Key Points
LCN2 acts to generate reactive oxygen species, leading to increased DNA strand breaks and apoptosis in normal CD34+ cells.
LCN2 promotes the generation of osteoblasts but diminishes adipogenesis, resembling the composition of the MF marrow microenvironment.
Abstract
Myelofibrosis (MF) is characterized by cytopenias, constitutional symptoms, splenomegaly, and marrow histopathological abnormalities (fibrosis, increased microvessel density, and osteosclerosis). The microenvironmental abnormalities are likely a consequence of the elaboration of a variety of inflammatory cytokines generated by malignant megakaryocytes and monocytes. We observed that levels of a specific inflammatory cytokine, lipocalin-2 (LCN2), were elevated in the plasmas of patients with myeloproliferative neoplasms (MF > polycythemia vera or essential thrombocythemia) and that LCN2 was elaborated by MF myeloid cells. LCN2 generates increased reactive oxygen species, leading to increased DNA strand breaks and apoptosis of normal, but not MF, CD34+ cells. Furthermore, incubation of marrow adherent cells or mesenchymal stem cells with LCN2 increased the generation of osteoblasts and fibroblasts, but not adipocytes. LCN2 priming of mesenchymal stem cells resulted in the upregulation of RUNX2 gene as well as other genes that are capable of further affecting osteoblastogenesis, angiogenesis, and the deposition of matrix proteins. These data indicate that LCN2 is an additional MF inflammatory cytokine that likely contributes to the creation of a cascade of events that results in not only a predominance of the MF clone but also a dysfunctional microenvironment.
Introduction
Cross talk between hematopoietic cells and nonhematopoietic marrow cells in myelofibrosis (MF) contributes to distinctive marrow microenvironmental changes that likely determine the function of specific marrow niches that support normal hematopoiesis.1-3 MF cells elaborate cytokines, which contribute to the development of marrow fibrosis, increased microvessel density, and osteosclerosis.3 These cytokines affect marrow mesenchymal cells that are not involved by the malignant process.3-9
Recently, neutrophil gelatinase-associated lipocalin (lipocalin-2; LCN2) has been implicated in the pathobiology of myeloproliferative neoplasms (MPNs).10-13 LCN2 promotes the proliferation of the malignant clone in chronic myeloid leukemia.14 In addition, LCN2 gene expression has been reported to be increased in CD34+ cells isolated from primary MF (PMF) and polycythemia vera (PV) patients, and LCN2 levels were elevated in the plasma of MPN patients.10-13 Furthermore, Kagoya and coworkers demonstrated in a mouse model that JAK2V617F-positive cells elaborate LCN2, which results in DNA damage in neighboring normal cells and cells belonging to the malignant clone by generating reactive oxygen species (ROS).11 We therefore further examined the role that LCN2 might play in MPNs.
Materials and methods
Specimen collection
Patient samples were collected at the Icahn School of Medicine at Mount Sinai. Written informed consent was obtained from patients according to guidelines established by the institutional review board of the Icahn School of Medicine at Mount Sinai. All patients met the World Health Organization diagnostic criteria for PV, essential thrombocythemia (ET), or PMF.1 CD34+ cells were isolated using a human CD34+ cell selection kit (STEMCELL Technologies, Vancouver, BC, Canada), and T- and B-cell–depleted MNCs were isolated using a human lymphocyte selection kit (STEMCELL Technologies). The severity of MF in 70 patients was assessed using prognostic parameters used for the development of the Dynamic International Prognostic Scoring System.15,16
Preparation of bone marrow ACs
Human bone narrow (BM) mononuclear cells (MNCs) were cultured in mesenchymal stem cell (MSC) growth medium (MSCGM) (Lonza) at a density of 2 to 5 × 104/mL in culture flasks or dishes. The cells that remained in suspension were removed by completely changing the medium at the time adherent cell (AC) layers were formed. The medium was changed twice weekly by replacing half of the medium with fresh MSCGM. Studies were performed with ACs that had not been passaged >4 times.
Cell proliferation assays
CD34+ cells were cultured with serum-free expansion medium (STEMCELL Technologies) containing stem cell factor, thrombopoietin, fms-like tyrosine kinase 3 ligand, and interleukin-3 (R&D Systems, Minneapolis, MN) with and without LCN2 (Sigma-Aldrich, St Louis, MO). Cell viability was determined using trypan blue (Sigma-Aldrich). The proportion of CD34+ cells was measured by flow cytometry after staining with an anti-CD34 antibody (BD Biosciences, San Jose, CA). AC proliferation was determined using the PrestoBlue Cell Viability Reagent (Life Technologies, CA).
JAK2V617F genotyping of hematopoietic colonies
CD34+ cells were plated in 30-mm dishes containing 1 mL of serum-free expansion medium with 1.1% methylcellulose, to which stem cell factor, thrombopoietin, fms-like tyrosine kinase 3 ligand, granulocyte macrophage–colony stimulating factor, interleukin-3, and erythropoietin were added with or without LCN2.17 Individual colonies were randomly plucked, and JAK2V617F was detected using nested allele-specific polymerase chain reaction (PCR).17
Flow cytometric analyses
Cells were collected and washed with MACS buffer (Miltenyi Biotec, San Diego, CA) and were stained with anti-CD34 antibody, annexin V (BD Biosciences), or LCN2 receptor antibody (Abcam, Cambridge, MA) directly. For intracellular staining, cells were fixed with 4% formaldehyde and permeabilized, and then stained with antibody to γH2AX or 2′,7′-dichlorofluorescein diacetate (Abcam) to evaluate the ROS activity. Data were acquired using a FACSCaliber analyzer (BD Biosciences).
Immunofluorescent and immunohistochemical staining
Cells were fixed with 4% formaldehyde, permeabilized, and then stained with primary antibodies. The primary antibodies were visualized with Alexa Fluor 546- or Alexa Fluor 488-conjugated immunoglobulin G (Life Technologies, Norwalk, CT). Stained slides were mounted using ProLong Gold antifade reagent (Life Technologies). Fluorescent images were acquired using a 1X71 fluorescence microscope (Olympus, Tokyo, Japan) and MicroSuite software (Olympus). Sections of formalin-fixed and paraffin-embedded MF marrow biopsy samples were baked and deparafinized. Immunohistochemical staining of LCN2 (Abcam) was performed using the Bond III autostainer (Leica Microsystems, Buffalo Grove, IL). The degree of fluorescence intensity was assessed using MetaMorph Microscopy Automation and Image Analysis Software (Molecular Devices, Sunnyvale, CA).
BM MSC differentiation assays
BM ACs were cultured in MSCGM with or without LCN2 for at least 10 days, and then in medium designed to favor either adipogenic or osteogenic differentiation (R&D Systems). The cells were fixed and immunostained.
Isolation of RNA and qRT-PCR
BM ACs were cultured with MSCGM alone or in medium containing LCN2 for 1 to 10 days. Total RNA was extracted from the ACs using an RNeasy kit (QIAGEN, Valencia, CA). Complementary DNA was reverse transcribed using EcoDry Premix kit (Clontech). The BMP2, COL1A1, OPG, PPARγ, RUNX2, TGF-β, VEGF, and GAPDH genes were evaluated by quantitative reverse-transcription (qRT)-PCR, which was performed using SYBR Green RT2 qPCR Mastermixes from QIAGEN and the RealPlex thermocycler (Eppendorf).
Statistical methods
Nonparametric Wilcoxon rank sum tests were used to assess 2 group differences; nonparametric Kruskal-Wallis analysis of variance F tests were used to assess multigroup differences. Correlations between LCN2 and hematologic parameters were determined using nonparametric Spearman correlation coefficients. Analysis of variance F tests were used to evaluate differences between cell samples. All pairwise comparisons were performed with Sidak correction in SPSS Version 20. P values <.05 after adjustment were considered statistically significant. Statistical analysis was performed using SAS 9.3.
For more details, please see the supplemental Material and methods, available on the Blood Web site.
Results
LCN2 levels are elevated in MF plasma and MF MNC-conditioned media
LCN2 levels were significantly greater in PV, ET, and PMF plasmas than in normal control plasma (P < .0001) (Figure 1A). PMF plasmas contained significantly higher levels of LCN2 (median, 93.8 ng/mL; range, 6.9-329.0 ng/mL) than PV plasmas (median, 54.8 ng/mL; range, 2.7-268.0 ng/mL) or ET plasmas (median, 47.4 ng/mL; range, 13.3-167.7 ng/mL; P = .0175). Furthermore, plasma LCN2 levels from patients with PV-MF (median, 160.7 ng/mL; range, 9.9-281.1 ng/mL) and ET-MF (median, 93.1 ng/mL, range: 31.7-173.5 ng/mL) were significantly greater than the levels present in PV and ET plasmas, respectively (P = .0002 and P = .0175, respectively) (Figure 1A). An inverse relationship between patient hemoglobin levels and LCN2 levels (P < .0001) (Figure 1B) and between platelet counts and LNC2 levels was observed (P = .0044) (Figure 1C). However, the plasma LCN2 levels were not significantly different in those patients who had more severe degrees of MF (Figure 1D). The levels of plasma LCN2 were not related to the degree of elevation of number of white blood cells (supplemental Figure 1), the type of treatment received (supplemental Figure 2), patient age (supplemental Figure 3), gender (supplemental Figure 4), or JAK2V617F status (supplemental Figure 5).
LCN2 levels are elevated in MPN plasma. (A) An enzyme-linked immunosorbent assay was used to quantitate LCN2 levels in plasma isolated from normal donors (n = 16) and patients with PV (n = 30), ET (n = 30), PMF (n = 30), PV-MF (n = 20), or ET-MF (n = 20) (Kruskal-Wallis nonparametric ANOVA; P < .0001 comparing normal plasma vs plasma from each type of MPN; P = .018 comparing PMF vs PV or ET). Plasma LCN2 levels in PV-MF and ET-MF were greater than those present in PV and ET plasmas, respectively (Wilcoxon rank sum tests; P = .0002 and P = .018, respectively).The numbers indicate the median LCN2 level present in the plasma of patients with a particular MPN. The nonparametric Spearman correlation coefficient analysis showed an inverse relationship between hemoglobin levels and LCN2 levels (coefficient, −0.35; P < .0001; n = 117) (B) and an inverse correlation between platelet counts and LCN2 levels (coefficient= −0.26, P = .0044, n = 117) (C). (D) The plasma LCN2 levels were not significantly different in those patients who had more severe degrees of MF as assessed by the number of prognostic variables that characterized their MF (Kruskal-Wallis nonparametric ANOVA; P > .05 at number 0 vs each of the others). ANOVA, analysis of variance.
LCN2 levels are elevated in MPN plasma. (A) An enzyme-linked immunosorbent assay was used to quantitate LCN2 levels in plasma isolated from normal donors (n = 16) and patients with PV (n = 30), ET (n = 30), PMF (n = 30), PV-MF (n = 20), or ET-MF (n = 20) (Kruskal-Wallis nonparametric ANOVA; P < .0001 comparing normal plasma vs plasma from each type of MPN; P = .018 comparing PMF vs PV or ET). Plasma LCN2 levels in PV-MF and ET-MF were greater than those present in PV and ET plasmas, respectively (Wilcoxon rank sum tests; P = .0002 and P = .018, respectively).The numbers indicate the median LCN2 level present in the plasma of patients with a particular MPN. The nonparametric Spearman correlation coefficient analysis showed an inverse relationship between hemoglobin levels and LCN2 levels (coefficient, −0.35; P < .0001; n = 117) (B) and an inverse correlation between platelet counts and LCN2 levels (coefficient= −0.26, P = .0044, n = 117) (C). (D) The plasma LCN2 levels were not significantly different in those patients who had more severe degrees of MF as assessed by the number of prognostic variables that characterized their MF (Kruskal-Wallis nonparametric ANOVA; P > .05 at number 0 vs each of the others). ANOVA, analysis of variance.
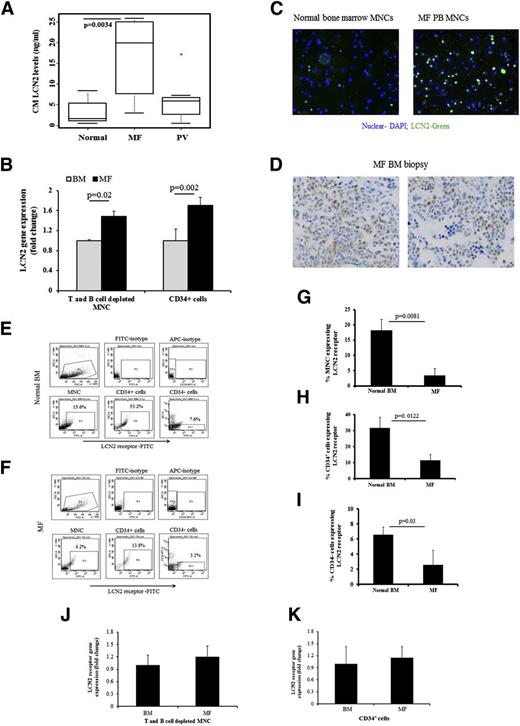
LCN2 levels were significantly greater in MF vs PV MNC–conditioned medium (CM) or normal BM MNCs (P = .0034) (Figure 2A). MF T- and B-cell–depleted peripheral blood MNCs expressed higher levels of LCN2 than normal BM MNCs depleted of T and B cells (Figure 2B). A greater proportion of MF MNCs compared to normal MNCs expressed LCN2 (Figure 2C). In addition, immunohistochemical analysis of PMF BM biopsy specimens revealed that LCN2 expression was restricted to cells belonging to the myeloid lineage rather than to erythroid or megakaryocytic cells (Figure 2D).
LCN2 levels are elevated in PMF MNC-CM, and LCN2 is localized to MF marrow myeloid cells. (A) LCN2 levels in media conditioned with MF or PV MNCs (n = 10 and n = 7, respectively) and normal bone marrow MNCs (n = 10) after incubation in serum-free expansion medium containing stem cell factor, thrombopoietin, fms-like tyrosine kinase 3 ligand, and interleukin-3 for 1 day (Kruskal-Wallis nonparametric ANOVA; P < .0034 comparing normal vs MF and PV vs MF). (B) qRT-PCR results showed that LCN2 transcript levels were greater in MF T- and B-cell–depleted MNCs, as well as in MF CD34+ cells (repeated-measures ANOVA F test; P = .02 and P = .002, respectively; n = 5). (C) Immunofluorescence staining with an anti-LCN2 antibody showing LCN2 (green) presented in normal and MF MNCs; nuclei were stained with DAPI (original magnification ×200). A greater proportion of MF MNCs expressed LCN2 compared to normal MNCs. (D) Photomicrograph demonstrating LCN2 expression by cells within a bone marrow biopsy specimen from an MF patient by staining with a LCN2 monoclonal antibody (clone 5G5, dilution 1:25, Abcam). LCN2 was expressed by myeloid cells but not by erythroid precursors or megakaryocytes (original magnification ×400). Flow cytometric analysis of LCN2 receptor expression by CD34+ cells and normal BM MNCs (E) and MF MNCs (F). (G) A greater proportion of normal BM MNCs (n = 5) expressed the LCN2 receptor as compared to MF MNCs (n = 6) (Wilcoxon rank sum test; P = .008). (H) Similarly, a greater proportion of normal BM CD34+ cells (n = 5) expressed the LCN2 receptor as compared to MF CD34+ cells (n = 5) (Wilcoxon rank sum test; P = .012). (I) A greater proportion of normal BM CD34− cells (n = 5) expressed the LCN2 receptor as compared to MF CD34− cells (n = 5) (Wilcoxon rank sum test; P = .03). (J) qRT-PCR studies indicated that LCN2 receptor gene expression was similar in both MF and normal T- and B-cell–depleted MNC cells samples. (K) qRT-PCR studies indicated that that LCN2 receptor gene expression was similar in both MF and normal CD34+ cells. DAPI, 4,6 diamidino-2-phenylindole.
LCN2 levels are elevated in PMF MNC-CM, and LCN2 is localized to MF marrow myeloid cells. (A) LCN2 levels in media conditioned with MF or PV MNCs (n = 10 and n = 7, respectively) and normal bone marrow MNCs (n = 10) after incubation in serum-free expansion medium containing stem cell factor, thrombopoietin, fms-like tyrosine kinase 3 ligand, and interleukin-3 for 1 day (Kruskal-Wallis nonparametric ANOVA; P < .0034 comparing normal vs MF and PV vs MF). (B) qRT-PCR results showed that LCN2 transcript levels were greater in MF T- and B-cell–depleted MNCs, as well as in MF CD34+ cells (repeated-measures ANOVA F test; P = .02 and P = .002, respectively; n = 5). (C) Immunofluorescence staining with an anti-LCN2 antibody showing LCN2 (green) presented in normal and MF MNCs; nuclei were stained with DAPI (original magnification ×200). A greater proportion of MF MNCs expressed LCN2 compared to normal MNCs. (D) Photomicrograph demonstrating LCN2 expression by cells within a bone marrow biopsy specimen from an MF patient by staining with a LCN2 monoclonal antibody (clone 5G5, dilution 1:25, Abcam). LCN2 was expressed by myeloid cells but not by erythroid precursors or megakaryocytes (original magnification ×400). Flow cytometric analysis of LCN2 receptor expression by CD34+ cells and normal BM MNCs (E) and MF MNCs (F). (G) A greater proportion of normal BM MNCs (n = 5) expressed the LCN2 receptor as compared to MF MNCs (n = 6) (Wilcoxon rank sum test; P = .008). (H) Similarly, a greater proportion of normal BM CD34+ cells (n = 5) expressed the LCN2 receptor as compared to MF CD34+ cells (n = 5) (Wilcoxon rank sum test; P = .012). (I) A greater proportion of normal BM CD34− cells (n = 5) expressed the LCN2 receptor as compared to MF CD34− cells (n = 5) (Wilcoxon rank sum test; P = .03). (J) qRT-PCR studies indicated that LCN2 receptor gene expression was similar in both MF and normal T- and B-cell–depleted MNC cells samples. (K) qRT-PCR studies indicated that that LCN2 receptor gene expression was similar in both MF and normal CD34+ cells. DAPI, 4,6 diamidino-2-phenylindole.
BCR-ABL protein activates the expression of LCN2 and represses LCN2 receptor expression, rendering BCR-ABL+ cells refractory to secreted LCN2.18 This finding led us to examine LCN2 receptor expression by both normal BM and MF MNCs and CD34+ cells (Figure E-F). The percentage of MF MNCs and CD34+ and CD34− cells expressing the LCN2 receptor was lower than that observed in normal BM MNCs (P = .0081, P = .0122, and P = .03, respectively; Figure 2G-I). However, LCN2 receptor messenger (m)RNA levels in MF CD34+ cells were similar to those observed in normal CD34+ cells (Figure 2J-K), suggesting that increased MF LCN2 receptor expression was a consequence of events that occurred posttranslationally. To further examine whether the downregulation of LCN receptor was the consequence of chronic exposure to LCN2, we incubated normal BM CD34+ cells from 3 different donors with LCN2 for varying periods of time (30 minutes to 3 days). We were unable to detect a significant reduction in the percentage of CD34+ cells expressing the LCN2 receptor (data not shown).
LCN2 promotes the proliferation of MF hematopoietic cells but not normal BM cells and peripheral blood cells
LCN2 increased PMF CD34+ cell numbers but decreased normal CD34+ cell numbers (P = .007) (Figure 3A). Also, LCN2 decreased the numbers of assayable normal marrow CFU-GM but not BFU-E (P = .001 and P > .05, respectively; Figure 3B; supplemental Table 1). By contrast, LCN2 did not inhibit, but rather promoted, MF colony formation derived from burst-forming unit–erythroid and CFU-GM (P = .02 and P = .009, respectively; Figure 3C; supplemental Table 2). Similarly, LCN2 treatment significantly decreased the numbers of CFU-GM-derived colonies generated from normal granulocyte colony-stimulating factor–mobilized peripheral blood CD34+ cells (P = .03 and P = .047, respectively; supplemental Figure 6; supplemental Table 3). The proliferative effect of LCN2 on MF hematopoietic progenitor cells (HPC) was observed irrespective of the patient’s JAK2V617F status (data not shown). Exposure of CD34+ cells from patients with JAK2V617F-positive MF to LCN2 did not alter the overall percentage of JAK2V617F-positive colonies but increased the proportion of homozygous JAK2V617F-positive colonies in 3 of 4 cases (Table 1).
LCN2 promotes the proliferation of MF hematopoietic cells but suppresses normal BM cell proliferation. (A) The addition of increasing doses of LCN2 increased MF CD34+ cell numbers (n = 7) but not normal BM CD34+ cell numbers (n = 6) (repeated-measures ANOVA F test, adjusted for normal BM vs MF; **P < .01). (B) LCN2 reduced normal BM CFU-GM–derived colony formation (repeated-measures ANOVA F test; P = .001 and P < .05; n = 6). (C) LCN2 promoted colony formation derived from MF burst-forming unit–erythroid (BFU-E) (repeated-measures ANOVA F test; P = .02 and P = .009; n = 11). CFU-GM, colony-forming unit–granulocyte-macrophage.
LCN2 promotes the proliferation of MF hematopoietic cells but suppresses normal BM cell proliferation. (A) The addition of increasing doses of LCN2 increased MF CD34+ cell numbers (n = 7) but not normal BM CD34+ cell numbers (n = 6) (repeated-measures ANOVA F test, adjusted for normal BM vs MF; **P < .01). (B) LCN2 reduced normal BM CFU-GM–derived colony formation (repeated-measures ANOVA F test; P = .001 and P < .05; n = 6). (C) LCN2 promoted colony formation derived from MF burst-forming unit–erythroid (BFU-E) (repeated-measures ANOVA F test; P = .02 and P = .009; n = 11). CFU-GM, colony-forming unit–granulocyte-macrophage.
Effect of LCN2 on the genotype of MF hematopoietic colonies
| Case . | JAK2 genotype of hematopoietic colonies . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control . | LCN2 20 ng/mL . | LCN2 100 ng/mL . | |||||||
| Homo . | Hete . | Wild-type . | Homo . | Hete . | Wild-type . | Homo . | Hete . | Wild-type . | |
| MF 1 | 0 (0) | 24 (100) | 0 (0) | 14 (61) | 9 (39) | 0 (0) | 13 (57) | 10 (43) | 0 (0) |
| MF 2 | 10 (43.5) | 13 (56.5) | 0 (0) | 4 (17) | 16 (67) | 4 (17) | 8 (33) | 16 (67) | 0 (0) |
| MF 3 | 11 (52) | 9 (43) | 1 (5) | 18 (100) | 0 (0) | 0 (0) | 24 (100) | 0 (0) | 0 (0) |
| MF 4 | 0 (0) | 12 (50) | 12 (50) | 8 (35.3) | 7 (29.4) | 8 (35.3) | 14 (64) | 6 (27) | 2 (9) |
| Case . | JAK2 genotype of hematopoietic colonies . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control . | LCN2 20 ng/mL . | LCN2 100 ng/mL . | |||||||
| Homo . | Hete . | Wild-type . | Homo . | Hete . | Wild-type . | Homo . | Hete . | Wild-type . | |
| MF 1 | 0 (0) | 24 (100) | 0 (0) | 14 (61) | 9 (39) | 0 (0) | 13 (57) | 10 (43) | 0 (0) |
| MF 2 | 10 (43.5) | 13 (56.5) | 0 (0) | 4 (17) | 16 (67) | 4 (17) | 8 (33) | 16 (67) | 0 (0) |
| MF 3 | 11 (52) | 9 (43) | 1 (5) | 18 (100) | 0 (0) | 0 (0) | 24 (100) | 0 (0) | 0 (0) |
| MF 4 | 0 (0) | 12 (50) | 12 (50) | 8 (35.3) | 7 (29.4) | 8 (35.3) | 14 (64) | 6 (27) | 2 (9) |
Twenty-four colonies were randomly selected for each condition. Values are represented as raw data (% of total).
Homo: homozygous; Hete: heterozygous.
LCN2 induces DNA double-strand breaks and apoptosis of normal BM cells, but not PMF MNCs and CD34+ cells
LCN2 significantly increased the proportion of normal BM cells, but not MF CD34+γ-H2AX+ cells (P = .007; Figure 4A; supplemental Table 4). We then assessed whether ROS were responsible for the accumulation of DNA strand breaks by measuring the levels of intracellular ROS in normal and MF MNCs by loading cells with 2′,7′-dichlorofluorescein diacetate. LCN2 treatment of normal, but not PMF, MNCs was associated with increased ROS activity (P = .011) (Figure 4B). PMF MNC-CM was capable of increasing the proportion of normal H2AX+ MNCs to a similar degree as purified LCN2. The administration of an LCN2 antibody, furthermore, reduced the proportion of CD34+γ-H2AX+ generated in the presence of MF CM (P = .001) (Figure 4C-D).
LCN2 induces DNA damage and increases ROS in normal, but not MF, CD34+ cells. (A) Treatment with LCN2 increased the proportion of normal, but not MF, CD34+ /γ-H2AX+ cells (repeated-measures ANOVA F test; **P < .01; n = 4). (B) Flow cytometric analysis of intensity of 2′,7′-dichlorofluorescein diacetate fluorescence, used to quantitate intracellular ROS, in normal and MF CD34+ cells (*P < .05, n = 5 and n = 6, respectively). (C) Flow cytometric analysis showing that treatment with MF CM increased the proportion of γ-H2AX+ normal BM MNCs to a similar degree as that induced by 20 ng/mL of LCN2. Prior incubation of an LCN2 antibody (R&D Systems) with MF MNC-CM reduced the proportion of γ-H2AX+ normal BM MNCs. (D) The increase in the proportion of normal γ-H2AX+ BM MNCs after the addition of LCN2 was not observed with the addition of an LCN2 antibody (repeated-measures ANOVA F test; P = .001; n = 4). (E-F) LCN2 treatment of normal BM CD34+ cells dramatically increased the percentage of CD34+annexinV+ cells (**P = .0081, ***P = .0008; n = 8); this increase was not observed with the addition of NAC to LCN2-containing cultures (repeated-measures ANOVA F test). (G-H) The percentage of MF annexinV+CD34+ cells was not increased after the addition of LCN2 or the addition of NAC to LCN2-containing cultures (repeated measures ANOVA F test; *P > .05; n = 6). NAC, N-acetylcysteine.
LCN2 induces DNA damage and increases ROS in normal, but not MF, CD34+ cells. (A) Treatment with LCN2 increased the proportion of normal, but not MF, CD34+ /γ-H2AX+ cells (repeated-measures ANOVA F test; **P < .01; n = 4). (B) Flow cytometric analysis of intensity of 2′,7′-dichlorofluorescein diacetate fluorescence, used to quantitate intracellular ROS, in normal and MF CD34+ cells (*P < .05, n = 5 and n = 6, respectively). (C) Flow cytometric analysis showing that treatment with MF CM increased the proportion of γ-H2AX+ normal BM MNCs to a similar degree as that induced by 20 ng/mL of LCN2. Prior incubation of an LCN2 antibody (R&D Systems) with MF MNC-CM reduced the proportion of γ-H2AX+ normal BM MNCs. (D) The increase in the proportion of normal γ-H2AX+ BM MNCs after the addition of LCN2 was not observed with the addition of an LCN2 antibody (repeated-measures ANOVA F test; P = .001; n = 4). (E-F) LCN2 treatment of normal BM CD34+ cells dramatically increased the percentage of CD34+annexinV+ cells (**P = .0081, ***P = .0008; n = 8); this increase was not observed with the addition of NAC to LCN2-containing cultures (repeated-measures ANOVA F test). (G-H) The percentage of MF annexinV+CD34+ cells was not increased after the addition of LCN2 or the addition of NAC to LCN2-containing cultures (repeated measures ANOVA F test; *P > .05; n = 6). NAC, N-acetylcysteine.
Exposure of normal BM CD34+ cells to LCN2 dramatically increased the number of annexin V+ cells in a dose-dependent fashion (Figure 4E-F). This effect was blocked by the addition of NAC, an ROS scavenger. By contrast, LCN2 with or without NAC did not increase the numbers of MF CD34+annexinV+ cells (Figure 4G-H). These data indicate that LCN2 not only selectively promotes the survival of PMF CD34+ cells but also leads to the apoptosis of normal BM CD34+ cells by increasing ROS.
LCN2 mediates bone marrow stromal cell proliferation by acting through the ROS pathway
We next determined whether the LCN2 receptor was expressed by normal MSCs. The LCN2 receptor was shown to be expressed by virtually all normal ACs (Figure 5A-B). We then monitored the effect of LCN2 on normal marrow ACs (MACs). After 3 weeks of incubation, increased proliferation of MACs was observed in cultures containing LCN2 alone (Figure 5C-D). Cultures with LCN2 contained fewer non-ACs but greater numbers of spindle-shaped ACs. Similar results were obtained with 6 different normal BM samples. In addition, the ability of LCN2 to promote MAC proliferation was blocked by the addition of NAC (Figure 5E-F). These data suggest that LCN2 acts on normal MSCs to promote the generation of ROS, which induces MAC proliferation. We then determined whether LCN2 secreted from PMF MNCs similarly affected the proliferation of MACs. MNCs from normal BM were cocultured with MF MNC samples known to elaborate LCN2 levels >20 ng/mL per 105 cells (supplemental Figure 7). The density of MACs was dramatically increased and appeared spindle shaped in wells containing LCN2-producing MF MNCs (supplemental Figure 7B). The density of normal BM MNCs that remained in suspension was reduced (supplemental Figure 7B). Similar changes were not observed following the culture of BM MNCs with normal or MF BM MNCs known to elaborate lower levels of LCN2 (supplemental Figure 7B).
LCN2 promotes the proliferation of BM stromal cells that express the LCN2 receptor. (A) Immunofluorescence staining using an anti-LCN2 receptor antibody (SLC22A17) indicating that normal BM ACs express the LCN2 receptor (green). The nuclei were stained with DAPI. Images represent the analysis of ACs from 2 different donors. (B) Western blot analysis of 4 different samples of normal BM ACs indicated the expression of the LCN2 receptor. (C) Phase-contrast microscopy showing AC confluence that occurred after the culture of BM MNCs with 0, 10, 20, and 50 ng/mL of LCN2 (original magnification ×200). (D) The degree of AC proliferation was assessed with the PrestoBlue Cell Viability Reagent; cell numbers were determined on the basis of fluorescence intensity at 560 nm. LCN2 treatment led to the generation of greater numbers of ACs by normal marrow MNCs (repeated-measures ANOVA F test; P = .008; n = 7). (E) Immunofluorescence analysis of vimentin expression showed that AC proliferation was impaired by the addition of NAC to LCN2-containing cultures. Similar results were obtained with 3 additional BM MNC samples; NAC alone did not affect AC proliferation. (F) Analysis of fluorescence intensity of vimentin expression showed a significantly increased expression in ACs from BM MNCs cultured with LCN2 alone (repeated-measures ANOVA F test; P = .007; n = 4), which was not observed with the addition of NAC alone or NAC plus LCN2. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
LCN2 promotes the proliferation of BM stromal cells that express the LCN2 receptor. (A) Immunofluorescence staining using an anti-LCN2 receptor antibody (SLC22A17) indicating that normal BM ACs express the LCN2 receptor (green). The nuclei were stained with DAPI. Images represent the analysis of ACs from 2 different donors. (B) Western blot analysis of 4 different samples of normal BM ACs indicated the expression of the LCN2 receptor. (C) Phase-contrast microscopy showing AC confluence that occurred after the culture of BM MNCs with 0, 10, 20, and 50 ng/mL of LCN2 (original magnification ×200). (D) The degree of AC proliferation was assessed with the PrestoBlue Cell Viability Reagent; cell numbers were determined on the basis of fluorescence intensity at 560 nm. LCN2 treatment led to the generation of greater numbers of ACs by normal marrow MNCs (repeated-measures ANOVA F test; P = .008; n = 7). (E) Immunofluorescence analysis of vimentin expression showed that AC proliferation was impaired by the addition of NAC to LCN2-containing cultures. Similar results were obtained with 3 additional BM MNC samples; NAC alone did not affect AC proliferation. (F) Analysis of fluorescence intensity of vimentin expression showed a significantly increased expression in ACs from BM MNCs cultured with LCN2 alone (repeated-measures ANOVA F test; P = .007; n = 4), which was not observed with the addition of NAC alone or NAC plus LCN2. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Phenotypic and functional analysis of LCN2-primed ACs and MSCs
Normal MACs in control cultures and cultures containing LCN2 were CD45− and CD34− but expressed vimentin, α-smooth muscle actin, CD90, CD105, and CD106 (Figure 6A-E). These phenotypic properties are consistent with adherent stromal cells with phenotypic properties associated with fibroblasts and MSCs.19
Phenotype of ACs treated with LCN2, and lineage differentiation potential of LCN2-treated ACs. Immunolabeling of MACs cultured in the presence or absence of LCN2. The ACs cultured under each of these conditions expressed vimentin+ (green) (A); α-smooth muscle actin (α-SMA+) (red) (B); CD105+ (red) (C); CD106 (green) (D); and CD90 (red) (E) (original magnification ×200). (F) Immunostaining of BM ACs that were preincubated with MSC culture medium alone for 10 days and then cultured with medium that promoted osteogenesis for 21 days. (G) Immunostaining of BM ACs that had been pretreated with LCN2 in MSC culture medium and then cultured under osteoblast-inducing conditions. Osteoblastic differentiation of MSCs was clearly enhanced by preincubation with LCN2 for 10 days; an anti-osteocalcin antibody was used to identify osteoblasts; cell nuclei were stained with DAPI. (H) The fluorescence intensity of osteocalcin was significantly increased in BM ACs pretreated with LCN2 (repeated-measures ANOVA F test; P = .0015; n = 6). (I) Immunostaining showed a greater number of adipocytes in BM ACs cultured in the MSC medium and then exposed to adipogenesis medium. (J) Immunostaining revealed that adipogenesis was diminished by pretreatment of BM ACs with LCN2 for 10 days and then cultured in adipogenesis medium. Anti-FABP4 (fatty acid–binding protein 4) antibody was used to identify adipocytes; cell nuclei were stained with DAPI. (K) Fluorescence intensity analysis revealed significantly decreased FABP4 protein expression in BM AC pretreated with LCN2 (repeated-measures ANOVA F test; P < .0001; n = 6).
Phenotype of ACs treated with LCN2, and lineage differentiation potential of LCN2-treated ACs. Immunolabeling of MACs cultured in the presence or absence of LCN2. The ACs cultured under each of these conditions expressed vimentin+ (green) (A); α-smooth muscle actin (α-SMA+) (red) (B); CD105+ (red) (C); CD106 (green) (D); and CD90 (red) (E) (original magnification ×200). (F) Immunostaining of BM ACs that were preincubated with MSC culture medium alone for 10 days and then cultured with medium that promoted osteogenesis for 21 days. (G) Immunostaining of BM ACs that had been pretreated with LCN2 in MSC culture medium and then cultured under osteoblast-inducing conditions. Osteoblastic differentiation of MSCs was clearly enhanced by preincubation with LCN2 for 10 days; an anti-osteocalcin antibody was used to identify osteoblasts; cell nuclei were stained with DAPI. (H) The fluorescence intensity of osteocalcin was significantly increased in BM ACs pretreated with LCN2 (repeated-measures ANOVA F test; P = .0015; n = 6). (I) Immunostaining showed a greater number of adipocytes in BM ACs cultured in the MSC medium and then exposed to adipogenesis medium. (J) Immunostaining revealed that adipogenesis was diminished by pretreatment of BM ACs with LCN2 for 10 days and then cultured in adipogenesis medium. Anti-FABP4 (fatty acid–binding protein 4) antibody was used to identify adipocytes; cell nuclei were stained with DAPI. (K) Fluorescence intensity analysis revealed significantly decreased FABP4 protein expression in BM AC pretreated with LCN2 (repeated-measures ANOVA F test; P < .0001; n = 6).
We next determined whether the incubation of normal MSCs with LCN2 altered their differentiation pattern. MSCs were preincubated with or without LCN2 for at least 10 days in MSCGM, and then in medium that favored osteocyte or adipocyte formation. The differentiation of MSCs toward osteoblast-like cells was enhanced by prior treatment with LCN2 as compared to MSCs not pretreated (Figure 6F-G). The fluorescence intensity of staining for osteocalcin, a protein marker of bone-forming cells, was significantly increased in MSCs pretreated with LCN2 (Figure 6H). By contrast, when LCN2-pretreated cells were cultured under differing conditions that favored adipocyte formation, adipogenesis was clearly impaired by the addition of LCN2 (Figure 6I-J). The fluorescence intensity of staining for the fatty acid–binding protein 4 was markedly decreased in LCN2-treated cells (Figure 6K). These data collectively indicate that LCN2 alters the differentiation pattern of normal MACs by promoting osteoblast and fibroblast, but not adipocyte, development.
The effects of LCN2 on MSC gene expression
As can be seen in Figure 7, after a single day of LCN2 exposure, the expression of runt-related transcription factor 2 (RUNX2), a master transcription factor associated with osteoblast differentiation,20-23 was significantly increased (Figure 7A). Similarly, the expression of TGF-β and VEGF transcripts (growth factors known to be important in remodeling the MF microenvironment)24-26 was also increased (Figure 7B), whereas bone morphogenetic protein 2 (BMP2), a member of the TGF-β superfamily involved in osteoblast differentiation,25-27 was increased on day 1 and day 6 of culture. In addition, 2 known products of osteoblasts, osteoprotegerin (OPG) and collagen type 1(COL1A1),20,21 were also increased after incubation with LCN2 (Figure 7C). By contrast, incubation with LCN2 markedly decreased PPARγ gene expression, which is associated with adipogenesis (Figure 7D). To further understand whether the ROS pathway plays a role in LCN2 alterations in BM MSC differentiation, NAC was added to LCN2-treated MSCs. NAC not only inhibited RUNX2 gene expression induced by LCN2 (Figure 7F) but also inhibited TGF-β, BMP2, VEGF, and OPG gene expression (Figure 7F). These data suggest that ROS is one of pathways by which LCN2 affects BM MSC differentiation. The pattern of gene expression illustrated in Figure 7E summarizes the pattern of genes that are expressed at different time points after incubation of MSCs.
LCN2 treatment of BM ACs induces expression of genes associated with osteoblastic, but not the adipocytic, differentiation program. qRT-PCR revealed that RUNX2 transcript levels were increased 1 day after incubation with LCN2 (A); VEGF, TGF-β, and BMP2 transcripts were also increased by the addition of LCN2 in a dose-dependent fashion after 1 day (B); LCN2 treatment increased OPG and COL1A1 gene expression after 10 days of incubation (C); and PPARγ gene levels were decreased after 1 day of incubation with LCN2 (D). (E) Three-dimensional area graph showing the time course of expression of various genes by BM ACs after incubation with LCN2 (50 ng/mL). (F) Addition of NAC blocked the expression of RUNX2, VEGF, TGF-β, BMP2, and OPG transcripts induced by LCN2 after 1 day. (G) Immunofluorescence staining with an anti-LCN2 receptor antibody (SLC22A17) revealed that MF BM ACs expressed the LCN2 receptor (green); nuclei were stained with DAPI. (H) qRT-PCR results revealed that RUNX2 transcription levels were increased in untreated MF BM ACs. (I) qRT-PCR showed that 10 days of treatment with LCN2 increased RUNX2, TGF-β, VEGF, and OPG gene expression but decreased PPARγ gene expression by MF BM ACs. (J) Diagram summarizing the proposed effects of LCN2 on hematopoietic progenitor cells and BM stromal cells in MF. DSB, DNA strand break; HPC, hematopoietic progenitor cells.
LCN2 treatment of BM ACs induces expression of genes associated with osteoblastic, but not the adipocytic, differentiation program. qRT-PCR revealed that RUNX2 transcript levels were increased 1 day after incubation with LCN2 (A); VEGF, TGF-β, and BMP2 transcripts were also increased by the addition of LCN2 in a dose-dependent fashion after 1 day (B); LCN2 treatment increased OPG and COL1A1 gene expression after 10 days of incubation (C); and PPARγ gene levels were decreased after 1 day of incubation with LCN2 (D). (E) Three-dimensional area graph showing the time course of expression of various genes by BM ACs after incubation with LCN2 (50 ng/mL). (F) Addition of NAC blocked the expression of RUNX2, VEGF, TGF-β, BMP2, and OPG transcripts induced by LCN2 after 1 day. (G) Immunofluorescence staining with an anti-LCN2 receptor antibody (SLC22A17) revealed that MF BM ACs expressed the LCN2 receptor (green); nuclei were stained with DAPI. (H) qRT-PCR results revealed that RUNX2 transcription levels were increased in untreated MF BM ACs. (I) qRT-PCR showed that 10 days of treatment with LCN2 increased RUNX2, TGF-β, VEGF, and OPG gene expression but decreased PPARγ gene expression by MF BM ACs. (J) Diagram summarizing the proposed effects of LCN2 on hematopoietic progenitor cells and BM stromal cells in MF. DSB, DNA strand break; HPC, hematopoietic progenitor cells.
MF BM ACs are capable of responding to LCN2 and expression increased RUNX2 transcripts
We then questioned whether BM MSCs from MF patients had similar responses to LCN2 as compared to normal MSCs. To address this question, the gene expression pattern of 2 individual MF BM ACs was compared to that of normal MACs. Similarly to normal ACs, the MF ACs expressed the LCN2 receptor (Figure 7G), and MF ACs also expressed higher levels of RUNX2, but not TGF-β and BMP2, transcripts as compared to normal BM ACs (Figure 7H). Importantly, after exposure to LCN2 for 10 days, RUNX2, TGF-β, OPG, VEGF, and COL1A1 gene expression was still increased, whereas BMP2 gene expression was modestly affected (Figure 7I). Similar to normal BM ACs, incubation with LCN2 decreased the expression of the PPARγ (Figure 7I). These data suggest that MF BM ACs are capable of responding to the effects of LCN2 by increasing the expression of several target genes.
Discussion
Several groups have reported that LCN2 gene expression is upregulated in MPNs and that plasma LCN2 levels are increased.10-13 In addition, a growing number of studies have provided evidence that a variety of solid tumors are characterized by overexpression of LCN2.28-31 Previously, LCN2 has been implicated in the pathobiology of another MPN, chronic myeloid leukemia, where this iron-containing protein has been shown not only to promote BCR/ABL+ hematopoiesis but also to inhibit the residual reservoir of normal hematopoietic progenitor cells, thereby likely contributing to the clonal dominance of the malignant clone.14,18 Beyond its effects on tumor cells, LCN2 expression has also been implicated as a mediator of renal, pulmonary, and cardiac fibrosis.32-34
In this study, we showed that the levels of LCN2 in the plasmas of MF patients (PMF, PV-MF, and ET-MF) were greater than those present in normal control plasma, findings that extend the observations of others.10-13 Furthermore, the LCN2 levels present in the MF plasmas were two- to threefold greater than those observed in patients with proliferative MPNs (PV and ET), providing a possible link between increased LCN2 levels and progression to MF. An inverse relationship between plasma LCN2 levels and hemoglobin levels and platelet counts, but not white blood cell numbers, in MPN patients was observed, suggesting that LCN2 might be related to the development of anemia and thrombocytopenia. The degree of increase in plasma LCN2 levels in patients was not related to gender, age, type of treatment received, or the severity of MF. Importantly, MF MNC-CM contained elevated LCN2 levels, and LCN2 mRNA expression levels were higher in lymphocyte-depleted MF MNCs. LCN2 was localized to myeloid cells within MF marrow cells. Although LCN2 transcripts were documented in this report to be detectable in normal and MF CD34+ cells,12,13 LCN2 synthesis has been previously shown to occur almost exclusively in marrow myelocytes and metamyelocytes.35-38 The addition of either purified LCN2 or MF CM containing LCN2 induced DNA damage and apoptosis in normal CD34+ cells, but not MF CD34+ cells, and affected hematopoietic colony formation in a corresponding fashion. These findings differ from those reported by Kagoya and coworkers11 who observed that LCN2 inhibited normal and JAK2V617F-positive hematopoiesis. These discrepancies can be attributed to studying cells from different species or to examining difference phases of MPNs (PV vs MF). Barosi and coworkers observed in a longitudinal study of MF patients a consistent rate of transformation from a heterozygous to a homozygous JAK2V617F mutational status, which was associated with disease progression.39 In the present study, the addition of LCN2 to MF CD34+ cells resulted in an increase in the proportion of hematopoietic colonies that were homozygous rather than heterozygous for JAK2V617, indicating that LCN2 favors the persistence of JAK2V617F homozygous rather than JAK2V617F heterozygous or JAK2 wild-type progenitor cells.
The inhibitory effect of MF CM on normal hematopoiesis was observed to be independent of the JAK2V617 status of the MF MNC donor. Kagoya and coworkers11 have reported that increased LCN2 levels were also present in MPN patient plasmas with calreticulin and JAK2 mutations. The expression of the LCN2 receptor by MF MNCs and CD34+ cells was observed to be downregulated, yet the levels of LCN2 receptor mRNA were similar in normal and MF CD34+ cells. These data suggested that downregulation of LCN2 receptor is a consequence of events that occurred posttranslationally. Sheng and coworkers have reported, however, that in murine cell lines, BCR/ABL repressed the LCN2 receptor expression by inducing a switch in binding from RUNX3, an activator of LCN2 receptor expression, to RUNX1, a repressor of LCN2 receptor expression.40 Our findings are, however, similar to the reduced expression of the thrombopoietin receptor that has been reported in the platelets of PV and MF patients, which was reported to not be a consequence of Mpl gene disruption or transcriptional repression, but rather associated with incomplete Mpl glycosylation.41,42 Furthermore, Pecquet et al43 and Royer et al44 have demonstrated that JAK2V617F is associated with an increase in Mpl degradation by the proteasome. Whether a similar mechanism could account for the reduced expression of the LCN2 receptor by MPN hematopoietic cells will require further study.
We demonstrated that LCN2 was able to damage normal cells, but not MF progenitor cells, by increasing the generation of ROS. Marty and coworkers have previously defined a role for ROS in JAK2V617F-positive hematopoiesis.45 They demonstrated in a mouse model that JAK2V617F induced the accumulation of ROS, oxidation of DNA, and accumulation of DNA strand breaks.45
As MF progresses, depletion of marrow hematopoietic cells is accompanied by progressive marrow fibrosis, increased marrow microvessel density, and osteosclerosis.1,46-48 MF-associated osteosclerosis has been associated with increased numbers of osteoblasts and reduced numbers of osteoclasts.1,49,50 The cells that make up the MF marrow microenvironment are not directly affected by the malignant process, but their differentiation patterns are influenced by cellular products elaborated by cells belonging to the MF clone.3 The coupling of the MF-associated clonal myeloproliferation with characteristic microenvironmental changes has been attributed to the intramedullary release of growth factors by dysplastic megakaryocytes and monocytes that subsequently activate MSCs. In addition, increased production of osteoprotegerin by stromal cells has been implicated in the unbalanced osteoblast production, which results in MF-related osteosclerosis.49 We provide evidence that the sequence of events leading to abnormalities of hematopoiesis and the microenvironment in MF can be attributed, at least in part, to LCN2 elaborated by malignant myeloid cells. We observed that MF MNC-CM containing high concentrations of LCN2, as well as purified LCN2, was able to promote stromal cell proliferation. These effects were eliminated by the addition of NAC, suggesting that the stromal cell proliferation was due to the generation of increased ROS. Furthermore, we demonstrated that LCN2 was capable of promoting osteoblast, but not adipocyte, differentiation of MSCs. The ability of LCN2 to increase osteoblastic differentiation is clearly reminiscent of the findings of Schepers and coworkers, who were able to show that malignant MPN myeloid cells elaborated a series of factors that stimulated murine MSCs to overproduce osteoblasts.51
We have documented that LCN2 can create a cascade of events that fuels the development of the MF phenotype. The rapid upregulation of RUNX2 within MSCs with LCN2 treatment is likely a pivotal step in their commitment to osteoblasts.21-23 Furthermore, the upregulation of TGF-β and BMP2, which follows the expression of RUNX2, likely further contributes to the amplification of the numbers of osteoblasts.26,27 In addition, the expression of BMP2 and VEGF may play a role in the development of increased marrow microvessel density.23,27 The coupling of osteoblast precursors with vascular repair has been previously reported,52 indicating that osteoblastogenesis and angiogenesis are likely directionally steered by the release of stimulatory signals such as VEGF and BMP2. The increased expression of OPG by LCN2-primed MSCs likely contributes to the development of osteosclerosis in MF because OPG promotes additional bone formation by binding to RANKL, thereby preventing osteoclast formation.53 Additionally, the increased expression of transcripts for the extracellular matrix protein collagen type 1 (COL1A1) by LCN2-stimulated MSCs is especially intriguing because type 1 collagen is increased within the marrows of patients with the most advanced forms of MF.1,21 Consistent with these observations, MF MACs contained a higher degree of expression of RUNX2 transcripts as compared to normal MSCs, which we speculate might be due to the priming of MF MSCs by LCN2 within the marrow microenvironment in humans. Furthermore, RUNX2, TGF-β, OPG, VEGF, and COL1A1 gene expression was increased, whereas PPARγ transcripts were decreased in primary normal and MF marrow MSCs treated in vitro with LCN2, suggesting that LCN2 might play a pivotal role in remodeling of the MF marrow microenvironment.
We demonstrated that normal and MF MSCs express the LCN2 receptor and that LCN2 affects MSCs by promoting the generation of ROS. ROS are well known to have diverse biological consequences, which are tissue specific.54-57 ROS has also been implicated previously as a determinant of MSC behavior58-61 in cancer patients. We document that a ROS inhibitor not only blocked BM MSC proliferation but also reduced the expression of genes associated with osteoblastic differentiation. These data are somewhat surprising because ROS has previously been reported to reduce adherence and adipocyte differentiation of rat MSCs.62,63 These discrepancies can be attributed to differences in the responses of rat and human MSCs to ROS or pathways, in addition to ROS contributing to the effects of LCN2 on human MSC differentiation.
The data presented here are illustrative of the complex interplay between the MF malignant hematopoietic cells and the marrow microenvironment (Figure 7J), which ultimately leads to the establishment and possible evolution of the MF clinical phenotype.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was support by National Institutes of Health National Cancer Institute grant 1P01CA108671 (R.H.).
Authorship
Contribution: M.L. designed and performed the experiments, analyzed the data, and wrote the paper; L.X. and Y.-C.L. performed the experiments; L.B., D.A., and J.L. assisted with the experiments; T.H. and J.D.G. analyzed the data, and participated in writing the paper; R.W. banked the plasma specimens and provided the samples; R.H. designed the experiments, analyzed the data, and wrote the paper; and all authors read and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald Hoffman, Division of Hematology/Oncology, Department of Medicine, The Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, One Gustave L. Levy Pl, Box 1079, New York, NY 10029; e-mail: ronald.hoffman@mssm.edu.