In this issue of Blood, Palmer et al provide encouragement that important chronic graft-versus-host disease (GVHD) patient outcomes (such as overall survival [OS] and failure-free survival [FFS]) are predicted by clinician-assessed response, patient-reported outcomes, and 2014 National Institutes of Health (NIH)-response criteria.1
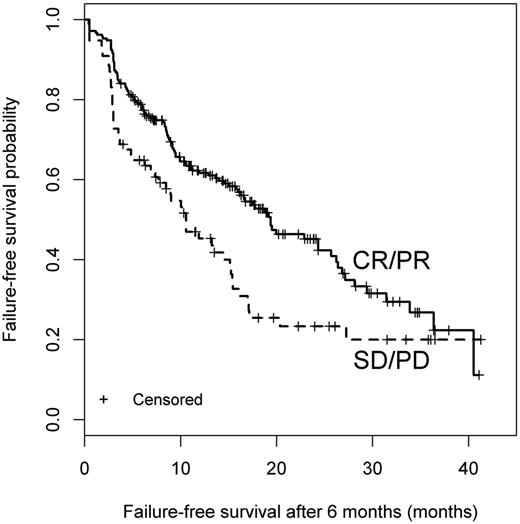
Patients with a clinician-reported response had higher likelihood of FFS than patients with SD or PD. CR, complete response; PR, partial response. See the complete Figure 1 in the article by Palmer et al that begins on page 160.
Patients with a clinician-reported response had higher likelihood of FFS than patients with SD or PD. CR, complete response; PR, partial response. See the complete Figure 1 in the article by Palmer et al that begins on page 160.
FFS has advantages for studies in chronic illness where reaching end points of OS and nonrelapse mortality (NRM) may take too long. The FFS end point takes a very global approach and incorporates objective end points (absence of systemic treatment change, NRM, and recurrent malignancy) into 1 composite end point.2-4 FFS is simple and takes into account addition of immunosuppression as well as significant (ie, life-threatening) toxicity from it. What the FFS end point does not tell us, however, is how chronic GVHD is impacting a particular patient, what manifestations are affecting them, and exactly in what order the manifestations are responding.
Because FFS does not address the symptoms and burden to the individual patient, it is very important to have shorter-term measures of disease that help predict FFS and OS. These measures are crucial to understand how immunosuppression is working on different patient groups (eg, patients with steroid-refractory disease or patients starting therapy at a particular stage in an organ), whether clinically or in a research study. These measures give us a snapshot at specific intervals on the overall burden of chronic GVHD in a specific patient.
What is so exciting from the work by Palmer et al is that criteria such as clinician-reported response, patient-reported response, and the 2014 NIH criteria are predictive of important long-term outcomes. For example, patients with a clinician-reported response had higher likelihood of FFS than patients with stable disease (SD) or progressive disease (PD) (see figure). The response measures were collected systematically in 575 patients enrolled in a prospective study of chronic GVHD.
The response measures that predict OS and FFS are simple to do in a busy clinic and are likely going to be very reproducible. In the past, chronic GVHD studies have been hampered by the lack of standardized and validated reproducible response criteria, which is particularly problematic given the pleomorphic and difficult to quantify manifestations of the disease (ie, sclerosis).5 Not surprisingly, data from the reported natural history study have shown that simple end points that also take into account the burden of disease on the patient (ie, how it is affecting their quality of life or activities of daily living) are proving to be most predictive of change and outcome. For example, NIH 0-3 skin score correlates better with physician and clinician perception of change as compared with skin body surface area measurements,6 and NIH 0-3 eye score is also more predictive of symptom change than Schirmer’s test.7 Based on these results, the 2005 NIH Consensus Criteria have recently been modified.8
Tremendous credit is due to the participants of the NIH Consensus for Chronic GVHD for developing the criteria and modifying them as data from Dr Stephanie Lee’s prospective natural history study emerged. Dr Lee and colleagues have done an excellent job systematically evaluating the NIH criteria and different elements of it and asking how they relate to long-term outcomes.
Chronic GVHD continues to be a major disability to many patients who have undergone allogeneic transplant. But we are now, compared with 10 years ago, in a much better place to study novel therapies to treat it. We now have short-term end points that correlate with important long-term outcomes and we know that the NIH response measures are sensitive to change. We have also had tremendous advances in the understanding of the basic immunopathology providing specific targets for treatment instead of the global and toxic immunosuppression currently in use. The timing is perfect to systematically study these novel therapies.
Conflict-of-interest disclosure: The author declares no competing financial interests.