Key Points
Survival of chronic GVHD patients was predicted by clinician-assessed response and changes in patient-reported outcomes.
FFS was predicted by clinician-assessed response, changes in patient-reported outcomes, and the 2014 NIH response criteria.
Abstract
Chronic graft-versus-host disease (GVHD) is a pleotropic syndrome that lacks validated methods of measuring response in clinical trials, although several end points have been proposed. To investigate the prognostic significance of these proposed end points, such as the 2005 National Institutes of Health (NIH) response measures, 2014 NIH response measures, clinician-reported response, and patient-reported response, we tested their ability to predict subsequent overall survival (OS), nonrelapse mortality (NRM), and failure-free survival (FFS). Patients (n = 575) were enrolled on a prospective chronic GVHD observational trial. At 6 months, clinician-reported response (P = .004) and 2014 NIH-calculated response (P = .001) correlated with subsequent FFS, and clinician-reported response predicted OS (P = .007). Multivariate models were used to identify changes in organ involvement, laboratory values, and patient-reported outcomes that were associated with long-term outcomes. At 6 months, a change in the 2005 NIH 0 to 3 clinician-reported skin score and 0 to 10 patient-reported itching score predicted subsequent FFS. Change in the Lee skin symptom score and Functional Assessment of Cancer Therapy–Bone Marrow Transplant score predicted subsequent OS. Change in the Lee skin symptom score predicted subsequent NRM. This study provides evidence that clinician-reported response and patient-reported outcomes are predictive of long-term survival. The trial was registered at www.clinicaltrials.gov as #NCT00637689.
Introduction
Chronic graft-versus-host disease (GVHD) is a significant cause of morbidity and mortality in survivors of allogeneic hematopoietic cell transplantation (HCT),1-8 and more effective treatments are needed. Although many clinical trials have been conducted, interpretation of results has been difficult because documentation of response in chronic GVHD has been particularly challenging. Because of the long time course of chronic GVHD, standard end points such as overall survival (OS) and nonrelapse mortality (NRM) require longer-term follow-up than might be desired in most early phase chronic GVHD trials. Therefore, efforts have been made to identify an interim response measure, such as failure-free survival (FFS). Thus far, there are no validated response measures; therefore, subjective clinical judgment is often used to determine response.9-16
In 2005, the National Institutes of Health (NIH) Chronic GVHD Consensus Conference recommended response measures based on serial organ assessments.17,18 Response is determined by comparing baseline and follow-up scores for each organ system to calculate overall response. In analysis of individual organ systems, this scoring system has been predictive of meaningful end points such as OS and NRM.19-24 However, when imputed into a calculated composite response, measures have had variable correlation with clinician-reported response and survival outcomes. Although 1 study demonstrated good correlation between response calculated per the 2005 NIH consensus criteria and clinician-reported response, and a survival advantage to those patients who had a favorable response (partial response [PR] or better) at 6 months, this study had a small number of patients evaluated.25 In other studies, these response criteria have not been associated with clinician-reported response,26 subsequent survival, or improved quality of life.27,28
In 2014, a second NIH consensus conference was held.29 Several changes to the NIH response algorithm were made. Notably, skin, mouth, and eye measurements were simplified; new joint measures were introduced; new mild symptoms in gastrointestinal (GI) and liver were not considered progression; and attribution of clinical manifestations (symptoms or signs) to causes other than chronic GVHD was captured and incorporated into scoring. The 2014 NIH response criteria have not been used in clinical trials yet.
FFS is a proposed intermediate end point of treatment success, defined as continued disease-free survival without addition of a new systemic immunosuppressive medication. FFS is appealing because it is easy to document and change of therapy has been associated with a higher mortality rate,30 but FFS also has the disadvantage of relying on the clinician’s treatment approach, which is subject to bias and variation in management styles. OS and NRM are attractive end points because they are definitive and acceptable to the US Food and Drug Administration, but survival is typically not the primary end point in trials of chronic illnesses such as chronic GVHD.29
We tested whether different aggregate measurements of response, including the 2005 and 2014 calculated NIH response, clinician-reported response, and patient-reported response measures were predictive of FFS, OS, and NRM. We also tested whether changes in individual organ assessments, laboratories, or patient-reported symptoms were predictive of FFS, OS, and NRM.
Methods
Chronic GVHD Consortium
A cohort of HCT recipients affected by chronic GVHD was enrolled in a multicenter observational study (#NCT00637689).31 Chronic GVHD was diagnosed according to 2005 NIH consensus criteria.32 The protocol was approved by the institutional review board at each site, and all subjects provided written informed consent. Patients enrolled in the cohort were allogeneic HCT recipients at least 2 years of age with chronic GVHD requiring systemic therapy, including both classic and overlap subtypes. For this analysis, only adults were included because of the small number of pediatric patients. Cases were classified as incident (enrollment <3 months after chronic GVHD diagnosis) or prevalent (enrollment 3 or more months after chronic GVHD diagnosis but <3 years after transplantation). Primary disease relapse, inability to comply with study procedures, and anticipated survival of <6 months were exclusion criteria. At enrollment and every 6 months thereafter, clinicians and patients reported standardized information summarizing chronic GVHD organ involvement and symptoms. Incident cases had an additional assessment time point at 3 months after enrollment.15 Objective medical data including ancillary testing, laboratory results and medical complications, and medication profiles were abstracted through standardized chart review after each visit.
End point definitions
For the FFS end point, failure was defined as malignancy relapse, death, or addition of a new immunosuppressive medication (eg, sirolimus, rituximab) or treatment (eg, extracorporeal photopheresis) intended for systemic treatment of chronic GVHD.33 Determination of failure was made by 2 separate reviewers (J. Palmer and S.J.L.) independently, and discrepancies were resolved by discussion. Treatment with pulse high-dose solumedrol (ie, 500 mg or 1000 mg for several days) was considered as a treatment change; steroid dose increases within the standard range were not considered a treatment change, consistent with other reports.34 Addition of topical therapies (eg, topical steroids, ophthalmic cyclosporine, topical GI steroids), supportive care treatments (eg, ursodeoxycholic acid), or systemic nonimmunosuppressive medications for management of GVHD involving specific organs (eg, montelukast or azithromycin for obstructive lung disease) was not considered a failure.
OS was defined as the time from enrollment to death from any cause. NRM was defined as time from enrollment to death with relapse as a competing risk.
Potential predictors
Overall response was determined in 3 ways: (1) NIH-calculated response was according to both the 200535 and 201429 consensus criteria algorithms that use changes in skin, mouth, eye, lungs, joints, GI, and liver measures to assign patients to the categories of complete response (CR), PR, stable disease (SD), and progressive disease (PD). Although the 2014 response criteria were not available when the study started, the relevant measures were collected in the study and available to calculate response using the 2014 algorithm. (2) Clinician-reported response was CR, PR, SD, and PD as reported on clinician-completed surveys. (3) Patients also reported whether their chronic GVHD was improving, stable, or worsening on a 7-point Likert scale. Supplemental Table 1 (see the Blood Web site) shows the cross tabulation of the 2014 calculated responses with the 2005 calculated responses, clinician-reported responses, and patient-reported responses.
To identify individual organ assessments, laboratory values, and patient-reported variables associated with FFS, OS, and NRM, univariable analyses used all available information. The complete list of variables may be found in supplemental Table 2. Briefly, all recommended measures from the 2005 NIH Consensus Conference on Clinical Trials in Chronic GVHD were included.17,32,36 In addition, other scales used in prior clinical trials were also collected and analyzed (eg, Vienna skin score37 and Hopkins scales38 ). Data regarding comorbidities, disease, and transplant characteristics were considered as potential predictors.
Statistical analysis
Cumulative incidence estimates of relapse, NRM, and addition of a new therapy as causes of failure were derived, treating each event as a competing risk for the other two.39
NIH-calculated, clinician-reported, and patient-reported changes in chronic GVHD disease activity were tested in landmark analyses for their ability to predict subsequent FFS, NRM, and survival after 3 and 6 months with P < .01 considered significant because of multiple testing. Only incident cases were included in the 3-month landmark analysis because data at 3 months were not collected for prevalent cases. Both incident and prevalent cases were included in the 6-month primary landmark analysis, but they were also tested separately. The FFS analysis was limited to patients who had not had a treatment change or recurrent malignancy before the landmark. Because FFS is a composite end point, we also tested whether response measures correlated with treatment change, considering relapse and death as competing risks. OS and NRM analyses included all patients who were alive without recurrent malignancy at the landmark, regardless of prior treatment change.
The analysis of organ assessments, laboratory values- and patient-reported variables was complicated because of the large number of variables. Supplemental Figure 1 shows how the large number of potential predictive covariates was reduced to the variables whose change scores independently predicted the outcomes of interest. Cox regression models were used to identify risk factors for various types of failure (FFS, NRM, mortality), using sequential selection processes within each organ because of the number of potential predictors (supplemental Table 2), many of which are correlated. First, the change in each variable was fit into a univariable Cox regression model while adjusting for the baseline value (supplemental Table 3). Second, within each organ system, factors associated with failure with a univariable likelihood ratio test P value ≤.05 were then fit into a multivariable model for each organ using a backward elimination procedure (supplemental Table 4). Lastly, variables still significant on multivariate analysis within each organ system were considered in building a multivariable Cox regression model including all organ systems. A backward elimination procedure was used to exclude risk factors until the P value of the likelihood ratio test for all remaining risk factors was ≤.05. The final multivariable model included all statistically significant variables (enrollment plus change scores) after backward elimination, as well as any statistically significant transplant characteristics (Table 3). We tested for interaction among significant variables and found none.
Agreement between overall responses at 3- or 6-month visit was tested by weighted κ statistic for ordinal measures with Fleiss-Cohen weights.40 Empirical interpretation was used for the κ coefficient (0, no agreement; 0-0.2, slight agreement; 0.2-0.4 fair agreement; 0.4-0.6, moderate agreement; 0.6-0.8, substantial agreement; 0.8-1.0, almost perfect agreement). SAS/STAT version 9.3 (SAS Institute Inc., Cary, NC) and R version 2.15.2 (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analyses.
Results
Patients
Between August 2007 and January 2013, 575 patients were enrolled in this chronic GVHD prospective study (Table 1). Four hundred and fifty-one patients had evaluations at 6 months, and 307 of those patients were alive without recurrent malignancy or prior treatment change. There were 1856 follow-up visits, for a total of 2431 visits. The cohort included 342 (59%) incident cases and 233 (41%) prevalent cases. The median time to enrollment was 11.9 months after transplant (range 2.9-294), median follow-up after enrollment for survivors was 44 months (range 0.9-76), and 149 (26%) have died.
Patient characteristics and outcomes
| Characteristic/category . | n . | Count (%) . |
|---|---|---|
| Study site | 575 | |
| Fred Hutchinson Cancer Research Center | 253 (44%) | |
| University of Minnesota | 61 (11%) | |
| Dana-Farber Cancer institute | 65 (11%) | |
| Stanford University Medical Center | 74 (13%) | |
| Vanderbilt University Medical Center | 48 (8%) | |
| Medical College of Wisconsin | 23 (4%) | |
| Washington University Medical Center | 4 (1%) | |
| Moffitt Cancer Center | 39 (7%) | |
| Memorial Sloan Kettering Cancer Center | 8 (1%) | |
| Case type | 575 | |
| Incident | 342 (59%) | |
| Prevalent | 233 (41%) | |
| Patient age at registration (y), median (range) | 575 | 52 (19-79) |
| Patient gender | 575 | |
| Female | 242 (42%) | |
| Male | 333 (58%) | |
| Diagnosis | 575 | |
| AML | 193 (34%) | |
| ALL | 62 (11%) | |
| CML | 30 (5%) | |
| CLL | 46 (8%) | |
| MDS | 89 (15%) | |
| NHL | 85 (15%) | |
| HL | 17 (3%) | |
| MM | 29 (5%) | |
| AA | 6 (1%) | |
| Other | 18 (3%) | |
| Disease status | 571 | |
| Early | 184 (32%) | |
| Intermediate | 248 (43%) | |
| Advanced | 139 (24%) | |
| Transplant source | 575 | |
| Bone marrow | 40 (7%) | |
| Cord blood | 28 (5%) | |
| Peripheral blood | 507 (88%) | |
| Transplant type | 571 | |
| Myeloablative | 297 (52%) | |
| Nonmyeloablative | 274 (48%) | |
| Donor-patient CMV status | 569 | |
| Patient and donor CMV both negative | 192 (34%) | |
| Patient or donor CMV positive | 377 (66%) | |
| Donor-patient gender combination | 569 | |
| Female into male | 167 (29%) | |
| Others | 402 (71%) | |
| Donor match | 573 | |
| Matched related | 238 (42%) | |
| Matched unrelated | 244 (43%) | |
| Mismatched | 91 (16%) | |
| Prior grade 2-4 acute GVHD | 575 | |
| Yes | 311 (54%) | |
| No | 264 (46%) | |
| 2005 NIH chronic GVHD global severity score | 575 | |
| Mild or less | 53 (9%) | |
| Moderate | 302 (53%) | |
| Severe | 220 (38%) |
| Characteristic/category . | n . | Count (%) . |
|---|---|---|
| Study site | 575 | |
| Fred Hutchinson Cancer Research Center | 253 (44%) | |
| University of Minnesota | 61 (11%) | |
| Dana-Farber Cancer institute | 65 (11%) | |
| Stanford University Medical Center | 74 (13%) | |
| Vanderbilt University Medical Center | 48 (8%) | |
| Medical College of Wisconsin | 23 (4%) | |
| Washington University Medical Center | 4 (1%) | |
| Moffitt Cancer Center | 39 (7%) | |
| Memorial Sloan Kettering Cancer Center | 8 (1%) | |
| Case type | 575 | |
| Incident | 342 (59%) | |
| Prevalent | 233 (41%) | |
| Patient age at registration (y), median (range) | 575 | 52 (19-79) |
| Patient gender | 575 | |
| Female | 242 (42%) | |
| Male | 333 (58%) | |
| Diagnosis | 575 | |
| AML | 193 (34%) | |
| ALL | 62 (11%) | |
| CML | 30 (5%) | |
| CLL | 46 (8%) | |
| MDS | 89 (15%) | |
| NHL | 85 (15%) | |
| HL | 17 (3%) | |
| MM | 29 (5%) | |
| AA | 6 (1%) | |
| Other | 18 (3%) | |
| Disease status | 571 | |
| Early | 184 (32%) | |
| Intermediate | 248 (43%) | |
| Advanced | 139 (24%) | |
| Transplant source | 575 | |
| Bone marrow | 40 (7%) | |
| Cord blood | 28 (5%) | |
| Peripheral blood | 507 (88%) | |
| Transplant type | 571 | |
| Myeloablative | 297 (52%) | |
| Nonmyeloablative | 274 (48%) | |
| Donor-patient CMV status | 569 | |
| Patient and donor CMV both negative | 192 (34%) | |
| Patient or donor CMV positive | 377 (66%) | |
| Donor-patient gender combination | 569 | |
| Female into male | 167 (29%) | |
| Others | 402 (71%) | |
| Donor match | 573 | |
| Matched related | 238 (42%) | |
| Matched unrelated | 244 (43%) | |
| Mismatched | 91 (16%) | |
| Prior grade 2-4 acute GVHD | 575 | |
| Yes | 311 (54%) | |
| No | 264 (46%) | |
| 2005 NIH chronic GVHD global severity score | 575 | |
| Mild or less | 53 (9%) | |
| Moderate | 302 (53%) | |
| Severe | 220 (38%) |
| . | . | Estimate (95% CI) . |
|---|---|---|
| FFS | ||
| At 1 y after enrollment | 45% (41%-49%) | |
| At 2 y after enrollment | 29% (25%-33%) | |
| At 4 y after enrollment | 11% (4%-22%) | |
| Relapse | ||
| At 1 y after enrollment | 6% (4%-8%) | |
| At 2 y after enrollment | 10% (8%-13%) | |
| At 4 y after enrollment | 13% (10%-17%) | |
| NRM | ||
| At 1 y after enrollment | 4% (3%-6%) | |
| At 2 y after enrollment | 6% (4%-8%) | |
| At 4 y after enrollment | 14% (7%-27%) | |
| Survival | ||
| At 1 y after enrollment | 89% (86%-91%) | |
| At 2 y after enrollment | 81% (78%-85%) | |
| At 4 y after enrollment | 71% (66%-76%) |
| . | . | Estimate (95% CI) . |
|---|---|---|
| FFS | ||
| At 1 y after enrollment | 45% (41%-49%) | |
| At 2 y after enrollment | 29% (25%-33%) | |
| At 4 y after enrollment | 11% (4%-22%) | |
| Relapse | ||
| At 1 y after enrollment | 6% (4%-8%) | |
| At 2 y after enrollment | 10% (8%-13%) | |
| At 4 y after enrollment | 13% (10%-17%) | |
| NRM | ||
| At 1 y after enrollment | 4% (3%-6%) | |
| At 2 y after enrollment | 6% (4%-8%) | |
| At 4 y after enrollment | 14% (7%-27%) | |
| Survival | ||
| At 1 y after enrollment | 89% (86%-91%) | |
| At 2 y after enrollment | 81% (78%-85%) | |
| At 4 y after enrollment | 71% (66%-76%) |
AA, aplastic anemia; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CI, confidence interval; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CMV, cytomegalovirus; HL, Hodgkin lymphoma; MDS, myelodysplastic syndrome; MM, multiple myeloma; NHL, non-Hodgkin lymphoma.
Landmark analysis
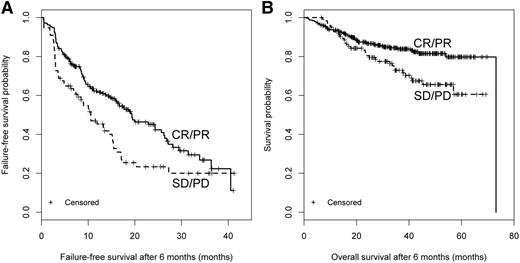
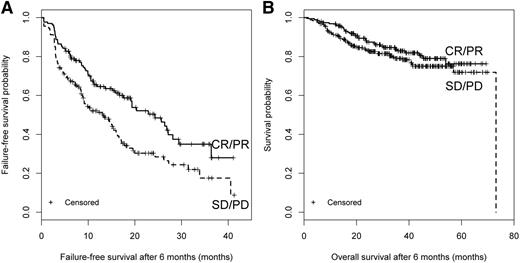
We first performed a landmark analysis evaluating how different response measurements at 3 months (incident cases only) or 6 months (both incident and prevalent cases) correlated with subsequent FFS, OS, and NRM (Table 2). At 3 months, clinician-reported (CR + PR vs SD + PD: HR = 0.34; 95% CI, 0.22-0.52; P < .001), patient-reported (improvement vs stable vs worsening: overall P < .001), and 2014 NIH-calculated (CR + PR vs SD + PD: HR = 0.60; 95% CI, 0.41-0.89; P = .01) response correlated with longer subsequent FFS but not with NRM or OS. Results were similar for treatment change. At 6 months, clinician-reported (CR + PR vs SD + PD: P = .004) and 2014 NIH-calculated (CR+PR vs SD + PD: HR = 0.58; 95% CI, 0.42-0.80; P = .001) response correlated with higher subsequent FFS but not NRM. Clinician-reported response was correlated with improved OS (HR = 0.55; 95% CI, 0.36-0.85; P = .007). The 2005 NIH-calculated response did not predict FFS, NRM, or OS but was predictive of treatment change (Table 2). The κ statistic between the 2005 and 2014 NIH-calculated responses was 0.32 suggesting poor to fair correlation. Kaplan-Meier plots for FFS and OS according to clinician-reported response and 2014 NIH-calculated response are shown in Figures 1 and 2, which also illustrate that the first FFS event occurred at a median of 16.3 months (95% CI, 13.5-19.4) after the 6-month assessment. Results were similar when the 6-month analysis was limited to incident cases (data not shown).
Landmark analysis after 3 months and 6 months
| Three-month landmark . | Treatment change after 3 mo . | FFS after 3 mo . | NRM after 3 mo . | OS after 3 mo . |
|---|---|---|---|---|
| NIH calculated (2005) | P = .37 | P = .49 | P = .55 | P = .51 |
| CR + PR vs SD + PD | ||||
| NIH calculated (2014) | HR 0.50 (0.30-0.80) | HR 0.60 (0.41-0.89) | P = .32 | P = .59 |
| CR + PR vs SD + PD | P = .003 | P = .01 | ||
| Clinician reported | HR 0.28 (0.18-0.47) | HR 0.34 (0.22-0.52) | P = .84 | P = .47 |
| CR + PR vs SD + PD | P < .001 | P < .001 | ||
| Patient-reported improvement (I) vs stable (S) vs worsening (W) | Overall P < .001 | Overall P < .001 | P = .62 | P = .36 |
| I vs S | HR 0.41 (0.24-0.74), P = .002 | HR 0.43 (0.27-0.70), P < .001 | ||
| S vs W | HR 0.24 (0.10-0.69), P = .003 | HR 0.25 (0.11-0.63), P = .001 |
| Three-month landmark . | Treatment change after 3 mo . | FFS after 3 mo . | NRM after 3 mo . | OS after 3 mo . |
|---|---|---|---|---|
| NIH calculated (2005) | P = .37 | P = .49 | P = .55 | P = .51 |
| CR + PR vs SD + PD | ||||
| NIH calculated (2014) | HR 0.50 (0.30-0.80) | HR 0.60 (0.41-0.89) | P = .32 | P = .59 |
| CR + PR vs SD + PD | P = .003 | P = .01 | ||
| Clinician reported | HR 0.28 (0.18-0.47) | HR 0.34 (0.22-0.52) | P = .84 | P = .47 |
| CR + PR vs SD + PD | P < .001 | P < .001 | ||
| Patient-reported improvement (I) vs stable (S) vs worsening (W) | Overall P < .001 | Overall P < .001 | P = .62 | P = .36 |
| I vs S | HR 0.41 (0.24-0.74), P = .002 | HR 0.43 (0.27-0.70), P < .001 | ||
| S vs W | HR 0.24 (0.10-0.69), P = .003 | HR 0.25 (0.11-0.63), P = .001 |
| Six-month landmark . | Treatment change after 6 mo . | FFS after 6 mo . | NRM after 6 mo . | OS after 6 mo . |
|---|---|---|---|---|
| NIH calculated (2005) | HR 0.61 (0.40-0.90) | P = .06 | P = .24 | P = .06 |
| CR + PR vs SD + PD | P = .01 | |||
| NIH calculated (2014) | HR 0.56 (0.37-0.83) | HR 0.58 (0.42-0.80) | P = .28 | P = .20 |
| CR + PR vs SD + PD | P = .003 | P = .001 | ||
| Clinician reported | HR 0.53 (0.36-0.81) | HR 0.61 (0.44-0.85) | P = .06 | HR 0.55 (0.36-0.85) |
| CR + PR vs SD + PD | P = .004 | P = .004 | P = .007 | |
| Patient-reported improvement vs stable vs worsening | P = .13 | P = .08 | P = .33 | P = .44 |
| Six-month landmark . | Treatment change after 6 mo . | FFS after 6 mo . | NRM after 6 mo . | OS after 6 mo . |
|---|---|---|---|---|
| NIH calculated (2005) | HR 0.61 (0.40-0.90) | P = .06 | P = .24 | P = .06 |
| CR + PR vs SD + PD | P = .01 | |||
| NIH calculated (2014) | HR 0.56 (0.37-0.83) | HR 0.58 (0.42-0.80) | P = .28 | P = .20 |
| CR + PR vs SD + PD | P = .003 | P = .001 | ||
| Clinician reported | HR 0.53 (0.36-0.81) | HR 0.61 (0.44-0.85) | P = .06 | HR 0.55 (0.36-0.85) |
| CR + PR vs SD + PD | P = .004 | P = .004 | P = .007 | |
| Patient-reported improvement vs stable vs worsening | P = .13 | P = .08 | P = .33 | P = .44 |
HR, hazard ratio.
Clinician-reported response. Response at 6 months and subsequent (A) FFS and (B) OS.
Clinician-reported response. Response at 6 months and subsequent (A) FFS and (B) OS.
The 2014 NIH-calculated response. Response at 6 months and subsequent (A) FFS and (B) OS.
The 2014 NIH-calculated response. Response at 6 months and subsequent (A) FFS and (B) OS.
Predictors
In order to identify the changes in individual variables at 6 months that are most predictive of subsequent outcomes, we performed a multivariate analysis to compare the performance of all the collected measures against one another.
All tested variables and the results of the organ-specific univariate and multivariate models are included in supplemental Tables 1-4. Overall, multivariate results are reported in Table 3. Improvements in the NIH 0 to 3 clinician-reported skin score and 0 to 10 patient-reported itching score at 6 months predicted longer subsequent FFS. Improvements in the Lee skin symptom score predicted longer subsequent OS and NRM, and the Functional Assessment of Cancer Therapy–Bone Marrow Transplant (FACT-BMT) trial outcome index score predicted longer subsequent OS.
Multivariate landmark analyses at 6 mo for subsequent FFS, OS, and NRM
| Outcome . | Parameter* . | No. events/no. at risk after excluding missing . | P . | HR (95% CI) . |
|---|---|---|---|---|
| FFS | Change in 2005 NIH 0 to 3 skin score | 112/211 | .001 | 1.53 (1.19-1.96) |
| Change in patient 0 to 10 skin itching | .002 | 1.15 (1.06-1.24) | ||
| OS | Change in Lee skin symptom score | 64/308 | .005 | 1.02 (1.01-1.04) |
| FACT-BMT total score | .04 | 0.98 (0.97-0.99) | ||
| NRM | Change in Lee skin symptom score | 48/326 | .001 | 1.03 (1.01-1.04) |
| Outcome . | Parameter* . | No. events/no. at risk after excluding missing . | P . | HR (95% CI) . |
|---|---|---|---|---|
| FFS | Change in 2005 NIH 0 to 3 skin score | 112/211 | .001 | 1.53 (1.19-1.96) |
| Change in patient 0 to 10 skin itching | .002 | 1.15 (1.06-1.24) | ||
| OS | Change in Lee skin symptom score | 64/308 | .005 | 1.02 (1.01-1.04) |
| FACT-BMT total score | .04 | 0.98 (0.97-0.99) | ||
| NRM | Change in Lee skin symptom score | 48/326 | .001 | 1.03 (1.01-1.04) |
Models include the enrollment value for the significant change scores and adjustment for baseline characteristics.
We added aggregate measures, such as clinician-reported overall response, patient-reported response, the 2014 NIH response measure, and the NIH global severity score, to the models to determine whether they could replace the organ-specific or patient-reported measures. None of the aggregate measures nor the NIH global severity score showed a statistically significant association with any outcome (all P > .09) in these models, whereas the individual measures remained statistically significant (P < .01), except for the FACT-BMT total score in the survival model, which had a P value of .08. These results suggest that changes in the individual measures have independent prognostic significance and cannot be replaced by knowledge of the summary response measures.
Discussion
Our primary goal in this analysis was to identify measurements at 3 or 6 months predictive of subsequent long-term outcomes. Many of the intermediate or long-term outcomes, such as FFS, NRM, and OS require longer follow-up times and are not practical for clinical trial design. Therefore, it is critical to identify 6-month surrogate end points that are able to predict long-term outcomes. Our analysis identified several surrogate end points that were predictive of the more critical end points such as FFS, NRM, and OS. At 6 months, the calculated 2014 NIH response and clinician-reported response predict subsequent FFS, whereas clinician-reported response predicts subsequent OS. These results suggest that the 2014 NIH response measures do reflect changes in chronic GVHD disease activity because patients who do not achieve a CR or PR are more likely to have their treatment regimen changed. Although the 2014 NIH response criteria were not associated with subsequent OS or NRM, it is important to remember that the NIH response measures were never designed to predict survival. They were instead designed to capture relevant changes in chronic GVHD disease activity as a result of chronic GVHD-directed therapy.
We proceeded to analyze which specific measured variables were most predictive of FFS, OS, and NRM by multivariate analysis. Surprisingly, we found that FFS, OS, and NRM were primarily predicted by changes in patient-reported measures. Patient-reported symptoms and quality of life may be more sensitive to overall health than clinician-reported chronic GVHD measures. Another possibility is that clinicians may aggressively immunosuppress more symptomatic patients leading to shorter FFS and worse survival.
The ability of the clinician-reported and patient-reported responses to predict FFS is not surprising, because therapy is likely to be changed if the clinician or patient concludes that the response to current therapy is not adequate. Interestingly, treatment changes occurred at a median of 16 months after the landmark, suggesting that even if the patient has not achieved a CR or PR by 6 months, it may still be some time before an actual treatment change is made. The ability of clinician-reported response at 6 months to predict OS is encouraging, because some chronic GVHD trials currently use clinician-reported end points. An earlier analysis of our cohort did not show an association between clinician-reported response and survival; however, this was likely because of shorter follow-up time27 because, in the current study, the survival benefit associated with clinician-reported response at 6 months did not appear until after 2 years. These findings demonstrate the prolonged disease course in patients with chronic GVHD and highlight the importance of identifying appropriate 6-month surrogate end points.
The 2014 NIH response criteria29 performed better than the 2005 criteria in our analysis. The improved performance of the 2014 NIH response measures is likely because of modifications based on data from studies done between the 2 consensus conferences. First, skin body surface area measurements were removed, and the NIH 0 to 3 skin score is now used to measure response. Second, change from 0 to 1 in GI and liver score is no longer considered progression. Third, Schirmer’s test has been replaced by change in 0 to 3 NIH eye score. Fourth, assessment of response in the lung is based on the forced expiratory volume in 1 second only and no longer includes diffusing capacity of the lungs for carbon monoxide. Finally, the NIH 0 to 3 joint score has been incorporated into the response criteria. As a result, overall response assignments derived from the 2014 algorithm show only poor to fair correlation with response assignments derived from the 2005 algorithm. Although the 2014 NIH response criteria also exclude an organ from the calculated response if the manifestation is entirely because of nonchronic GVHD causes, we could not ascertain in our data set whether signs or symptoms were related to chronic GVHD. Despite this limitation, the 2014 NIH response was predictive of subsequent FFS in our study population.
We also analyzed individual variables to understand which factors were most associated with long-term outcomes. We were able to identify a few individual measures whose change predicted subsequent FFS, OS, and NRM. Notably, of the 5 identified variables, 1 was a clinician-reported skin measure and 4 were patient-reported measures. A change in the NIH 0 to 3 skin score and patient reported 0 to 10 itching score predicted subsequent FFS. Skin manifestations are bothersome to patients, easily noted on exam, and likely drive treatment changes. We did not expect that patient-reported measures would predict OS and NRM and were surprised to find that these were the only identified predictors. Specifically, change in the Lee skin symptom score and the FACT-BMT score predicted OS. Worsening of the Lee skin symptom score predicted NRM, as has been previously reported in an earlier analysis of this patient cohort.23 These associations may be because of the increased immunosuppression given to patients who have advanced and symptomatic chronic GVHD. Alternatively, worsening symptoms and quality of life may simply reflect declining health with its associated higher mortality rates.
Several findings were unexpected. First, baseline disease risk, which usually predicts relapse, did not predict FFS, OS, or NRM. FFS is largely determined by the addition of other systemic treatment and not by relapse or death. Also, because the median time to enrollment was 11.9 months, patients who relapsed early after transplant were not enrolled in our cohort. Second, factors that have historically predicted survival in chronic GVHD, such as platelet count,41 hyperbilirubinemia,22 overlap syndrome,42 and lower GI involvement,22 were not associated with survival in multivariate analysis. We previously reported that FFS was associated with enrollment NIH scores for skin and GI tract, range of motion, forced vital capacity, bronchiolitis obliterans syndrome, hepatic dysfunction, female donor into male recipient, prior grade 2-4 acute GVHD, and quality of life, but in the current analysis, only change in the NIH skin score was found to be associated with FFS. Although several of these variables were significant in organ-specific univariate analysis, they were not significant in the multivariate analysis. These apparent discrepancies may be partially explained by differences in the analytic approaches. In the current analysis, patients who experienced death or treatment change before the landmark were excluded from the analysis, potentially eliminating the statistical associations previously observed when the models started at enrollment. Another possibility is that what matters for prognosis is whether an organ is involved, not how it responds over time. Finally, it is possible that not enough change occurred in the chronic GVHD activity of the organ by 6 months to demonstrate an association with FFS or OS.
Our study has several limitations. First, this analysis was conducted as a discovery exercise. Although the results are informative, they will need to be validated in a separate independent cohort prior to drawing definitive conclusions, and such a study is ongoing. The timing of assessments in the study was calendar driven and not influenced by the patient’s clinical status or changes in therapy. Therefore, the measured and reported responses may not accurately recapitulate the circumstances of a clinical trial. Additionally, sequential assessments might have been done by different providers, causing inconsistency, especially because forms from the previous assessment were not routinely made available for reference. No direct instructions regarding subjective response assessments were provided to clinicians in assigning a clinical CR, PR, SD, or PD. Finally, some patient-reported outcome measures were missing. Despite these limitations, our data derive strength from the prospective collection of data with the use of standardized forms, the detailed chronic GVHD assessments that were performed, and the large number of patients from multiple centers.
In summary, our data show that the 2014 NIH response measures and clinician-reported response at 3 and 6 months correlate with subsequent FFS. Patient-reported response at 3 months predicted subsequent FFS. Clinician-reported response at 6 months predicted OS. Additionally, this study demonstrates the importance of specific patient-reported measures such as the Lee skin symptom score, for which changes predict OS and NRM, and the FACT BMT, for which changes predict OS. These results lend credence to the 2014 NIH response measures as reflective of disease activity, although not predictive of OS. They also emphasize the critical contribution of patient-reported measures to the assessment of patients with chronic GVHD. Based on these data, we recommend that for now, the 2014 NIH response measures, clinician-reported responses, and patient-reported outcomes be collected in therapeutic trials of chronic GVHD to ensure that relevant data are available once the best algorithm to capture a meaningful objective response is determined.43
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from the National Institutes of Health, National Cancer Institute (CA118953). The Chronic GVHD Consortium (grant U54 CA163438) is a part of the National Institutes of Health Rare Disease Clinical Research Network, supported through collaboration between the Office of Rare Diseases Research, the National Center for Advancing Translational Sciences, and the National Cancer Institute.
Authorship
Contribution: J. Palmer and S.J.L. designed research and drafted the manuscript; all authors contributed to analysis and interpretation of data and critical review of the manuscript; J. Palmer, P.J.M., Y.I., J. Pidala, S.Z.P., M.A., I.P., M.E.D.F., and S.J.L. contributed patients; and X.C. and B.S. performed statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeanne Palmer, Mayo Clinic Phoenix, 5777 East Mayo Blvd, Phoenix, AZ 85054; e-mail: palmer.jeanne@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal