benefit of HD daunorubicin in NPM1 and FLT3-ITD mutant patients considering mutations in the other gene. We confirmed in the multivariable analysis that the benefit of HD daunorubicin in NPM1 mutant patients exists regardless of FLT3-ITD mutant status (FLT3-ITD mutant: HR, 0.54; 95% CI, 0.27-1.08; Wald P = .08; FLT3-ITD wild type: HR, 0.45; 95% CI, 0.22-0.94; Wald P = .03; supplemental Table 2). We also assessed the impact of HD daunorubicin in NPM1 wild-type patients by FLT3-ITD status. We found significant benefit of HD daunorubicin in NPM1 wild-type patients exists for FLT3-ITD wild-type patients (HR, 0.69; 95% CI, 0.52-0.91; Wald P = .009) in the adjusted analysis. No statistically significant dose benefit was observed in NPM1 wild-type patients with FLT3-ITD mutation (HR, 0.69; 95% CI, 0.38-1.27; Wald P = .23). Of note, within the FLT3-ITD mutant subgroup, a test of interaction between treatment and NPM1 status was not significant (adjusted P = .27), indicating that the benefit of HD daunorubicin in FLT3-ITD mutant does not require concurrent NPM1 mutation.
Additionally, we assessed the benefit of HD daunorubicin in patients with IDH mutations given a previous report suggesting favorable prognosis in patients with NPM1+/FLT3-ITD−/IDH+ AML.18 Patients with IDH+ AML (n = 83) benefited from HD daunorubicin (HR, 0.58; 95% CI, 0.33-0.99; Wald P = .047); multivariable analysis confirmed benefit (HR, 0.56; 95% CI, 0.30-1.04; Wald P = .067). In the small subgroup of NPM1+/FLT3-ITD−/IDH+ patients (n = 27), a benefit was not confirmed on univariate analysis, but trended toward benefit in adjusted analysis (HR, 0.18; 95% CI, 0.03-1.24; Wald P = .08).
Impact of HD daunorubicin is concordant whether measured by an OS or an EFS end point
The benefit of HD daunorubicin was generally concordant whether assessed via an OS or an EFS end point (Table 2). Only patients with favorable cytogenetics demonstrated an OS benefit that was not confirmed in the EFS analysis (HR, 0.77; 95% CI, 0.45-1.32; Wald P = .34). Benefit of HD daunorubicin for patients with intermediate cytogenetics and for patients with NPM1, FLT3-ITD, and DNMT3A mutant AML was confirmed using an EFS end point.
Median EFS and HRs by treatment (HD vs SD), according to subgroup
| Subgroup . | Standard dose (45 mg/m2 per day) . | High dose (90 mg/m2 per day) . | P for rlps† . | Log-rank P for EFS . | Univariate . | Multivariable‡ . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4-year cum rlps rate* . | Median EFS (months) (no. of events/total) . | 4-year EFS . | 4-y Cum Rlps Rate . | Median EFS (months) (no. of events/total) . | 4-year EFS . | HR (95% CI) . | Wald P . | No. of events/total . | HR (95% CI) . | Wald P . | |||
| All patients | 57% | 4.5 (272/330) | 20% | 55% | 11.3 (235/327) | 28% | .26 | <.0001 | 0.71 (0.60, 0.85) | .0001 | 503/650 | 0.70 (0.59, 0.84) | <.0001 |
| Age | |||||||||||||
| <50 years | 58% | 5.0 (152/188) | 20% | 51% | 13.9 (112/172) | 36% | .06 | <.0001 | 0.62 (0.48, 0.79) | .0001 | 263/359 | 0.63 (0.49, 0.82) | .0004 |
| ≥50 years | 54% | 4.0 (120/142) | 19% | 58% | 9.4 (123/155) | 19% | .70 | .15 | 0.83 (0.65, 1.07) | .15 | 240/294 | 0.83 (0.64, 1.08) | .16 |
| Cytogenetic | |||||||||||||
| Favorable | 54% | 14.0 (25/38) | 36% | 39% | 17.2 (28/51) | 44% | .21 | .33 | 0.77 (0.45, 1.32) | .34 | 53/89 | 0.75 (0.42, 1.31) | .31 |
| Intermediate | 56% | 3.7 (116141) | 20% | 55% | 16.1 (84/127) | 33% | .22 | .0002 | 0.59 (0.44, 0.78) | .0002 | 199/267 | 0.63 (0.47, 0.84) | .002 |
| Indeterminate | 59% | 4.9 (74/91) | 20% | 69% | 8.7 (69/85) | 18% | .42 | .55 | 0.91 (0.65, 1.26) | .55 | 142/175 | 0.90 (0.64, 1.26) | .53 |
| Unfavorable | 54% | 0.8 (56/59) | 8% | 49% | 4.5 (53/63) | 16% | .77 | .052 | 0.69 (0.47, 1.01) | .06 | 109/122 | 0.63 (0.43, 0.93) | .02 |
| Gene mutation | |||||||||||||
| NPM1 | 61% | 4.0 (56/65) | 18% | 46% | 21.0 (38/65) | 41% | .19 | <.0001 | 0.45 (0.30, 0.68) | .0002 | 94/130 | 0.44 (0.29, 0.69) | .0003 |
| FLT3-ITD | 70% | 1.9 (78/83) | 8% | 61% | 6.8 (47/64) | 23% | .24 | .0009 | 0.55 (0.38, 0.79) | .001 | 124/146 | 0.51 (0.34, 0.75) | .0007 |
| DNMT3A | NA | 4.4 (58/61) | 5% | 69% | 9.4 (46/58) | 21% | .84 | .03 | 0.66 (0.45, 0.97) | .04 | 103/118 | 0.70 (0.46, 1.05) | .09 |
| MLL-PTD | NA | 5.2 (16/16) | 0% | NA | 8.7 (12/15) | 20% | .81 | .19 | 0.60 (0.28, 1.31) | .20 | 27/30 | 0.71 (0.27, 1.87) | .49 |
| Subgroup . | Standard dose (45 mg/m2 per day) . | High dose (90 mg/m2 per day) . | P for rlps† . | Log-rank P for EFS . | Univariate . | Multivariable‡ . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4-year cum rlps rate* . | Median EFS (months) (no. of events/total) . | 4-year EFS . | 4-y Cum Rlps Rate . | Median EFS (months) (no. of events/total) . | 4-year EFS . | HR (95% CI) . | Wald P . | No. of events/total . | HR (95% CI) . | Wald P . | |||
| All patients | 57% | 4.5 (272/330) | 20% | 55% | 11.3 (235/327) | 28% | .26 | <.0001 | 0.71 (0.60, 0.85) | .0001 | 503/650 | 0.70 (0.59, 0.84) | <.0001 |
| Age | |||||||||||||
| <50 years | 58% | 5.0 (152/188) | 20% | 51% | 13.9 (112/172) | 36% | .06 | <.0001 | 0.62 (0.48, 0.79) | .0001 | 263/359 | 0.63 (0.49, 0.82) | .0004 |
| ≥50 years | 54% | 4.0 (120/142) | 19% | 58% | 9.4 (123/155) | 19% | .70 | .15 | 0.83 (0.65, 1.07) | .15 | 240/294 | 0.83 (0.64, 1.08) | .16 |
| Cytogenetic | |||||||||||||
| Favorable | 54% | 14.0 (25/38) | 36% | 39% | 17.2 (28/51) | 44% | .21 | .33 | 0.77 (0.45, 1.32) | .34 | 53/89 | 0.75 (0.42, 1.31) | .31 |
| Intermediate | 56% | 3.7 (116141) | 20% | 55% | 16.1 (84/127) | 33% | .22 | .0002 | 0.59 (0.44, 0.78) | .0002 | 199/267 | 0.63 (0.47, 0.84) | .002 |
| Indeterminate | 59% | 4.9 (74/91) | 20% | 69% | 8.7 (69/85) | 18% | .42 | .55 | 0.91 (0.65, 1.26) | .55 | 142/175 | 0.90 (0.64, 1.26) | .53 |
| Unfavorable | 54% | 0.8 (56/59) | 8% | 49% | 4.5 (53/63) | 16% | .77 | .052 | 0.69 (0.47, 1.01) | .06 | 109/122 | 0.63 (0.43, 0.93) | .02 |
| Gene mutation | |||||||||||||
| NPM1 | 61% | 4.0 (56/65) | 18% | 46% | 21.0 (38/65) | 41% | .19 | <.0001 | 0.45 (0.30, 0.68) | .0002 | 94/130 | 0.44 (0.29, 0.69) | .0003 |
| FLT3-ITD | 70% | 1.9 (78/83) | 8% | 61% | 6.8 (47/64) | 23% | .24 | .0009 | 0.55 (0.38, 0.79) | .001 | 124/146 | 0.51 (0.34, 0.75) | .0007 |
| DNMT3A | NA | 4.4 (58/61) | 5% | 69% | 9.4 (46/58) | 21% | .84 | .03 | 0.66 (0.45, 0.97) | .04 | 103/118 | 0.70 (0.46, 1.05) | .09 |
| MLL-PTD | NA | 5.2 (16/16) | 0% | NA | 8.7 (12/15) | 20% | .81 | .19 | 0.60 (0.28, 1.31) | .20 | 27/30 | 0.71 (0.27, 1.87) | .49 |
cum rlps rate, cumulative relapse rate; NA, not applied due to no relapse time over 4 years on any patient in this group.
Among patients with complete remission reported.
Using the competing risk analysis by taking into account of death as a competing event.
Adjusted for sex, age, hemoglobin level, leukocyte count, platelet count, and cytogenetics.
HD daunorubicin is required for the favorable impact of NPM1 mutation
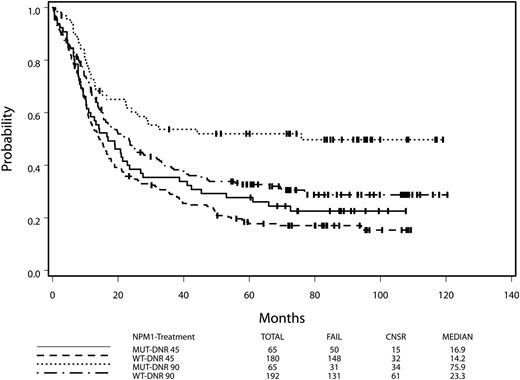
NPM1 mutant AML is associated with a favorable outcome compared with NPM1 wild-type AML.27 However, we found that the favorable impact of an NPM1 mutation depended on treatment assignment, although the interaction between the treatment assignment and the NPM1 mutation status was not significant (P = .17). In the HD group, the HR for death of NPM1 mutant AML compared with NPM1 wild-type AML was 0.60 (95% CI, 0.41-0.89; Wald P = .01), with NPM1 mutant patients having a median OS of 75.9 months (4-year OS, 52%) compared with 23.3 months in the wild-type group (4-year OS, 34%). In contrast, for the SD group, the HR for death (mutant/wild type) was not significant (HR, 0.84; 95% CI, 0.61-1.16; Wald P = .30; Figure 2), with median OS of 16.9 and 14.2 months for the mutant group and the wild-type group, respectively (log-rank P = .30). After adjusting for other prognostic factors plus FLT3-ITD, MLL-PTD, and DNMT3A, the prognostic impact of NPM1 was even stronger in the HD group (the HD group: HR [mutant/wild type] = 0.48; 95% CI, 0.30-0.78; Wald P = .003; the SD group: HR = 0.93, 95% CI, 0.63-1.38; Wald P = .71).
HD daunorubicin is necessary for benefit of the NPM1 mutation. Data for overall survival from the intention-to-treat analysis are shown for patients with NPM1 mutant and NPM1 wild-type AML by receipt of HD daunorubicin or SD daunorubicin.
HD daunorubicin is necessary for benefit of the NPM1 mutation. Data for overall survival from the intention-to-treat analysis are shown for patients with NPM1 mutant and NPM1 wild-type AML by receipt of HD daunorubicin or SD daunorubicin.
In contrast to NPM1, the prognostic impact of FLT3-ITD, MLL-PTD, and DNMT3A did not depend on treatment assignment (Wald P values for interaction are .35, .46, and .79, respectively).
Table 3 summarizes the HRs for death for each of the target genes stratified by treatment dose. In the context of SD treatment, FLT3-ITD and DNMT3A (but not NPM1) contribute to the OS prediction after adjusting for other confounding factors in the model. In contrast, in the setting of HD treatment, NPM1 and DNMT3A (but not FLT3-ITD) contribute to OS prediction after adjusting for other confounding factors.
HRs (mutant/wild type) for death, according to treatment dose and gene
| Gene . | Mutant . | Wildtype . | Log-rank P . | Univariate . | Multivariable* . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median OS (months) (no. of events/total) . | 4-year OS . | Median OS (months) (no. of events/total) . | 4-year OS . | HR (95% CI) . | Wald P . | No. of events/total . | HR (95% CI) . | Wald P . | ||
| Main effect | ||||||||||
| NPM1 | 23.5 (81/130) | 40% | 16.9 (279/372) | 29% | .01 | 0.73 (0.57, 0.94) | .01 | 0.68 (0.50, 0.92) | .01 | |
| FLT3-ITD | 11.7 (118/147)) | 22% | 25.0 (303/456)) | 38% | <.0001 | 1.65 (1.33, 2.04) | <.0001 | 338/477 | 1.67 (1.28, 2.19) | .0002 |
| DNMT3A | 15.3 (95/119) | 23% | 22.8 (254/371) | 36% | .03 | 1.31 (1.03, 1.66) | .03 | 338/477338/477 | 1.54 (1.18, 2.01) | .002 |
| MLL-PTD | 16.9 (26/31) | 19% | 21.0 (405/586) | 35% | .13 | 1.36 (0.92, 2.03) | .13 | 338/477 | 0.80 (0.49, 1.30) | .36 |
| Standard dose | ||||||||||
| NPM1 | 16.9 (50/65) | 29% | 14.2 (148/180) | 24% | .30 | 0.84 (0.61, 1.16) | .30 | 186/232 | 0.93 (0.63, 1.38) | .71 |
| FLT3-ITD | 10.1 (74/83) | 17% | 20.3 (156/215)) | 34% | <.0001 | 1.77 (1.34, 2.33) | <.0001 | 186/232 | 2.10 (1.45, 3.05) | <.0001 |
| DNMT3A | 14.1 (55/61) | 13% | 16.1 (137/177) | 30% | .06 | 1.35 (0.98, 1.85) | .06 | 186/232 | 1.47 (1.03, 2.10) | .04 |
| MLL-PTD | 16.2 (16/16) | 6% | 16.5 (220/290) | 30% | .08 | 1.58 (0.95, 2.63) | .08 | 186/232 | 1.01 (0.54, 1.90) | .98 |
| High dose | ||||||||||
| NPM1 | 75.9 (31/65) | 52% | 23.3 (131/192) | 34% | .01 | 0.60 (0.41, 0.89) | .01 | 152/245 | 0.48 (0.30, 0.78) | .003 |
| FLT3-ITD | 15.2 (44/64) | 28% | 31.7 (147/241) | 42% | .04 | 1.43 (1.02, 2.01) | .04 | 152/245 | 1.35 (0.89, 2.04) | .16 |
| DNMT3A | 16.5 (40/58) | 33% | 29.2 (117/194) | 41% | .23 | 1.25 (0.87, 1.79) | .23 | 152/245 | 1.51 (0.99, 2.31) | .06 |
| MLL-PTD | 20.6 (10/15) | 33% | 27.4 (185/296) | 40% | .68 | 1.14 (0.61, 2.16) | .68 | 152/245 | 0.61 (0.27, 1.36) | .23 |
| Gene . | Mutant . | Wildtype . | Log-rank P . | Univariate . | Multivariable* . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median OS (months) (no. of events/total) . | 4-year OS . | Median OS (months) (no. of events/total) . | 4-year OS . | HR (95% CI) . | Wald P . | No. of events/total . | HR (95% CI) . | Wald P . | ||
| Main effect | ||||||||||
| NPM1 | 23.5 (81/130) | 40% | 16.9 (279/372) | 29% | .01 | 0.73 (0.57, 0.94) | .01 | 0.68 (0.50, 0.92) | .01 | |
| FLT3-ITD | 11.7 (118/147)) | 22% | 25.0 (303/456)) | 38% | <.0001 | 1.65 (1.33, 2.04) | <.0001 | 338/477 | 1.67 (1.28, 2.19) | .0002 |
| DNMT3A | 15.3 (95/119) | 23% | 22.8 (254/371) | 36% | .03 | 1.31 (1.03, 1.66) | .03 | 338/477338/477 | 1.54 (1.18, 2.01) | .002 |
| MLL-PTD | 16.9 (26/31) | 19% | 21.0 (405/586) | 35% | .13 | 1.36 (0.92, 2.03) | .13 | 338/477 | 0.80 (0.49, 1.30) | .36 |
| Standard dose | ||||||||||
| NPM1 | 16.9 (50/65) | 29% | 14.2 (148/180) | 24% | .30 | 0.84 (0.61, 1.16) | .30 | 186/232 | 0.93 (0.63, 1.38) | .71 |
| FLT3-ITD | 10.1 (74/83) | 17% | 20.3 (156/215)) | 34% | <.0001 | 1.77 (1.34, 2.33) | <.0001 | 186/232 | 2.10 (1.45, 3.05) | <.0001 |
| DNMT3A | 14.1 (55/61) | 13% | 16.1 (137/177) | 30% | .06 | 1.35 (0.98, 1.85) | .06 | 186/232 | 1.47 (1.03, 2.10) | .04 |
| MLL-PTD | 16.2 (16/16) | 6% | 16.5 (220/290) | 30% | .08 | 1.58 (0.95, 2.63) | .08 | 186/232 | 1.01 (0.54, 1.90) | .98 |
| High dose | ||||||||||
| NPM1 | 75.9 (31/65) | 52% | 23.3 (131/192) | 34% | .01 | 0.60 (0.41, 0.89) | .01 | 152/245 | 0.48 (0.30, 0.78) | .003 |
| FLT3-ITD | 15.2 (44/64) | 28% | 31.7 (147/241) | 42% | .04 | 1.43 (1.02, 2.01) | .04 | 152/245 | 1.35 (0.89, 2.04) | .16 |
| DNMT3A | 16.5 (40/58) | 33% | 29.2 (117/194) | 41% | .23 | 1.25 (0.87, 1.79) | .23 | 152/245 | 1.51 (0.99, 2.31) | .06 |
| MLL-PTD | 20.6 (10/15) | 33% | 27.4 (185/296) | 40% | .68 | 1.14 (0.61, 2.16) | .68 | 152/245 | 0.61 (0.27, 1.36) | .23 |
Sex, age, hemoglobin level, leukocyte count, platelet count, cytogenetics, and all 4 genes were fitted into the model.
Effect of molecular mutation on benefit of gemtuzumab
Of 307 patients registered to autologous HCT, 153 (49.8%) received planned therapy. In an intention-to-treat analysis, we confirmed no benefit of GO overall, and no benefit was seen in any age or cytogenetic strata except that patients with intermediate cytogenetics had inferior OS if they were assigned to receive GO (supplemental Table 3). Additionally, there was no GO benefit in patients with NPM1, DNMT3A, FLT3-ITD, or MLL-PTD mutant AML. Results were similar when analysis was restricted to patients who actually received autologous transplant, although the unfavorable impact of GO on patients with intermediate cytogenetics was no longer seen (supplemental Table 4).
Discussion
Many factors impact survival after an AML diagnosis including patient characteristics, disease characteristics, and therapy received. Although patient and disease characteristics are typically immutable, modification of the therapy administered has the potential to improve outcomes. Here, we present updated results of the E1900 trial and demonstrate the broad ability of anthracycline intensification during induction to improve OS in patients age 60 or younger with de novo AML, including those with favorable and intermediate cytogenetics and those with mutations in FLT3-ITD, NPM1, and DNMT3A. A benefit in those with unfavorable cytogenetic risk is suggested on multivariable analysis; however, a larger study focused on this patient population would be needed to confirm the benefit.
The ECOG-ACRIN study, E1900, joins a series of studies attempting to improve the anthracycline plus cytarabine regimen that has long been a standard for AML induction in younger patients.1,2 At the time of study initiation, all previous attempts to improve OS with the addition of a third drug or by increasing the dose of cytarabine had been unsuccessful (although since the initial publication of E1900, an OS benefit of gemtuzumab ozogamicin, cladribine, and HD cytarabine has been demonstrated in subgroups).1,3-12 E1900 studied whether an increased dose of daunorubicin used during induction could increase rates of remission and, more importantly, improve OS. The results of E1900 reported in 2009 confirmed the benefit of anthracycline intensification in younger patients in AML: an exciting and long-awaited improvement in a decades-old regimen.1,2 The results were additionally significant because HD daunorubicin was not associated with additional toxicity, obviating difficult discussions about benefit vs potential morbidity. The benefit of HD daunorubicin has since been confirmed prospectively in both younger28 and older patients,29 as well as by a retrospective meta-analysis.30
The celebration of the original results was tempered by the determination that not all subgroups benefited; in particular, patients with high-risk features such as older age (age ≥50 years), unfavorable cytogenetics, and FLT3-ITD mutant AML did not appear to derive benefit.17 However, we continued to follow patients, and mature data now suggest broader benefit in high-risk patients: patients with unfavorable cytogenetics were shown to benefit in multivariable analysis (suggesting need for further study and confirmation) and benefit was confirmed on both univariate and multivariable analysis for those with FLT3-ITD mutant AML. The 4-year OS (which is arguably a surrogate for cure) is 10% higher in the patients with FLT3-ITD mutant AML receiving HD therapy.
We also demonstrate a benefit for HD daunorubicin in patients with NPM1 and DNMT3A mutant AML, with a particularly marked advantage seen in patients with NPM1 mutant AML. NPM1 mutant patients receiving HD daunorubicin had a remarkable increase in median OS (75.9 vs 16.9 months) and a >20% increase in 4-year OS (52% vs 29%), indicating that HD daunorubicin offers a meaningful increase in OS (and likely cure) to the approximately one-fourth of AML patients who carry an NPM1 mutation, regardless of FLT3-ITD status.
The findings in the NPM1 mutant group were additionally significant because we demonstrate that the known prognostic impact of NPM1 depended on treatment assignment, an effect that persisted after adjusting for other prognostic factors and common co-occurring mutations (FLT3-ITD and DNMT3A).27 This highlights the importance of interpreting prognostic studies in the context of the specific treatment received. Indeed, after adjustment, we found that FLT3-ITD was only prognostic in the setting of SD treatment, whereas DNMT3A was prognostic regardless of daunorubicin dose. Additionally, our updated analysis confirms that, although patients aged 50 to 60 years do not benefit overall, a substantial subgroup of older patients with NPM1 or FLT3-ITD mutant AML do benefit from HD daunorubicin. Finally, we show that the benefit of HD daunorubicin as measured by OS is largely concordant to conclusions drawn by analysis of an EFS end point, suggesting that EFS may be a reliable end point in AML clinical trials.
In contrast to our study, the UK AML17 trial comparing 90 to 60 mg/m2 of daunorubicin during the first induction course for untreated AML has reported excess early mortality without improvement in OS. It is important to recognize the significant differences in induction strategy and cumulative anthracycline dose between E1900 and UK AML 17. In E1900, only patients with residual leukemic blasts at nadir marrow assessment received a second course of induction such that only patients positioned to benefit were exposed to additional toxicity (38% of those who ultimately achieved CR received a second cycle). In contrast, in UK AML 17, a second course of induction including 50 mg/m2 per day of daunorubicin for 3 days was given regardless of response to first induction; this approach may have led to overtreatment and excess toxicity in the cohort that negated any OS benefit. In addition, the standard arm in UK AML 17 received a cumulative dose of 110 mg/m2 of daunorubicin; this dose exceeds the dose that the majority of patients assigned to the HD arm in E1900 received. In essence, both arms of UK AML 17 received HD daunorubicin by E1900 definition, and the UK AML 17 investigators demonstrated that further dose escalation (via a standard second induction course for a cumulative daunorubicin dose of 140 mg/m2 in the HD arm) did not provide further benefit.25
We additionally present results regarding the impact of HD daunorubicin on OS for a larger group of gene mutations; these results, however, need to be interpreted with caution due to the rarity of mutation incidences. Further study is required to confirm the impact of daunorubicin dose on these subgroups. We also lack data on FLT3-ITD allelic ratio, and therefore the impact of this variable on the benefit of HD daunorubicin in the FLT3-ITD subgroup will require further investigation.
With longer follow-up, we were still unable to confirm a benefit of HD daunorubicin in older patients (aged 50-60 years), whereas Lowenberg et al29 demonstrated a significant improvement in OS in patients aged 60 to 65 years receiving HD daunorubicin. The reason for this discrepancy is not entirely clear, although the administration of HD daunorubicin in Lowenberg et al was in the context of a higher dose of cytarabine (200 mg/m2 per day for 7 days), and additionally, all patients received a second cycle of chemotherapy (cytarabine) without additional daunorubicin.
In sum, in this updated analysis of the E1900 trial, we demonstrate that HD daunorubicin benefits AML patients with FLT3-ITD mutations, may benefit patients with unfavorable cytogenetics, and additionally demonstrates benefit in a significant subgroup of older patients aged 50 to 60 years with FLT3-ITD or NPM1 mutations. We also demonstrate that NPM1 mutant patients particularly benefit from HD therapy and that the favorable prognosis associated with presence of an NPM1 is uniquely associated with higher-dose therapy.
With demonstration of the expanded benefit of HD daunorubicin, we suggest that HD daunorubicin be the standard for all eligible adult patients up to 60 years of age and be the standard of comparison for future clinical trials. Additionally, as we enter an era of targeted therapy, including for FLT3-ITD mutant AML in the upfront setting,31 it is important that these agents be tested in the context of optimal chemotherapy.
Presented in abstract form at the 56th annual meeting of the American Society of Hematology, San Francisco, CA, December 6-9, 2014.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was conducted by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD, and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA180820, CA180794, CA180867, CA180791, CA180790, CA180583, and CA189859, the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services.
This study’s content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute
Authorship
Contribution: M. R. Luskin, S.M.L., J.-W.L., and Z.S. designed the analysis and wrote the manuscript; H.F.F., H.M.L., M.R.L., J.R., M.S.T., and S.M.L. provided patients; M. R. Luskin, S.M.L., J.-W.L., Z.S., H.F.F., O.A.-W., J.M.B., R.P.K., H.M.L., R.L.L., M. R. Litzow, E.M.P., J.P.P., J.R., J.M.R., and M.S.T. analyzed and interpreted data and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Selina Luger, Division of Hematology and Oncology, Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, 3400 Civic Center Blvd, Philadelphia, PA 19104; e-mail: selina.luger@uphs.upenn.edu.