Key Points
Germline GATA2 mutations account for 15% of advanced and 7% of all primary pediatric MDS and do not influence overall survival.
The majority (72%) of adolescents with MDS and monosomy 7 carry an underlying GATA2 deficiency.
Abstract
Germline GATA2 mutations cause cellular deficiencies with high propensity for myeloid disease. We investigated 426 children and adolescents with primary myelodysplastic syndrome (MDS) and 82 cases with secondary MDS enrolled in 2 consecutive prospective studies of the European Working Group of MDS in Childhood (EWOG-MDS) conducted in Germany over a period of 15 years. Germline GATA2 mutations accounted for 15% of advanced and 7% of all primary MDS cases, but were absent in children with MDS secondary to therapy or acquired aplastic anemia. Mutation carriers were older at diagnosis and more likely to present with monosomy 7 and advanced disease compared with wild-type cases. For stratified analysis according to karyotype, 108 additional primary MDS patients registered with EWOG-MDS were studied. Overall, we identified 57 MDS patients with germline GATA2 mutations. GATA2 mutations were highly prevalent among patients with monosomy 7 (37%, all ages) reaching its peak in adolescence (72% of adolescents with monosomy 7). Unexpectedly, monocytosis was more frequent in GATA2-mutated patients. However, when adjusted for the selection bias from monosomy 7, mutational status had no effect on the hematologic phenotype. Finally, overall survival and outcome of hematopoietic stem cell transplantation (HSCT) were not influenced by mutational status. This study identifies GATA2 mutations as the most common germline defect predisposing to pediatric MDS with a very high prevalence in adolescents with monosomy 7. GATA2 mutations do not confer poor prognosis in childhood MDS. However, the high risk for progression to advanced disease must guide decision-making toward timely HSCT.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1518.
Disclosures
The authors, Associate Editor Mary C. Dinauer, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Describe the prevalence of germline GATA2 mutations among children and adolescents with myelodysplastic syndrome (MDS), based on 2 consecutive prospective studies.
Compare other genetic features and clinical characteristics of germline GATA2 mutation carriers with wild-type cases of MDS, based on 2 consecutive prospective studies and stratified analysis according to karyotype.
Determine the clinical implications of genetic findings on pediatric MDS, based on 2 consecutive prospective studies and stratified analysis according to karyotype.
Release date: March 17, 2016; Expiration date: March 17, 2017
Introduction
The study of cancer genomes has identified a countless number of somatic gene mutations associated with malignancy.1 Inherited genetic drivers predisposing to clonal evolution, are, however, less well understood. Myelodysplastic syndromes (MDSs) represent a heterogeneous group of clonal hematopoietic disorders occurring with various frequencies in all age groups. Significant differences between primary MDSs in children and adults are evident for morphology, cytogenetics, and therapeutic approaches. In children and adolescents, refractory cytopenia of childhood (RCC) represents the most common subtype usually associated with pancytopenia and hypocellular marrow2,3 ; monosomy 7 is the most frequent aberration in children, whereas aberrations typically observed in adult MDS such as complex karyotypes or isolated del(5q) are rare.4 The somatic mutation landscape also differs between children and adults with myeloid diseases.5-7 Inherited genetic predisposition was thus far not considered a likely cause of de novo primary MDS, in contrast to MDS evolving from known inherited bone marrow failure (IBMF) syndromes such as Fanconi anemia or dyskeratosis congenita8 and the familial MDS/acute myeloid leukemia (AML) syndromes.9,10 The latter result from deficiencies of the hematopoietic transcription factors CEBPA, RUNX1,11,12 and GATA2.13 The spectrum of GATA2-related disease additionally involves immunodeficiencies14-16 and syndromic features such as congenital deafness and lymphedema originally defining Emberger syndrome.17 Patients present early in life with symptoms affecting hematopoietic, immune, and lymphatic systems, complicated by systemic infections and a high risk of developing MDS/AML18,19 However, no estimates exist on the prevalence and clinical significance of germline GATA2 mutations within consecutive MDS cohorts. This makes it difficult giving advice on the expected outcomes and recommendations on evidence-based clinical care.
The GATA2 gene encodes a chief hematopoietic transcription factor that through its 2 zinc fingers (ZFs) can occupy GATA DNA motifs in several thousand genes.20,21 Its major role in early hematopoiesis22-24 is attained by cooperating in a complex network of transcription factors in a dose-dependent manner.23-27 The GATA2 germline mutational landscape involves truncating mutations presumably resulting in loss of the second ZF (ZF2).15 In addition, missense mutations within ZF2 and noncoding variants in the +9.5-kb regulatory region of GATA2 are thought to result in haploinsufficiency.13,17 Hence, the term GATA2 deficiency or haploinsufficiency has been widely accepted to describe GATA2-spectrum disorders; in the following, both GATA2-mutated and GATA2-deficient are used interchangeably. It is noteworthy that somatic GATA2 mutations in adult myeloid neoplasia can occur in both ZF regions28,29 and can have loss- or gain-of-function effects.28,30,31 In this study we aimed to assess the frequency of germline GATA2 mutations that underlie pediatric MDS and to define clinical characteristics, therapeutic response, and prognostic consequences of this predisposition syndrome.
Methods
Study population
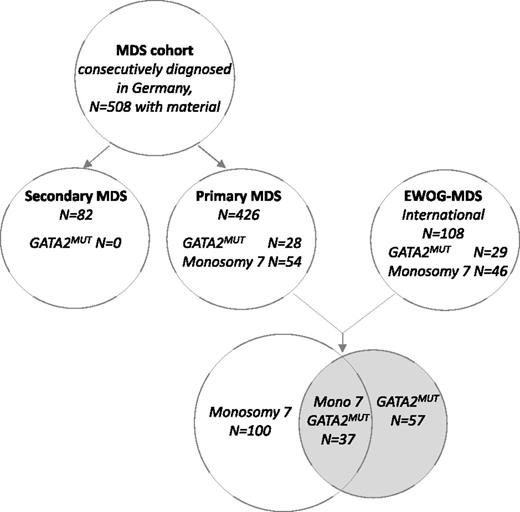
MDS was classified according to the World Health Organization criteria for pediatric MDS and included RCC and the advanced MDS subtypes, namely refractory anemia with excess blasts (RAEB) and RAEB in transformation (RAEB-t).32 We studied a consecutive cohort of 508 children and adolescents with material available for genetic testing (out of 544 enrolled) diagnosed with MDS in Germany between July 1, 1998 and June 30, 2013 and enrolled in the prospective studies 98 or 2006 (www.clinicaltrials.gov; #NCT00047268, #NCT00662090) of the European Working Group of MDS in Childhood (EWOG-MDS). Of the 508 patients, 426 had been diagnosed with primary MDS (including familial MDS defined as ≥1 first-degree relative suffering from hematological neoplasia) and 82 with MDS secondary to chemo- or radiation therapy or to acquired severe aplastic anemia. Patients with known IBMF disorders had been excluded from all analyses. For stratified analysis according to karyotype, we next extended the cohort of primary MDS by including a group of 108 additional patients registered in EWOG-MDS trials (Figure 1). The additional group was purposely biased toward the major cytogenetic aberrations (monosomy 7 and trisomy 8) encountered in patients with GATA2 deficiency.
For metaphase karyotyping, chromosome banding analyses of diagnostic bone marrow (BM) specimens had been performed according to standard procedures.4 Hematopoietic stem cell transplantation (HSCT) was performed in accordance with the EWOG-MDS recommendations (www.ewog-mds.org). All studies were approved by the institutional ethics committees of the respective institutions. Written informed consent was obtained from patients or patients’ guardians.
Genetic studies
Sanger sequencing of exons 2 to 6 and intron 4 in the GATA2 gene (NM_032638.4) was applied to DNA from BM or peripheral blood (PB) granulocytes. The presence of heterozygous polymorphisms ruled out a whole gene deletion and simplified selection of patients for copy number analysis (single nucleotide polymorphism array-based deletion analysis; Affymetrix 6.0, Santa Clara, CA). For germline investigations, nonmyeloid specimens were used according to the following priority: (1) cultured primary skin fibroblasts, (2) hair follicles, and (3) purified T cells. The feasibility of T-cell DNA to discriminate between germline and somatic events in patients prior to HSCT had previously been established for patients with somatic mutations such as ASXL1, detected only in the myeloid lineage but not in T cells (V.P., S.H., M.W.W., unpublished results, July 1, 2015). Novel GATA2 variants reported here were scored as likely pathogenic and disease associated according to the following criteria: (1) presence of identical mutation in other family member with GATA2-related MDS, (2) presence of known syndromic GATA2-related phenotype (eg, lymphedema in the affected individuals), (3) mutation previously reported in GATA2 deficiency, (4) novel mutation not present in controls in the Exome Aggregation Consortium browser (exac.broadinstitute.org) nor in the dbSNP database (ncbi.nlm.nih.gov/SNP) and exome variant server (evsgs.washington.edu), and (5) high conservation across other species and high pathogenicity score (scale-invariant feature transform, mutation taster).
Statistical analysis
Overall survival (OS) was defined as the time from diagnosis to death or last follow-up, whereas event-free survival (EFS) was defined as the time from HSCT to death, disease recurrence, or last follow-up. The Kaplan-Meier method was used to estimate survival rates, and the 2-sided log-rank test was used to evaluate the equality of the survivorship functions in different subgroups. Time-to-event outcomes for relapse and transplant-related mortality (TRM) were estimated using cumulative incidence curves, using relapse and TRM as the respective competing risks. The χ2 test was used to examine the statistical significance of the association between GATA2 status and categorized factors. Fisher's exact test was calculated for 2 × 2 contingency analyses. Nonparametric statistics were used to test for differences in continuous variables in terms of mutational status (Mann-Whitney U test). All P values were 2 sided, and values <.05 were considered to be statistically significant. Software packages SPSS for Windows 20.0.0 (IBM Corp, New York, NY) and NCSS 2004 (NCSS, Kaysville, UT) were used.
Results
Prevalence and phenotype of GATA2-related MDS
BM or PB samples of 508 children and adolescents with primary or secondary MDS enrolled in 2 consecutive prospective studies were analyzed (Figure 1). In 28 of the 426 (7%) patients with primary MDS, 24 distinct GATA2 mutations were identified (Table 1). The constitutional nature of mutations was established in all cases tested. In contrast, no mutations were found among the 82 patients with secondary MDS. Accounting for the MDS subtype, GATA2 mutations were more prevalent in advanced MDS (15%, 13 of 85) compared with RCC (4%, 15 of 341, P < .01). GATA2 mutated (GATA2mut) patients were older at diagnosis (median age, 12.3 vs 10.3 years; P < .01) and presented more often with advanced MDS (46% vs 18%, P < .01) and with monosomy 7 (70% vs 11%, P < .01; Table 1) compared with the GATA2 wild-type (GATA2WT) group. Surprisingly, in the majority (71%) of the GATA2mut group, MDS occurred sporadically without family history of hematologic malignancy.
Characteristics of primary MDS with GATA2 deficiency in a consecutive population-based cohort
| . | Parameter . | GATA2mut (N = 28) . | GATA2WT (N = 398) . | P value . |
|---|---|---|---|---|
| Mutations | Total/distinct | 28/24 | 0 | |
| Age | Median (range), years | 12.3 (5.2-17.4) | 10.3 (0.2-18.1) | <.05 |
| Sex | Males, N = 248 | 15 (54%) | 233 (58%) | n.s. |
| Females, N = 178 | 13 (46%) | 165 (42%) | ||
| Subtype | RCC, N = 341 | 15 (54%) | 326 (82%) | <.01 |
| RAEB/RAEB-t, N = 85 | 13 (46%) | 72 (18%) | ||
| Karyotype* | Monosomy 7,† N = 54 | 19 (70%) | 35 (11%) | <.01 |
| Structural complex, N = 9 | 0 (0%) | 9 (3%) | ||
| Other, N = 30 | 1 (4%)‡ | 29 (9%) | ||
| Normal, N = 253 | 7 (26%) | 246 (77%) | ||
| Familial disease | Index patients (%) | 8 (29%) | 8 (2%) | <.01 |
| . | Parameter . | GATA2mut (N = 28) . | GATA2WT (N = 398) . | P value . |
|---|---|---|---|---|
| Mutations | Total/distinct | 28/24 | 0 | |
| Age | Median (range), years | 12.3 (5.2-17.4) | 10.3 (0.2-18.1) | <.05 |
| Sex | Males, N = 248 | 15 (54%) | 233 (58%) | n.s. |
| Females, N = 178 | 13 (46%) | 165 (42%) | ||
| Subtype | RCC, N = 341 | 15 (54%) | 326 (82%) | <.01 |
| RAEB/RAEB-t, N = 85 | 13 (46%) | 72 (18%) | ||
| Karyotype* | Monosomy 7,† N = 54 | 19 (70%) | 35 (11%) | <.01 |
| Structural complex, N = 9 | 0 (0%) | 9 (3%) | ||
| Other, N = 30 | 1 (4%)‡ | 29 (9%) | ||
| Normal, N = 253 | 7 (26%) | 246 (77%) | ||
| Familial disease | Index patients (%) | 8 (29%) | 8 (2%) | <.01 |
n.s., not significant.
Karyotypes at diagnosis were informative in 346 of 426 patients (GATA2 mutated: n = 27; wild type: n = 319).
Includes monosomy 7 with 1 or 2 additional aberrations.
Includes 1 patient with der(1;7)(q10;p10) and trisomy 8.
Hematologic phenotype associated with GATA2 deficiency is determined by monosomy 7
Investigating PB findings of the consecutive cohort at diagnosis, we observed significantly higher hemoglobin concentrations and platelet counts, as well as higher numbers of myeloid precursors among patients with GATA2 deficiency compared with GATA2WT (supplemental Table 1, available on the Blood Web site). Unexpectedly, there were more GATA2mut cases with monocytosis (>1.0 × 109/L) compared with GATA2WT (P < .01).
Because the majority of GATA2mut patients presented with MDS and monosomy 7, we reassessed the above findings in an extended cohort purposely augmented with this cytogenetic category. Within the subgroup of patients with monosomy 7, hematologic parameters did not differ according to mutational status with the exception of a lower ratio of myeloid to erythroid cells in the BM of GATA2mut patients (supplemental Figure 1; supplemental Table 2). Thus, the above differences in hematologic phenotype between GATA2mut and GATAWT groups appears to have been determined by monosomy 7 rather than by GATA2 mutations.
GATA2-related MDS is associated with der(1;7) and trisomy 8 in addition to monosomy 7
A total of 57 pediatric MDS patients with GATA2 deficiency could be identified in the extended cohort (Figure 1). The abnormal karyotypes of these cases fall in only 3 categories: monosomy 7 in 68% of patients, unbalanced aberration der(1;7) (resulting in loss of the q-arm of chromosome 7) in 7%, and trisomy 8 in 9% of patients (Table 2). Neither del(5q) nor structurally complex aberrations were identified.
Cytogenetic abnormalities in GATA2-related childhood MDS
| Karyotype . | Patients, N (%) N = 56 . | MDS subtype . | |
|---|---|---|---|
| RCC . | RAEB/RAEB-t . | ||
| Monosomy 7* | 38 (68%) | 17 | 16/5 |
| Der(1;7)(q10;p10)† | 4 (7%) | 1 | 3/0 |
| Trisomy 8‡ | 5 (9%) | 3 | 1/1 |
| Normal | 9 (16%) | 9 | 0/0 |
| Karyotype . | Patients, N (%) N = 56 . | MDS subtype . | |
|---|---|---|---|
| RCC . | RAEB/RAEB-t . | ||
| Monosomy 7* | 38 (68%) | 17 | 16/5 |
| Der(1;7)(q10;p10)† | 4 (7%) | 1 | 3/0 |
| Trisomy 8‡ | 5 (9%) | 3 | 1/1 |
| Normal | 9 (16%) | 9 | 0/0 |
Karyotypes were available for 56 of 57 GATA2-deficient patients.
Includes 5 patients with an additional aberration [trisomy 8, trisomy 21, trisomy 22, del(12)(p11p13), del(18q)].
Includes 2 patients with additional trisomy 8 and 1 patient each with trisomy 11 or a marker chromosome.
Includes 1 patient with a marker chromosome.
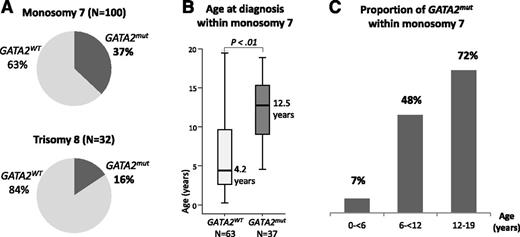
We next calculated the prevalence of GATA2 deficiency within the specific cytogenetic categories. Of the 449 patients analyzed, 100 had monosomy 7 and 32 had trisomy 8. Within the monosomy 7 subgroup, one third (37%) carried GATA2 mutations (Figure 2A), and the median age at diagnosis was significantly higher for GATA2mut patients compared with the wild-type group (12.5 vs 4.2 years, P < .01; Figure 2B). Of note, the youngest child with GATA2-related MDS with monosomy 7 was 4.4 years old at time of diagnosis. The prevalence of GATA2 mutations increased dramatically with age, reaching 72% in adolescents aged 12 to 19 years at diagnosis of MDS associated with monosomy 7 (Figure 2C). Among MDS patients with trisomy 8, 16% (5/32) had an underlying GATA2 deficiency (Figure 2A). Their median age was 15.3 (11.4-17.1) years. The 4 patients with MDS and der(1;7) translocation were also carriers of GATA2 mutations (data not shown). Thus, 7% of the GATA2mut patients carried a der(1;7) translocation (Table 2).
Prevalence of GATA2 deficiency in MDS patients with monosomy 7 and trisomy 8. (A) Proportion of GATA2 mutated and wild-type patients within subgroups of (top) monosomy 7 and (bottom) trisomy 8. (B) Boxplots with age distribution of patients with monosomy 7 according to GATA2 mutational status. (C) Prevalence of GATA2 deficiency in patients with monosomy 7 subgroup stratified by age groups.
Prevalence of GATA2 deficiency in MDS patients with monosomy 7 and trisomy 8. (A) Proportion of GATA2 mutated and wild-type patients within subgroups of (top) monosomy 7 and (bottom) trisomy 8. (B) Boxplots with age distribution of patients with monosomy 7 according to GATA2 mutational status. (C) Prevalence of GATA2 deficiency in patients with monosomy 7 subgroup stratified by age groups.
Landscape of germline GATA2 mutations in childhood MDS
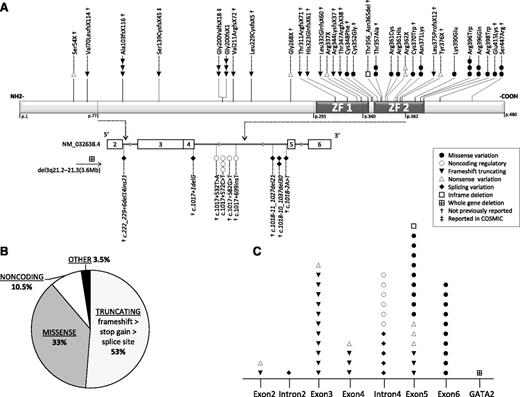
In total, we identified 57 GATA2mut patients with 44 distinct (31 novel) germline mutations (Figure 3A-B; Table 3) with the following predicted effects: 18 (16 novel) truncating frameshift/stop-gain in 22 patients; 5 (all novel) splice site in 6 patients; 13 (5 novel) missense in 17 patients; 4 (3 novel) noncoding intron 4 variants in 6 cases, and in 1 patient each a novel 30-bp in-frame deletion and a novel heterozygous germline deletion involving the entire GATA2 locus: del(3)(q21.2q21.3), chr3:126 637 078-130 282 197(NCBI36/hg18). All missense mutations were confined to exons 5 and 6 (Figure 3A,C), corresponding to ZF2 (amino acids 347-398) with the exception of mutations C-terminal to ZF2: Glu415Lys (novel) and a previously reported mutation Ser447Arg33 (Figure 3A). Both mutations affect highly conserved amino acids and were scored as deleterious by prediction algorithms. Noncoding mutations in intron 4 clustered between nucleotides +526 and +699 adjacent to the donor splice site c.1017 (Figure 3A, bottom). All the other mutations with predicted structural effect (nonsense, splice site, deletions) occurred randomly prior to the C-terminal end of ZF2. Two different truncating mutations, counted as novel in our cohort, had been previously reported in solid tumors in the COSMIC somatic mutation database; however, information on material source and germline status was inconclusive.34 Finally, no correlation between the type of GATA2 mutation and the hematologic phenotype was identified (data not shown).
Landscape of GATA2 mutations in childhood MDS. (A) Distinct mutations identified in 57 patients are shown. The presumed functional effect of mutations is depicted by symbols as shown in the legend box. Top panel depicts the GATA2 protein structure with the known DNA-binding zinc fingers 1 (ZF1) and 2 (ZF2). GATA2 gene structure (NM_032638.4) is shown below and begins with exon 2 (first coding exon). Relevant introns represented by gray line are stretched out (ie, intron 4 that contains the regulatory sites E-box, GATA, and ETS). Italic font depicts splice site variants. Mutations previously reported in the Catalogue of Somatic Mutations in Cancer (COSMIC) database were Ser139CysfsX45 in pancreas carcinoma (ID COSS2068125), and Gly200ValfsX18 in a patient with adenocarcinoma of the colon (ID COSS1650947); both mutations were confirmed as germline in our patients. (B) Pie chart depicting the proportion of patients with different mutation types; 53% of patients carry truncating mutations, among them, frameshift being the most common followed by stop gain and splice site mutations. (C) Distribution of distinct mutations identified in 57 patients in relation to their localization within the gene.
Landscape of GATA2 mutations in childhood MDS. (A) Distinct mutations identified in 57 patients are shown. The presumed functional effect of mutations is depicted by symbols as shown in the legend box. Top panel depicts the GATA2 protein structure with the known DNA-binding zinc fingers 1 (ZF1) and 2 (ZF2). GATA2 gene structure (NM_032638.4) is shown below and begins with exon 2 (first coding exon). Relevant introns represented by gray line are stretched out (ie, intron 4 that contains the regulatory sites E-box, GATA, and ETS). Italic font depicts splice site variants. Mutations previously reported in the Catalogue of Somatic Mutations in Cancer (COSMIC) database were Ser139CysfsX45 in pancreas carcinoma (ID COSS2068125), and Gly200ValfsX18 in a patient with adenocarcinoma of the colon (ID COSS1650947); both mutations were confirmed as germline in our patients. (B) Pie chart depicting the proportion of patients with different mutation types; 53% of patients carry truncating mutations, among them, frameshift being the most common followed by stop gain and splice site mutations. (C) Distribution of distinct mutations identified in 57 patients in relation to their localization within the gene.
Detailed mutational and clinical outcome data of pediatric patients with MDS and GATA2 deficiency
| Patient no. . | Sex . | GATA2 mutation . | Genotype . | Age at dx (years) . | Initial (highest) MDS subtype . | Karyotype at dx . | Therapy . | Preparative regimen . | Donor . | SC source . | Outcome . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A044 | F | FsX* | c.303delC | p.Ala103fsX116 | 12.4 | RCC | Der(1;7) add | HSCT | Bu-based | MSD | BM | CR (>9.5 y) |
| A056 | M | Splice site* | c.1018-11_1027del21 | p.? | 16.1 | RAEB | −7 | HSCT | Bu-based | MSD | BM | CR (>4.5 y) |
| B002 | M | FsX* | c.968dupA | p.His323GlnfsX61 | 14.5 | RCC(RAEB) | −7 | HSCT | Bu-based | MSD | PB | CR (>6.6 y) |
| B032 | F | Missense* | c.1046G>T | p.Cys348Phe | 12.7 | RAEB-t | −7 | HSCT | Bu-based | MSD | BM | CR (>4.0 y) |
| CZ041 | M | Inframe deletion* | c.1066_1095del30 | p.Thr356_Asn365del | 15.7 | RCC(RAEB) | −7 | HSCT | Bu-based | UD | BM | CR (>9.0 y) |
| CZ053 | M | FsX* | c.1035_1038dupCGGC | p.Thr347ArgfsX38 | 4.4 | RAEB | −7 | HSCT | Bu-based | MSD | BM | CR (>7.2 y) |
| CZ054 | M | Intron 4 | c.1017+572C>T | p.= | 17 | RCC | −7 | HSCT | Bu-based | UD | BM | CR (>7.0 y) |
| CZ061 | M | Intron 4 | c.1017+572C>T | p.= | 17.5 | RCC | Normal | HSCT | Bu-based | MSD | BM | CR (>3.9 y) |
| CZ087 | M | Splice site* | c.222_229+6del14ins21 | p.? | 11.4 | RCC | +8 | HSCT | Treo-based | UD | PB | CR (>1.0 y) |
| D076 | M | FsX* | c.627_630dupCGGC | p.Val211ArgfsX72 | 12.5 | RCC(RAEB) | −7 | HSCT | Bu-based | UD | PB | CR (>13.4 y) |
| D1010 | M | Stop gain* | c.1128C>G | p.Tyr376X | 12.7 | RCC | Normal | HSCT | Treo-based | UD | BM | CR (>0.4 y) |
| D314 | F | FsX | c.599dupG | p.Gly200fsX1 | 10 | RAEB | −7, add | HSCT | Bu-based | UD | PB | Died, TRM (infection 3.5 mo after HSCT) |
| D342 | M | FsX† | c.416_417delCT | p.Ser139CysfsX45 | 16.6 | RCC(RAEB) | −7 | HSCT | Bu-based | UD | BM | Died, relapse 4 y after HSCT |
| D350 | M | FsX* | c.207_208delCG | p.Val70LeufsX114 | 11 | RAEB | Der(1;7) add | HSCT | Bu-based | MSD | PB | CR (>5.3 y) |
| D415 | F | Missense | c.1341C>A | p.Ser447Arg | 13.6 | RAEB | −7 | HSCT | Bu-based | UD | PB | CR (>7.0 y) |
| D427 | F | Missense | c.1187G>A | p.Arg396Gln | 12.9 | RAEB | −7, add | HSCT | Bu-based | MSD | PB | CR (>10.1 y) |
| D429 | F | FsX† | c.599delG | p.Gly200ValfsX18 | 16.3 | RCC | Normal | HSCT | RIC | UD | PB | CR (>9.6 y) |
| D479 | F | Missense | c.1186C>T | p.Arg396Trp | 16.8 | RCC | Normal | HSCT | Other | UD | PB | CR (>4.9 y) |
| D569 | F | FsX* | c.1031_1049del19 | p.Arg344LysfsX37 | 10.3 | RCC | Normal | HSCT | RIC | MSD | BM | CR (>5.0 y) |
| D612 | M | FsX* | c.685delC | p.Leu229CysfsX5 | 7.5 | RCC | −7 | HSCT | Bu-based | MSD | BM | CR (>6.3 y) |
| D680 | M | Splice site* | c.1018-10_1037del30 | p.? | 12.1 | RCC | −7 | HSCT | Bu-based | UD | BM | CR (>6.5 y) |
| D726 | F | FsX* | c.303delC | p.Ala103fsX116 | 9.6 | RAEB | −7 | HSCT | Bu-based | MSD | BM | CR (>6.3 y) |
| D731 | F | FsX* | c.306delA | p.Ala103fsX116 | 5.2 | RAEB | −7, add | HSCT | Bu-based | UD | PB | CR (>4.3 y) |
| D794 | F | Stop gain | c.1084C>T | p.Arg362X | 6.1 | RCC(RAEB) | −7, add | HSCT | Bu-based | UD | BM | CR (>3.8 y) |
| D801 | M | Missense | c.1187G>A | p.Arg396Gln | 15.6 | RCC | Normal | HSCT | Treo-based | UD | PB | CR (>1.0 y) |
| D807 | M | Splice site* | c.1018-10_1037del30 | p.? | 13.8 | RCC | Normal | HSCT | Treo-based | UD | BM | CR (>1.4 y) |
| D983 | M | Intron 4* | c.1017+582G>T | p.= | 13.4 | RCC | Normal | HSCT | RIC | MSD | BM | CR (>1.9 y) |
| I112 | F | Stop gain* | c.802G>A | p.Gly268X | 17.1 | RAEB-t | +8 | HSCT | Bu-based | UD | BM | Died, 1.4 y after HSCT |
| I198 | M | FsX* | c.970_994dup25 | p.Leu332GlnfsX60 | 18.6 | RCC | −7 | HSCT | Bu-based | MSD | BM | Died, TRM (infection 6.5 mo after HSCT) |
| I301 | M | Missense | c.1081C>T | p.Arg361Cys | 10.7 | RAEB | −7 | HSCT | n.a. | n.a. | n.a. | Died, TRM (GVHD 1.5 mo after HSCT) |
| I305 | M | Stop gain* | c.161C>A | p.Ser54X | 18 | RAEB-t(MDR-AML) | −7 | HSCT | Treo-based | UD | BM | CR (>3.6 y) |
| I306 | M | Missense | c.1186C>T | p.Arg396Trp | 14.3 | RCC | Normal | HSCT | Treo-based | UD | PB | CR (>2.8 y) |
| NL097 | M | Missense | c.1341C>A | p.Ser447Arg | 12.9 | RCC(RAEB-t) | −7 | HSCT | Bu-based | MSD | BM | Died, TRM (infection 2 mo after HSCT) |
| NL113 | F | Missense | c.1168A>G | p.Lys390Glu | 12.8 | RAEB | Der(1;7) add | HSCT | Bu-based | UD | PB | CR (>6.2 y) |
| PL027 | M | Stop gain | c.1009C>T | p.Arg337X | 14.9 | RCC | −7 | HSCT | Bu-based | UD | PB | CR (>5.9 y) |
| SC152 | F | FsX | c.599dupG | p.Gly200fsX1 | 12.2 | RAEB | −7 | HSCT | Bu-based | UD | BM | CR (>2.0 y) |
| D271 | M | Missense | c.1186C>T | p.Arg396Trp | 12.5 | RAEB | −7 | HSCT | Bu-based | UD | PB | Relapse 1.8 y after 1.HSCT; CR after 2.HSCT (>4.2 y) |
| D492 | M | Splice site* | c.1017+1delG | p.? | 17.4 | RCC(RAEB) | −7 | HSCT | Bu-based | MMFD | BM | Relapse 2 y after 1.HSCT; Died, relapse 6 mo after 2.HSCT |
| D762 | M | Missense | c.1113C>G | p.Asn371Lys | 9.7 | RAEB(RAEB-t) | −7 | HSCT | Treo-based | UD | PB | Relapse 1.7 y after 1.HSCT; CR after 2.HSCT |
| D420 | F | Gene Δ* | del3q21.2–21.3 | p.= | 15 | RCC(RAEB-t) | −7 | HSCT | TBI-based | MMFD§ | PB | CR (>7.3 y) |
| I126 | F | Splice site* | c.1018-2A>T | p.? | 5.1 | RCC(RAEB) | −7 | HSCT | TBI-based | MMFD§ | PB | CR (>10.2 y) |
| I199 | M | FsX* | c.1124delT | p.Leu375PrsX12 | 7.8 | RAEB(RAEB-t) | −7 | HSCT | TBI-based | MMFD§ | PB | CR (>4.7 y) |
| D621 | M | Missense | c.1192C>T | p.Arg398Trp | 15.9 | RCC | −7 | HSCT | Other | MMFD§ | PB | GF after 1.HSCT; Died, TRM (GF/microangiopathy after 2.HSCT) |
| D147 | M | Stop gain | c.1084C>T | p.Arg362X | 14.2 | RCC(RAEB) | −7, add | AML-th, HSCT | TBI-based | UD | BM | Died, infection 11.5 mo after HSCT |
| D151 | F | Missense | c.1113C>A | p.Asn371Lys | 16 | RCC(MDR-AML) | +8, add | AML-th, HSCT | Bu-based | UD | PB | CR (>0.1 y) |
| D245 | M | Missense* | c.1110C>G | p.Cys370Trp | 8.7 | RAEB-t | −7 | AML-th, HSCT | n.a. | UD | n.a. | Died, TRM (GVHD/infection, 4 mo after HSCT |
| D770 | F | FsX† | c.599delG | p.Gly200ValfsX18 | 10.2 | RAEB | −7, add | AML-th, HSCT | Bu-based | MSD | PB | Died, relapse 13 mo after HSCT |
| D907 | F | FsX* | c.932_937delinsG | p.Thr311ArgfsX71 | 7.5 | RAEB(MDR-AML) | −7 | AML-th, HSCT | Bu-based | UD | BM | CR (>3.5 y) |
| D955 | F | FsX | c.599dupG | p.Gly200fsX1 | 11.4 | RAEB-t | −7 | AML-th, HSCT | Treo-based | UD | BM | Died, relapse 1 y after HSCT |
| NL116 | F | Missense* | c.1069A>G | p.Thr357Ala | 15.3 | RAEB | Der(1;7) add | AML-th, HSCT | Treo-based | MMFD§ | PB | CR (>5.3 y) |
| CZ057 | M | Intron 4 | c.1017+572C>T | p.= | 17.4 | RAEB(MDR-AML) | −7 | AML-th | Died, disease progress 6 mo after dx | |||
| D184 | F | Missense* | c.1054T>G | p.Cys352Gly | 8.8 | RAEB(MDR-AML) | −7 | AML-th | Died, disease progress 2.2 y after dx | |||
| SC021 | F | Missense* | c.1243G>A | p.Glu415Lys | 13.7 | RAEB(MDR-AML) | +8 | AML-th | Died, infection during AML-th, 2 y after dx | |||
| D418 | M | Missense | c.1082G>A | p.Arg361His | 7.3 | RAEB-t | −7 | Supportive | Died, disease progress 1 mo after dx | |||
| D506 | M | Intron 4* | c.1017+699insT | p.= | 13.5 | RCC | n.a. | w&w | Alive with disease (last FUP 8 y after dx) | |||
| D609 | M | Intron 4* | c.1017+532T>A | p.= | 3.1 | RCC | Normal | w&w | Alive with disease (last FUP 16 y after dx) | |||
| NL134 | F | Missense* | c.1069A>G | p.Thr357Ala | 15.3 | RCC | +8 | w&w | Alive with disease (last FUP 5 y after dx) | |||
| Patient no. . | Sex . | GATA2 mutation . | Genotype . | Age at dx (years) . | Initial (highest) MDS subtype . | Karyotype at dx . | Therapy . | Preparative regimen . | Donor . | SC source . | Outcome . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A044 | F | FsX* | c.303delC | p.Ala103fsX116 | 12.4 | RCC | Der(1;7) add | HSCT | Bu-based | MSD | BM | CR (>9.5 y) |
| A056 | M | Splice site* | c.1018-11_1027del21 | p.? | 16.1 | RAEB | −7 | HSCT | Bu-based | MSD | BM | CR (>4.5 y) |
| B002 | M | FsX* | c.968dupA | p.His323GlnfsX61 | 14.5 | RCC(RAEB) | −7 | HSCT | Bu-based | MSD | PB | CR (>6.6 y) |
| B032 | F | Missense* | c.1046G>T | p.Cys348Phe | 12.7 | RAEB-t | −7 | HSCT | Bu-based | MSD | BM | CR (>4.0 y) |
| CZ041 | M | Inframe deletion* | c.1066_1095del30 | p.Thr356_Asn365del | 15.7 | RCC(RAEB) | −7 | HSCT | Bu-based | UD | BM | CR (>9.0 y) |
| CZ053 | M | FsX* | c.1035_1038dupCGGC | p.Thr347ArgfsX38 | 4.4 | RAEB | −7 | HSCT | Bu-based | MSD | BM | CR (>7.2 y) |
| CZ054 | M | Intron 4 | c.1017+572C>T | p.= | 17 | RCC | −7 | HSCT | Bu-based | UD | BM | CR (>7.0 y) |
| CZ061 | M | Intron 4 | c.1017+572C>T | p.= | 17.5 | RCC | Normal | HSCT | Bu-based | MSD | BM | CR (>3.9 y) |
| CZ087 | M | Splice site* | c.222_229+6del14ins21 | p.? | 11.4 | RCC | +8 | HSCT | Treo-based | UD | PB | CR (>1.0 y) |
| D076 | M | FsX* | c.627_630dupCGGC | p.Val211ArgfsX72 | 12.5 | RCC(RAEB) | −7 | HSCT | Bu-based | UD | PB | CR (>13.4 y) |
| D1010 | M | Stop gain* | c.1128C>G | p.Tyr376X | 12.7 | RCC | Normal | HSCT | Treo-based | UD | BM | CR (>0.4 y) |
| D314 | F | FsX | c.599dupG | p.Gly200fsX1 | 10 | RAEB | −7, add | HSCT | Bu-based | UD | PB | Died, TRM (infection 3.5 mo after HSCT) |
| D342 | M | FsX† | c.416_417delCT | p.Ser139CysfsX45 | 16.6 | RCC(RAEB) | −7 | HSCT | Bu-based | UD | BM | Died, relapse 4 y after HSCT |
| D350 | M | FsX* | c.207_208delCG | p.Val70LeufsX114 | 11 | RAEB | Der(1;7) add | HSCT | Bu-based | MSD | PB | CR (>5.3 y) |
| D415 | F | Missense | c.1341C>A | p.Ser447Arg | 13.6 | RAEB | −7 | HSCT | Bu-based | UD | PB | CR (>7.0 y) |
| D427 | F | Missense | c.1187G>A | p.Arg396Gln | 12.9 | RAEB | −7, add | HSCT | Bu-based | MSD | PB | CR (>10.1 y) |
| D429 | F | FsX† | c.599delG | p.Gly200ValfsX18 | 16.3 | RCC | Normal | HSCT | RIC | UD | PB | CR (>9.6 y) |
| D479 | F | Missense | c.1186C>T | p.Arg396Trp | 16.8 | RCC | Normal | HSCT | Other | UD | PB | CR (>4.9 y) |
| D569 | F | FsX* | c.1031_1049del19 | p.Arg344LysfsX37 | 10.3 | RCC | Normal | HSCT | RIC | MSD | BM | CR (>5.0 y) |
| D612 | M | FsX* | c.685delC | p.Leu229CysfsX5 | 7.5 | RCC | −7 | HSCT | Bu-based | MSD | BM | CR (>6.3 y) |
| D680 | M | Splice site* | c.1018-10_1037del30 | p.? | 12.1 | RCC | −7 | HSCT | Bu-based | UD | BM | CR (>6.5 y) |
| D726 | F | FsX* | c.303delC | p.Ala103fsX116 | 9.6 | RAEB | −7 | HSCT | Bu-based | MSD | BM | CR (>6.3 y) |
| D731 | F | FsX* | c.306delA | p.Ala103fsX116 | 5.2 | RAEB | −7, add | HSCT | Bu-based | UD | PB | CR (>4.3 y) |
| D794 | F | Stop gain | c.1084C>T | p.Arg362X | 6.1 | RCC(RAEB) | −7, add | HSCT | Bu-based | UD | BM | CR (>3.8 y) |
| D801 | M | Missense | c.1187G>A | p.Arg396Gln | 15.6 | RCC | Normal | HSCT | Treo-based | UD | PB | CR (>1.0 y) |
| D807 | M | Splice site* | c.1018-10_1037del30 | p.? | 13.8 | RCC | Normal | HSCT | Treo-based | UD | BM | CR (>1.4 y) |
| D983 | M | Intron 4* | c.1017+582G>T | p.= | 13.4 | RCC | Normal | HSCT | RIC | MSD | BM | CR (>1.9 y) |
| I112 | F | Stop gain* | c.802G>A | p.Gly268X | 17.1 | RAEB-t | +8 | HSCT | Bu-based | UD | BM | Died, 1.4 y after HSCT |
| I198 | M | FsX* | c.970_994dup25 | p.Leu332GlnfsX60 | 18.6 | RCC | −7 | HSCT | Bu-based | MSD | BM | Died, TRM (infection 6.5 mo after HSCT) |
| I301 | M | Missense | c.1081C>T | p.Arg361Cys | 10.7 | RAEB | −7 | HSCT | n.a. | n.a. | n.a. | Died, TRM (GVHD 1.5 mo after HSCT) |
| I305 | M | Stop gain* | c.161C>A | p.Ser54X | 18 | RAEB-t(MDR-AML) | −7 | HSCT | Treo-based | UD | BM | CR (>3.6 y) |
| I306 | M | Missense | c.1186C>T | p.Arg396Trp | 14.3 | RCC | Normal | HSCT | Treo-based | UD | PB | CR (>2.8 y) |
| NL097 | M | Missense | c.1341C>A | p.Ser447Arg | 12.9 | RCC(RAEB-t) | −7 | HSCT | Bu-based | MSD | BM | Died, TRM (infection 2 mo after HSCT) |
| NL113 | F | Missense | c.1168A>G | p.Lys390Glu | 12.8 | RAEB | Der(1;7) add | HSCT | Bu-based | UD | PB | CR (>6.2 y) |
| PL027 | M | Stop gain | c.1009C>T | p.Arg337X | 14.9 | RCC | −7 | HSCT | Bu-based | UD | PB | CR (>5.9 y) |
| SC152 | F | FsX | c.599dupG | p.Gly200fsX1 | 12.2 | RAEB | −7 | HSCT | Bu-based | UD | BM | CR (>2.0 y) |
| D271 | M | Missense | c.1186C>T | p.Arg396Trp | 12.5 | RAEB | −7 | HSCT | Bu-based | UD | PB | Relapse 1.8 y after 1.HSCT; CR after 2.HSCT (>4.2 y) |
| D492 | M | Splice site* | c.1017+1delG | p.? | 17.4 | RCC(RAEB) | −7 | HSCT | Bu-based | MMFD | BM | Relapse 2 y after 1.HSCT; Died, relapse 6 mo after 2.HSCT |
| D762 | M | Missense | c.1113C>G | p.Asn371Lys | 9.7 | RAEB(RAEB-t) | −7 | HSCT | Treo-based | UD | PB | Relapse 1.7 y after 1.HSCT; CR after 2.HSCT |
| D420 | F | Gene Δ* | del3q21.2–21.3 | p.= | 15 | RCC(RAEB-t) | −7 | HSCT | TBI-based | MMFD§ | PB | CR (>7.3 y) |
| I126 | F | Splice site* | c.1018-2A>T | p.? | 5.1 | RCC(RAEB) | −7 | HSCT | TBI-based | MMFD§ | PB | CR (>10.2 y) |
| I199 | M | FsX* | c.1124delT | p.Leu375PrsX12 | 7.8 | RAEB(RAEB-t) | −7 | HSCT | TBI-based | MMFD§ | PB | CR (>4.7 y) |
| D621 | M | Missense | c.1192C>T | p.Arg398Trp | 15.9 | RCC | −7 | HSCT | Other | MMFD§ | PB | GF after 1.HSCT; Died, TRM (GF/microangiopathy after 2.HSCT) |
| D147 | M | Stop gain | c.1084C>T | p.Arg362X | 14.2 | RCC(RAEB) | −7, add | AML-th, HSCT | TBI-based | UD | BM | Died, infection 11.5 mo after HSCT |
| D151 | F | Missense | c.1113C>A | p.Asn371Lys | 16 | RCC(MDR-AML) | +8, add | AML-th, HSCT | Bu-based | UD | PB | CR (>0.1 y) |
| D245 | M | Missense* | c.1110C>G | p.Cys370Trp | 8.7 | RAEB-t | −7 | AML-th, HSCT | n.a. | UD | n.a. | Died, TRM (GVHD/infection, 4 mo after HSCT |
| D770 | F | FsX† | c.599delG | p.Gly200ValfsX18 | 10.2 | RAEB | −7, add | AML-th, HSCT | Bu-based | MSD | PB | Died, relapse 13 mo after HSCT |
| D907 | F | FsX* | c.932_937delinsG | p.Thr311ArgfsX71 | 7.5 | RAEB(MDR-AML) | −7 | AML-th, HSCT | Bu-based | UD | BM | CR (>3.5 y) |
| D955 | F | FsX | c.599dupG | p.Gly200fsX1 | 11.4 | RAEB-t | −7 | AML-th, HSCT | Treo-based | UD | BM | Died, relapse 1 y after HSCT |
| NL116 | F | Missense* | c.1069A>G | p.Thr357Ala | 15.3 | RAEB | Der(1;7) add | AML-th, HSCT | Treo-based | MMFD§ | PB | CR (>5.3 y) |
| CZ057 | M | Intron 4 | c.1017+572C>T | p.= | 17.4 | RAEB(MDR-AML) | −7 | AML-th | Died, disease progress 6 mo after dx | |||
| D184 | F | Missense* | c.1054T>G | p.Cys352Gly | 8.8 | RAEB(MDR-AML) | −7 | AML-th | Died, disease progress 2.2 y after dx | |||
| SC021 | F | Missense* | c.1243G>A | p.Glu415Lys | 13.7 | RAEB(MDR-AML) | +8 | AML-th | Died, infection during AML-th, 2 y after dx | |||
| D418 | M | Missense | c.1082G>A | p.Arg361His | 7.3 | RAEB-t | −7 | Supportive | Died, disease progress 1 mo after dx | |||
| D506 | M | Intron 4* | c.1017+699insT | p.= | 13.5 | RCC | n.a. | w&w | Alive with disease (last FUP 8 y after dx) | |||
| D609 | M | Intron 4* | c.1017+532T>A | p.= | 3.1 | RCC | Normal | w&w | Alive with disease (last FUP 16 y after dx) | |||
| NL134 | F | Missense* | c.1069A>G | p.Thr357Ala | 15.3 | RCC | +8 | w&w | Alive with disease (last FUP 5 y after dx) | |||
add, one additional cytogenetic aberration (excluding translocations); AML-th, AML-like therapy; Bu-based, busulfan-based conditioning; CR, complete remission (time from last HSCT); dx, diagnosis; fsX, frameshift truncating mutation; FUP, follow-up; Gene Δ, whole gene deletion; GF, graft failure; intron 4, noncoding mutation in intron 4; MDR-AML, MDS-related acute myeloid leukemia; MMFD, mismatched family donor; MSD, matched sibling donor; n.a., data not available; RIC, reduced intensity conditioning; SC source, stem cell source; TBI-based, total body irradiation–based conditioning; trunc, truncating; w&w, watch and wait; −7, monosomy 7; +8, trisomy 8; treo-based, treosulfan-based conditioning; UD, unrelated donor.
Novel mutation, previously not reported.
Mutation previously reported only in somatic setting (COSMIC database).
Haploidentical transplantation.
Inheritance and penetrance
Detailed family history was available for 53 GATA2mut patients. Familial MDS/AML had been identified in 12 study patients of 9 pedigrees (supplemental Figure 2A). All affected relatives were diagnosed with MDS/AML as children or young adults. The remaining 41 study patients with sporadic MDS showed unremarkable family history. For 11 of those, the unaffected family members (parents ± siblings) were investigated for the presence of GATA2 mutations (supplemental Figure 2B). Although no silent carriers for coding mutations were identified, in 3 families with noncoding mutations found in the index case, the same variant was also present in the respective fathers who were clinically healthy. In an additional 7 patients with sporadic MDS and coding GATA2 mutations who received HSCT from a matched sibling, the respective mutation was not identified in donor blood.
Nonhematologic phenotype in pediatric MDS cohort with GATA2 deficiency
Previously reported nonhematologic phenotypes of GATA2 deficiency (syndromic by means of Emberger-like features or signs of immunodeficiency associated with MonoMAC [monocytopenia, B and natural killer cell lymphopenia and mycobacterial, fungal, and viral infections]/DCML [dendritic cell, monocyte, and lymphocyte deficiency]) were identified in a total of 51% (29 of 57) of studied patients. In detail, 9% (5 of 57) of patients presented with deafness, 23% (13 of 57) with lymphedema/hydrocele, and 39% (22 of 57) with immunodeficiency; some patients presented with multiple symptoms. Additional novel clinical features recurrently present in the investigated cohort included anomalies of the urogenital tract in 12% (7 of 57) and relevant behavioral problems (eg, autism and aggressive behavior) in 19% (11 of 57) of GATA2mut patients.
GATA2 deficiency is not a poor prognostic factor for OS
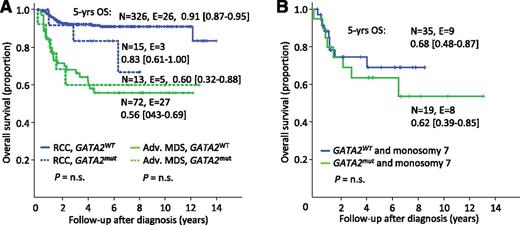
Among the 426 consecutively diagnosed children with primary MDS (Figure 1), 5-year OS was, as expected, significantly superior in the RCC subgroup compared with the advanced MDS subgroup (91% vs 57%, P < .01; supplemental Figure 3A). When evaluating OS according to mutational status alone, the GATA2mut group had an inferior OS compared with GATA2WT (73% vs 84%, P < .05; supplemental Figure 3B). However, when accounting for MDS subtype and presence of monosomy 7, there was no difference in OS in relation to GATA2 mutational status (Figure 4A-B).
OS according to MDS subtypes and GATA2 mutational status. (A) OS in 426 children and adolescents consecutively diagnosed with primary MDS according to MDS subtypes and GATA2 mutational status. (B) Overall survival in 100 children and adolescents with monosomy 7 according to GATA2 mutational status. Adv. MDS, advanced MDS subtypes; n.s., not significant; N, patients under risk; E, events; GATA2mut, mutation carriers; GATA2WT, patients with wild-type status.
OS according to MDS subtypes and GATA2 mutational status. (A) OS in 426 children and adolescents consecutively diagnosed with primary MDS according to MDS subtypes and GATA2 mutational status. (B) Overall survival in 100 children and adolescents with monosomy 7 according to GATA2 mutational status. Adv. MDS, advanced MDS subtypes; n.s., not significant; N, patients under risk; E, events; GATA2mut, mutation carriers; GATA2WT, patients with wild-type status.
Stable disease is rare in GATA2-related MDS
Of the 57 children with MDS and GATA2 deficiency, 7 were not transplanted. Four of those children died of disease progression or infection after AML-like chemotherapy or supportive care. The remaining 3 patients with stable non–transfusion-dependent RCC are still alive without severe infectious complications 5 to 16 years from diagnosis (Table 3). Of note, 2 of these 3 patients had novel noncoding mutations in GATA2 intron 4 (D506 and D609).
Outcome of HSCT is not influenced by GATA2 mutational status
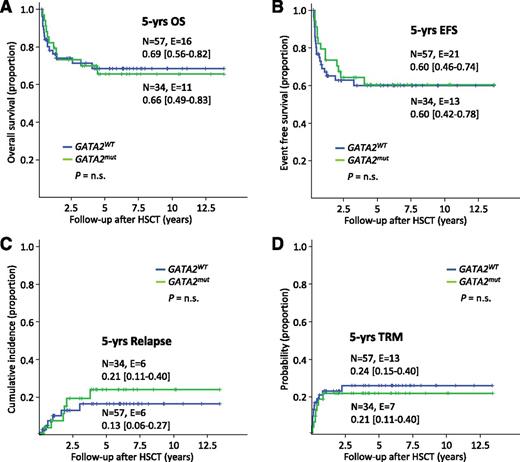
Among the cohort of 100 patients with monosomy 7 (Figure 1), 91 including 34 GATA2 mutation carriers had been transplanted. The 5-year OS (66% vs 69%) and EFS (60% for both) were comparable between the GATA2mut and GATA2WT groups (Figure 5A-B). The relapse rate did not significantly differ between both groups (21% vs 13%; Figure 5C). Of note, TRM was independent of underlying GATA2 deficiency (21% vs 24%; Figure 5D) as was the rate of infectious complications (66% vs 61%, P = not significant). Specifically, evaluating viral, bacterial, fungal, parasitic infections, cytomegalovirus reactivation, cytomegalovirus disease, Epstein-Barr virus infections/post-transplant lymphoproliferative disorder, and adenovirus infections, the rate of complication was 66% (33 of 50) in the GATA2mut and 61% (168 of 276) in the GATA2WT groups. Across all karyotypes, AML-like chemotherapy prior to HSCT was administered to 7 patients only; 4 of those died due to TRM or relapse.
Outcome from HSCT based on GATA2 status in patients with monosomy 7. Kaplan-Meier survival curves and cumulative incidence curves according to GATA2 mutational status in 91 patients with monosomy 7 undergoing HSCT. (A) OS, (B) EFS, (C) incidence of relapse, and (D) probability of TRM. n.s., not significant; N, patients under risk; E, events.
Outcome from HSCT based on GATA2 status in patients with monosomy 7. Kaplan-Meier survival curves and cumulative incidence curves according to GATA2 mutational status in 91 patients with monosomy 7 undergoing HSCT. (A) OS, (B) EFS, (C) incidence of relapse, and (D) probability of TRM. n.s., not significant; N, patients under risk; E, events.
Discussion
Outside the setting of known IBMF disorders and familial MDS/AML, little has been known about the contribution of hereditary predisposition to the etiology of primary MDS. This study shows for the first time that GATA2 germline mutations underlie 7% of consecutively diagnosed pediatric cases with primary MDS, and 15% of advanced MDS. In comparison, the prevalence of germline GATA2 mutations in a cohort of 586 adult patients with MDS and MDS/MPN patients reached only 0.5% (3 of 7 patients with GATA2 mutations; J.P.M., Cleveland Clinic MDS registry, personal communication, January 1, 2015). The absence of GATA2 mutations in secondary MDS of childhood and their rarity in adult MDS reinforces the notion that different etiologies are involved in these conditions. Drawing from histories of our patients and from published findings, it can be concluded that GATA2-related disease generally manifests in adolescence or young adulthood and is unlikely to contribute to MDS in older patients. Median age at diagnosis of GATA2 deficiency in previous reports is estimated at 29 years.18 In our prospective childhood MDS cohort, GATA2mut patients had a median age at diagnosis of 12 years. In conclusion, the age peak for hemato-immunologic disease manifestation in individuals with GATA2 mutations lies between the second and third decade of life.
Strikingly, in the majority of GATA2-deficient patients, MDS occurred sporadically without preexisting family history of MDS, and in half of all patients, no known clinical features pointing toward GATA2 deficiency were present. Sporadic disease had previously been reported in few patients with GATA2-related hematologic malignancy without additional accessory phenotypes and in patients with immunodeficiency.14,35
The high rate of de novo GATA2 mutations in patients with MDS and the low number of silent carriers are consistent with a disease exposing high penetrance and expressivity. Although the high incidence of sporadic MDS and the full penetrance for coding (exonic) mutations in our cohort must be interpreted with caution due to referral bias, it is also true that relatively few (to our knowledge, 8 reported) clinically silent carriers with coding mutations were identified among >200 reported GATA2mut cases.18 Finally, our findings suggestive of reduced penetrance for noncoding mutations are consistent with a previous report of 2 silent carriers with such variants.36
Previous reports suggested that cytopenias and immunodeficiencies result from GATA2 haploinsufficiency. This notion is consistent with the loss of hematopoietic stem cells (HSC) observed in mouse models of GATA2 haploinsufficiency and conditional GATA2 inactivation.23,27 On the opposite, GATA2 overexpression inhibits HSC output37 and erythroid differentiation.38 Like others, we found 3 types of monoallelic mutations: truncating mutations, missense mutations in ZF2, and noncoding mutations in the enhancer region of intron 4. Thus far, only a handful of GATA2 mutations have been functionally validated. Loss of function was demonstrated for the ZF2 mutations Thr354Met and Thr355del found in familial MDS/AML,13 Arg361Leu and Cys373Arg in Emberger syndrome,17 and Arg350_Asn351ins8 in de novo AML with unknown germline status.39 Of these, only Thr354Met was present in 3 of our patients. On the other hand, somatic GATA2 mutations identified in myeloid diseases were shown to enhance (Gly320Asp/ZF1 and Leu359Val/ZF2), reduce (Ala318Thr/ZF1 and Leu321Phe/ZF1), or not to alter (Arg308Pro/ZF1) the transactivation activity of GATA2 protein.28,31,39 The fact that all truncating mutations identified abolish ZF2 emphasizes its functional significance. Noncoding GATA2 mutations were initially reported by Holland’s group40 in the +9.5 intronic region shown to act as transcriptional enhancer.41 Notably, 10.5% of affected patients in our study carried these noncoding variants. For most of them the clinical presentation was similar to patients with coding mutations, pointing toward comparable functional effect. However 2 patients (D506 and D609) with novel intronic variants showed stable hematologic disease and long-term survival between 8 and 16 years, an observation certainly deserving further functional analyses. The variant c.1017+532T>A in D609 falls within the highly conserved E-box region of GATA2 spanning CATCTGC nucleotides starting at position c.1017+530, for which important regulatory/enhancer function has been ascribed; in addition, a family with mutation in this region (c.1017+512del28) presenting with MonoMAC/MDS was previously reported.42 The pathogenic significance of the variant c.1017+699insT in D506 is not clear. Although it affects a highly conserved nucleotide and has not been previously reported in control databases, it is also possible that it might represent a nonpathogenic private variant.
The recounted difficulty in identifying genotype-phenotype association was also observed in our study when, for example, hematologic phenotypes of different patients with identical mutations were assessed. Although the same mutation was associated with MDS developing after years of preexisting cytopenias in one child, it caused MDS without prodromal symptoms in another patient. Possibly, GATA2 genotypes can have various functional consequences in different biological contexts. We therefore propose the category “MDS following GATA2 disease” or “GATA2-related MDS.” In this study, we focused on the systematic review of hematologic findings in GATA2mut patients within a large prospective MDS cohort. The known MonoMAC-/Emberger-like phenotypes were also observed in half of our patients, but they did not negatively affect the clinical outcome. Strikingly, we encountered more cases with monocytosis rather than monocytopenia in patients with GATA2-related MDS, which can be attributed to the more advanced disease with monosomy 7. This finding emphasizes the broad heterogeneity of GATA2 disease phenotype. In addition, we report for the first time novel recurrent phenotypes of GATA2 deficiency, namely urogenital tract malformations and behavioral problems.
Advanced MDS and monosomy 7 were highly overrepresented in GATA2-related MDS. By contrast, in the recent National Institutes of Health series of GATA2mut patients evaluated for immunodeficiency, trisomy 8 (24%) was more frequent than monosomy 7 (16%).36 Although the prevalence of monosomy 7 in previous reports on GATA2 deficiency was estimated at 29%,18 this karyotype was found at least twice as frequent in our pediatric cohort. Moreover, several GATA2mut patients in this study presented with the unbalanced aberration der(1;7)(q10;p10) leading to trisomy 1q and loss of 7q, which is otherwise rare in pediatric MDS4 and was not found in GATA2WT patients. Given the high prevalence of germline GATA2 mutations in adolescents with monosomy 7 (72%), an underlying GATA2 deficiency should be excluded during the diagnostic workup in this subgroup.
Accounting for MDS subtypes and cytogenetics, OS was not affected by GATA2 mutations, indicating that MDS subtype rather than GATA2 status was the relevant prognostic factor. HSCT is the only curative option for GATA2-related MDS. The National Institutes of Health reported a survival rate of 54% at 4 years after HSCT in 21 GATA2mut patients that were transplanted for MDS/AML, pulmonary alveolar proteinosis, or recurrent infections.36 The comparably favorable 5-year OS of 66% in our pediatric cohort transplanted for MDS with monosomy 7 can be likely explained by the younger age and the lower prevalence of GATA2-related systemic complications. Our findings indicate that GATA2 deficiency itself does not increase TRM in affected children. We concur with Grossman and colleagues43 that the ideal time window for HSCT in GATA2 deficiency is during the hypocellular MDS phase and before manifestation of severe complications (ie, invasive infections) and support the need for a close monitoring of GATA2mut patients for the occurrence of any of these events. Interestingly, we observed a slightly higher proportion of relapse after HSCT within GATA2mut group with monosomy 7 (21% vs 13%; P = not significant), an observation deserving further validation in larger cohorts.
Finally, our findings should be interpreted in the light of some limitations. One is the fact that children with MDS and advanced disease are subjected to HSCT in a very timely manner. In addition, although our results show comparable survival for GATA2mut and GATA2WT children with MDS, this finding might not be translated into the setting of adult patients with other GATA2-related phenotypes (ie, immunodeficiency or pulmonary complications).
In summary, we show that GATA2 deficiency is a predisposing condition for MDS and is responsible for 7% of all primary pediatric MDS cases and prevalent in two third of adolescents with monosomy 7. Our results redefine the paradigm of MDS in children and adolescents and highlight the contribution of de novo germline mutations with a delayed latency oncogenic effect. GATA2 mutational status does not negatively affect the outcome of MDS in children and adolescents. We suggest that decisions on timing and preparative regimen for HSCT in GATA2-related MDS should be guided, as for all children with MDS, by the known risk factors such as cytogenetic evolution, severity of cytopenias, and advanced disease. Ideally, the implementation of early diagnosis of GATA2 deficiency can avoid unnecessary diagnostic procedures, enable tailored surveillance strategy, and limit the use of noncurative therapies specifically avoiding immunosuppression. We propose that GATA2 analysis should be included in the workup of all children and young adults presenting with MDS and monosomy 7, der(1;7), or trisomy 8, irrespective of family history or accessory GATA2 disease phenotype.
Presented in part at the 54th annual meeting of the American Society of Hematology, Atlanta, GA, December 8-11, 2012.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
For excellent technical assistance and data management, the authors thank Sandra Zolles, Gunda Ruzaike, Alexandra Fischer, Wilfried Truckenmüller, and Anne Strauss (Freiburg), and Hideki Makishima (Cleveland).
This work was supported by Deutsche Krebshilfe (Max Eder grant 109005 to M.W.W. and MDS consortium grant 110293 to C.M.N.), German Federal Ministry of Education and Research (DKTK German cancer consortium, molecular diagnostics of pediatric malignancies to C.M.N. and M.W.W.), and intramural grant CCIAP4 to M.W.W. This study was supported by the project (Ministry of Health, Czech Republic) for conceptual development of research organization 00064203 (University Hospital Motol, Prague, Czech Republic). M.W.W. is a recipient of the 2015 European Hematology Association–American Society of Hematology fellowship on Translational Research Training in Hematology.
Authorship
Contribution: M.W.W., B. Strahm, and C.M.N. conceived and designed the study; all authors collected clinical data; M.W.W., S.H., G.G., B. Schlegelberger, B. Strahm, and C.M.N. analyzed and interpreted the data; P.N. provided statistical analysis; and all authors wrote the manuscript and provided final approval.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.H. is Department of Pediatrics, St. Luke's International Hospital, Tokyo, Japan.
A complete list of the members of the EWOG-MDS appears in “Appendix.”
Correspondence: Marcin W. Wlodarski, Department of Pediatrics and Adolescent Medicine, Division of Pediatric Hematology and Oncology, University of Freiburg, Mathildenstrasse 1, 79106 Freiburg, Germany; e-mail: marcin.wlodarski@uniklinik-freiburg.de.
Appendix
The members of the EWOG-MDS are: Marcin W. Wlodarski, Shinsuke Hirabayashi, Victor Pastor, Jan Starý, Henrik Hasle, Riccardo Masetti, Michael Dworzak, Markus Schmugge, Marry van den Heuvel-Eibrink, Marek Ussowicz, Barbara De Moerloose, Albert Catala, Owen P. Smith, Petr Sedlacek, Arjan C. Lankester, Marco Zecca, Victoria Bordon, Susanne Matthes-Martin, Jonas Abrahamsson, Jörn Sven Kühl, Karl-Walter Sykora, Michael H. Albert, Stephan Schwarz, Gudrun Göhring, Brigitte Schlegelberger, Annámaria Cseh, Peter Noellke, Ayami Yoshimi, Franco Locatelli, Irith Baumann, Brigitte Strahm, and Charlotte M. Niemeyer.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal