Key Points
When possible, ATG should be avoided in adults undergoing UCBT following Cy/Flu/TBI200 regimen.
Abstract
We analyzed 661 adult patients who underwent single-unit (n = 226) or double-unit (n = 435) unrelated cord blood transplantation (UCBT) following a reduced-intensity conditioning (RIC) consisting of low-dose total body irradiation (TBI), cyclophosphamide, and fludarabine (Cy/Flu/TBI200). Eighty-two patients received rabbit antithymocyte globulin (ATG) as part of the conditioning regimen (ATG group), whereas 579 did not (non-ATG group). Median age at UCBT was 54 years, and diagnoses were acute leukemias (51%), myelodysplastic syndrome/myeloproliferative neoplasm (19%), and lymphoproliferative diseases (30%). Forty-four percent of patients were transplanted with advanced disease. All patients received ≥4 antigens HLA-matched UCBT. Median number of collected total nucleated cells was 4.4 × 107/kg. In the ATG group, on 64 evaluable patients, ATG was discontinued 1 (n = 27), 2 (n = 20), or > 2 days before the graft infusion (n = 17). In multivariate analyses, the use of ATG was associated with decreased incidence of acute graft-versus-host disease (hazard ratio [HR], 0.31; 95% confidence interval [CI], 0.17-0.55; P < .0001), higher incidence of nonrelapse mortality (HR, 1.68; 95% CI, 1.16-2.43; P = .0009), and decreased overall survival (HR, 1.69; 95% CI, 1.19-2.415; P = .003). Collectively, our results suggest that the use of ATG could be detrimental, especially if given too close to graft infusion in adults undergoing UCBT following Cy/Flu/TBI200 regimen.
Introduction
Allogeneic hematopoietic stem cell transplantation offers a curative option for several hematologic malignancies. In patients lacking a suitable HLA-matched donor, unrelated cord blood transplantation (UCBT) represents a valid alternative source of stem cells.1-7 UCBT following reduced-intensity conditioning (RIC) has been shown to be a feasible approach.8,9 However, cord blood (CB) contains significantly fewer CD34+ cells and mature lymphocytes than the other sources of stem cells,10 leading to a delayed posttransplant immune reconstitution with high incidence of early nonrelapse mortality (NRM) and an increased risk of late engraftment and graft failure.11-15 In vivo recipient T-cell depletion by using antithymocyte globulin (ATG) is one of the strategies that has been established to overcome the risk of graft rejection.16,17 However, the use of ATG, incorporated within the conditioning regimen prior to UCBT, is still controversial, especially for adults receiving a RIC single-unit allo–cord blood transplant.18-20 The aim of this multicenter retrospective report was to assess the impact of ATG on the outcomes of 661 patients who underwent a single- or double-unit UCBT following a RIC in 79 European Society for Blood and Marrow Transplantation (EBMT) centers (a list of participating centers is included online in the supplemental Appendix; see the Blood Web site).
Patients and methods
Data collection
Eurocord and the EBMT Group provided data on CB recipients. Participating centers were asked to verify the data referred to EBMT Registry and to provide additional information on each patient. Indeed, a specific questionnaire containing details on ATG type, source, dose, and timing was also sent to all participating centers, as well as queries regarding inaccurate or missing data.
Inclusion criteria
Data of 661 patients who underwent transplantation between January 2004 and December 2011 in 79 EBMT centers were analyzed.
Inclusion criteria were the following: (1) age over 18 years at the time of transplantation and (2) first single- or a double-unit UCBT after RIC for hematologic malignancies. To make our population as homogeneous as possible, we included only adults who received a conditioning associating fludarabine plus cyclophosphamide and low-dose (<6 Gy) total body irradiation (TBI; Cy/Flu/TBI200 conditioning), as previously described by Brunstein et al.21 Because ATG could be part of the conditioning regimen, 82 patients received ATG (the “ATG group”) and 579 did not receive ATG (the “non-ATG group”).
Details regarding timing of ATG infusion were available for 64 patients. The median number of days of treatment was 3 (range 1-5), and the drug was stopped at a median time of 2 days before the graft infusion (range 1-7), that is, at D-2 (range D-7 to D-1), considering D0 the day of stem cell infusion. Patients were divided into 3 categories according to the time of ATG discontinuation before graft infusion: 27 patients stopped ATG at D-1 or D0; 20 stopped it at D-2, and the remaining 17 patients stopped ATG between D-7 and D-3.
Data regarding the dose and type of ATG were available for 58 patients. Median dose was 11.7 mg/kg (range 2.5-60). Patients receiving ATG Fresenius (n = 20) had higher doses at a median of 22.9 mg/kg of recipient body weight (range 5-60 mg/kg). Thirty-eight patients received ATG Thymoglobuline (Genzyme) at a median dose of 7.1 mg/kg (range 4-15 mg/kg). There was no difference between the 2 types of ATG in terms of timing of administration.
End points and definitions
Neutrophil recovery was defined as achieving an absolute neutrophil count of at least 500/mm3 for 3 consecutive days, and neutrophil engraftment was defined as neutrophil recovery excluding patients with autologous recovery. Data on patients who received a second transplantation for graft failure were censored at the time of the second transplantation. Graft failure was defined as absence of neutrophil recovery 60 days after transplantation. Acute graft-versus-host disease (GVHD) was diagnosed and graded according to published criteria22 ; chronic GVHD was diagnosed according to standard criteria23 and evaluated in patients who survived >100 days with sustained engraftment.
NRM was defined as any death without relapse. Relapse was defined on the basis of morphologic evidence of hematologic malignancies. Overall survival (OS) was defined as the time elapsed from transplant to death, whatever the cause of death, and event-free survival (EFS) was defined as survival with no evidence of relapse.
Statistical analysis
Follow-up was considered from the time of UCBT to the last assessment or death. The median follow-up for survivors was 36 months (2 to 99).
Besides the use of ATG, the following variables were considered in a risk factor analysis for outcomes: (1) patients’ characteristics including age at UCBT, gender, and cytomegalovirus (CMV) serostatus of recipient; (2) disease characteristics including diagnosis and disease status at transplant (complete remission, disease present, refractory or advanced disease); and (3) transplantation characteristics including year of UCBT, type of graft (single or double unit), GVHD prophylaxis, sex mismatch as a male patient undergoing transplantation with CB of female donor, HLA matching, ABO compatibility, total nucleated cell (TNC) dose, and CD34 infused cell dose.
Median values and ranges were used for continuous variables and percentages for categorical variables. With the use of Pearson’s χ2 test for categorical and the Mann-Whitney U test for continuous variables, we compared the differences between the ATG and the non-ATG groups. Cumulative incidence curves were built in a competing risks setting, with death treated as a competing event, to calculate the probability of neutrophil recovery, acute GVHD, chronic GVHD, and relapse.24 Probabilities of EFS and OS were estimated by the Kaplan-Meier method; the log-rank test was used for univariate comparisons. Variables found to have a P value <.10 were included in multivariate analyses, with the use of Cox proportional-hazards regression to adjust for EFS and OS and Fine and Gray’s proportional-hazards model for subdistribution of a competing risk for neutrophil engraftment, relapse, and acute and chronic GVHD.25
Statistical analyses were performed with SPSS (SPSS Inc., Chicago, IL) and R software packages.
Results
Characteristics of the patients
Table 1 shows patients’ characteristics and transplantation modalities according to the use of ATG. Median age at UCBT was 52 years (range 18-72), and patients in the ATG group tended to be older (52 for the non-ATG group vs 54 for the ATG group; P = .068).
Patients, transplant, and graft characteristics according to the use of ATG in the Cy/Flu/TBI200 regimen for UCBT in adults with hematologic malignancies
| Characteristics . | Non-ATG group (n = 579) . | ATG group (n = 82) . | Total (N = 661) . | P* . |
|---|---|---|---|---|
| Patient related | ||||
| Age at UCBT | ||||
| Median | 52 | 54 | 52 | .068 |
| Range | 18-69 | 19-72 | 18-72 | |
| Male sex, n (%) | 289 (50) | 49 (60) | 338 (51) | .10 |
| Recipient-CMV serostatus at transplant, n (%) | 338 (60) | 54 (67) | 392 (61) | .18 |
| Diagnosis, n (%) | ||||
| Acute leukemias | 294 (51) | 40 (49) | 334 (51) | .009 |
| MDS/MPD or CML | 101 (17) | 25 (31) | 126 (19) | |
| Lymphoproliferative disorders | 184 (32) | 17 (21) | 201 (30) | |
| Disease status at UCBT, n (%) | ||||
| Advanced | 245 (43) | 44 (54) | 289 (44) | .063 |
| CR1/2 | 328 (57) | 38 (46) | 366 (56) | |
| Previous ASCT, n (%) | 180 (32) | 19 (23) | 199 (30) | .12 |
| Graft related | ||||
| Single UCBT, n (%) | 197 (34) | 29 (35) | 226 (34) | .81 |
| HLA compatibility, n (%)† | ||||
| 6/6 or 5/6 | 152 (28) | 21 (30) | 173 (29) | .76 |
| ≤4/6 | 386 (72) | 49 (70) | 435 (72) | |
| ABO incompatibility, n (%) | ||||
| Compatibles/minor | 152 (29) | 10 (20) | 162 (28) | .21 |
| Major | 374 (71) | 39 (80) | 413 (72) | |
| Sex mismatch, n (%) | 187 (33) | 36 (47) | 223 (35) | .013 |
| TNC collected, median (range) | 4.42 (0.41-12.21) | 4.14 (1.99-13.67) | (0.41-13.67) | .51 |
| TNC infused, median (range) | 3.5 (0.38-9.44) | 3.54 (1.1-6.6) | (0.38-9.44) | .95 |
| Characteristics . | Non-ATG group (n = 579) . | ATG group (n = 82) . | Total (N = 661) . | P* . |
|---|---|---|---|---|
| Patient related | ||||
| Age at UCBT | ||||
| Median | 52 | 54 | 52 | .068 |
| Range | 18-69 | 19-72 | 18-72 | |
| Male sex, n (%) | 289 (50) | 49 (60) | 338 (51) | .10 |
| Recipient-CMV serostatus at transplant, n (%) | 338 (60) | 54 (67) | 392 (61) | .18 |
| Diagnosis, n (%) | ||||
| Acute leukemias | 294 (51) | 40 (49) | 334 (51) | .009 |
| MDS/MPD or CML | 101 (17) | 25 (31) | 126 (19) | |
| Lymphoproliferative disorders | 184 (32) | 17 (21) | 201 (30) | |
| Disease status at UCBT, n (%) | ||||
| Advanced | 245 (43) | 44 (54) | 289 (44) | .063 |
| CR1/2 | 328 (57) | 38 (46) | 366 (56) | |
| Previous ASCT, n (%) | 180 (32) | 19 (23) | 199 (30) | .12 |
| Graft related | ||||
| Single UCBT, n (%) | 197 (34) | 29 (35) | 226 (34) | .81 |
| HLA compatibility, n (%)† | ||||
| 6/6 or 5/6 | 152 (28) | 21 (30) | 173 (29) | .76 |
| ≤4/6 | 386 (72) | 49 (70) | 435 (72) | |
| ABO incompatibility, n (%) | ||||
| Compatibles/minor | 152 (29) | 10 (20) | 162 (28) | .21 |
| Major | 374 (71) | 39 (80) | 413 (72) | |
| Sex mismatch, n (%) | 187 (33) | 36 (47) | 223 (35) | .013 |
| TNC collected, median (range) | 4.42 (0.41-12.21) | 4.14 (1.99-13.67) | (0.41-13.67) | .51 |
| TNC infused, median (range) | 3.5 (0.38-9.44) | 3.54 (1.1-6.6) | (0.38-9.44) | .95 |
ASCT, autologous stem cell transplantation; CML, chronic myeloid leukemia; CR1/2, first and second complete remission; MDS/MPD, myelodysplastic syndrome/myeloproliferative neoplasm.
The χ2 test was used for categorical variables, and the Mann-Whitney nonparametric test for continuous variables.
HLA compatibility was analyzed by serology or low-resolution typing level for HLA-A and -B and by allelic (DNA) or high-resolution typing for HLA-DRB1.
Patients in the ATG group presented a higher proportion of MDS/MPN (31% vs 17%; P = .009) and a trend to have more advanced disease (P = .063) when compared with the non-ATG group. The proportion of patients with acute leukemia was similar in the 2 groups (49% vs 51%).
Transplantation modalities
All CB units were transplanted following a RIC regimen consisting of cyclophosphamide (50 mg/kg), fludarabine (200 mg/m2), and a single fraction of TBI (200 cGy in 86%, 400 cGy in 12%, and 600 cGy in 2% of patients). Patients in the ATG group were transplanted more recently (P = .015) and received more sex-matched CB unit (P = .013). Otherwise, all transplantation characteristics were similar. The median number of infused TNCs was 3.5 × 107/kg (range 0.38-9.44), and most patients received grafts with 4 out of 6 HLA compatibilities. HLA compatibility was analyzed by serology or low-resolution typing level for HLA-A and -B and by allelic or high-resolution typing for HLA-DRB1.
Patients’ outcomes
Neutrophil engraftment.
Cumulative incidence of 60-day neutrophil engraftment was 83 ± 1% with no difference according to the use of ATG (84 ± 1 for the non-ATG vs 82 ± 4% for the ATG group; P = .17). The median time to neutrophil engraftment was 20 days (range 1-80). Graft failure occurred in 103 patients (15.6%), 88 in the non-ATG and 15 in the ATG group. Of them, 30 were alive at a median of 33 months after transplantation. Patients with lymphoproliferative diseases presented a higher rate of neutrophil engraftment when compared with patients with acute leukemia or MDS/MPN (60-day cumulative incidence of neutrophil engraftment 90 ± 2%, 80 ± 2%, 84 ± 3%, respectively). The percentage of patients receiving previous ASCT was significantly higher in those with lymphoproliferative disorders (69.3% vs 13.4% for patients with acute leukemias or MDS/MPN; P < .0001). In multivariate analysis, previous ASCT and not diagnosis, was associated with greater neutrophil engraftment (HR, 1.40; 95% CI, 1.17-1.48; P = .0002). We found no impact of the use of ATG on the relative risk of neutrophil engraftment.
GVHD
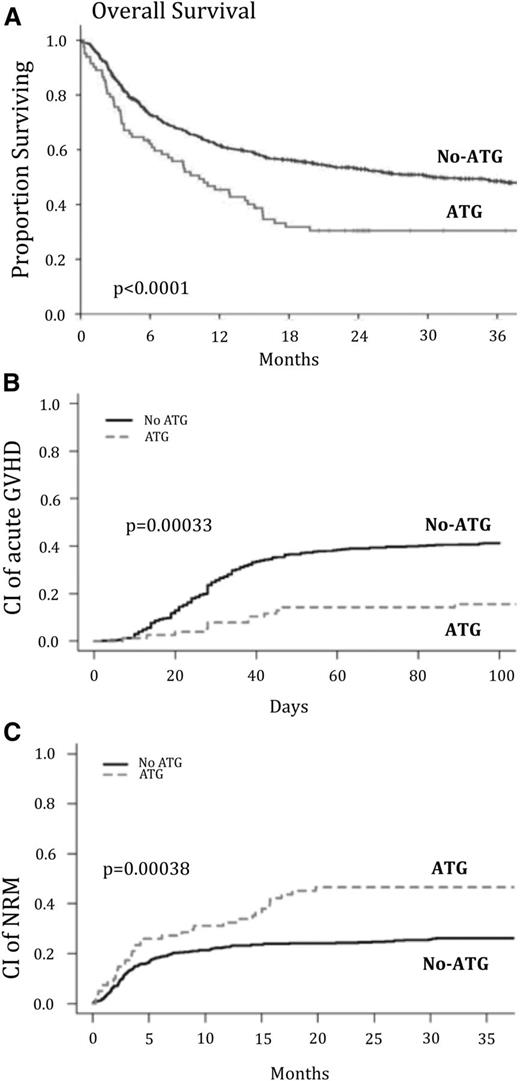
The cumulative incidence of acute grade 2 to 4 GVHD was 15 ± 4% and 41 ± 2% in the ATG and the non-ATG groups, respectively (P = .00033; Figure 1B). Likewise, acute grade 3-4 GVHD was 1.3% and 16% in the ATG and the non-ATG groups, respectively (P = .0005). In the multivariate analysis, the risk of acute grade 2 to 4 GVHD was significantly lower in the ATG group (HR, 0.31; 95% CI, 0.17-0.55; P < .0001; Table 2).
Outcome after transplantation of CB according to the presence or absence of ATG in conditioning. The unadjusted cumulative incidence of OS (A), acute GVHD (B), and NRM (C) is shown after CB transplantation after RIC.
Outcome after transplantation of CB according to the presence or absence of ATG in conditioning. The unadjusted cumulative incidence of OS (A), acute GVHD (B), and NRM (C) is shown after CB transplantation after RIC.
Multivariate analyses for outcomes after UCBT for adults with hematologic malignancies according to the use of ATG in the Cy/Flu/TBI200 regimen
| Characteristics . | 3-Year OS . | 3-Year NRM . | Acute 3-4 GVHD . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| ATG in conditioning | |||||||||
| No | 1 | [1.19-2.41] | .003 | 1 | [1.16-2.43] | .0009 | 1 | [0.17-0.55] | .000 |
| Yes | 1.69 | 1.68 | 0.31 | ||||||
| Characteristics . | 3-Year OS . | 3-Year NRM . | Acute 3-4 GVHD . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| ATG in conditioning | |||||||||
| No | 1 | [1.19-2.41] | .003 | 1 | [1.16-2.43] | .0009 | 1 | [0.17-0.55] | .000 |
| Yes | 1.69 | 1.68 | 0.31 | ||||||
Among the patients who survived >100 days, cumulative incidence of chronic GVHD was 20 ± 4% and 29 ± 2% in the ATG and the non-ATG groups, respectively (P = .072). Multivariate analysis confirmed no impact of ATG on cumulative incidence of chronic GVHD.
OS
The unadjusted 3-year probability of OS was different between the 2 groups (30 ± 5% in the ATG group vs 48 ± 2% in the non-ATG group; P < .0001) (Figure 1). In univariate analysis, other risk factors associated with a significant decrease in OS were the following: age >51 years at UCBT, CMV positive serostatus, diagnosis of MDS/MPN, advanced disease status at UCBT, and the presence of ≥2 out of 6 HLA mismatches (Table 1).
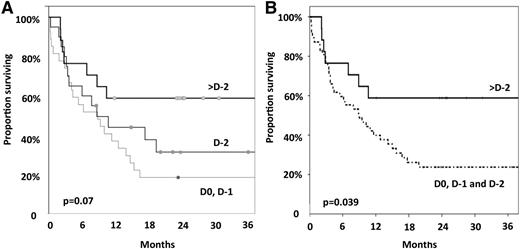
The multivariate analysis confirmed the detrimental impact of ATG on OS (HR, 1.69; 95% CI, 1.19-2.41; P = .003 for the ATG group). For the 64 patients with available data, the interval from the last dose of ATG and graft infusion seems to have impact on OS. Thus, OS was 18 ± 7%, 31 ± 11%, and 59 ± 12% in patients who stopped ATG, 1 day (at D0 or D-1), 2 days (at D-2), and >2 days (before D-2) before graft infusion, respectively (P = .07; Figure 2). When analyzing these patients in 2 groups (last dose of ATG at D-2, D-1, or D0) vs last dose of ATG before D-2 OS was 26 ± 7% and 59 ± 12%, respectively (P = .039; Figure 2).
Impact of the timing of the last ATG infusion on survival. (A) The day of transplant and the day before (D0, D-1) vs 2 days before the transplant (D-2) vs >2 days before the transplant (>D-2). (B) Within 2 days before the transplant (D0, D-1, and D-2) vs >2 days before the transplant.
Impact of the timing of the last ATG infusion on survival. (A) The day of transplant and the day before (D0, D-1) vs 2 days before the transplant (D-2) vs >2 days before the transplant (>D-2). (B) Within 2 days before the transplant (D0, D-1, and D-2) vs >2 days before the transplant.
NRM and relapse
NRM was 46% and 26 ± 2%, in the ATG and the non-ATG groups, respectively (P = .00038) (Figure 1C). This difference was confirmed by multivariate analysis (HR, 1.68; 95% CI, 1.16-2.43; P = .0009 for the ATG group). The interval from the last dose of ATG and graft infusion also seems to impact NRM. This latter was 59 ± 9%, 51 ± 12%, and 23 ± 10% in patients who stopped ATG, 1 day (at D-1), 2 days (at D-2), and >2 days (before D-2) before graft infusion, respectively (P = .12). Again, when grouping the patients into those who received ATG closer to CB cells infusion (at D-2, D-1, or D0) vs patients receiving last those of ATG before D-2 NRM was 56 ± 7 vs 23 ± 10, respectively (P = .05).
The cumulative incidence of relapse was not significantly different between the 2 groups (29 ± 5% in the ATG group vs 34 ± 2% in the non-ATG group; P = .60). In the multivariate analysis, the risk of relapse was similar in the 2 groups.
Causes of death
As shown in Table 3, 20 of the 82 patients of the ATG group (24%) died of relapse or progression, and while 158 of the 579 patients of the non-ATG group (27%) died of relapse or progression (P = .58).
Causes of death in the 2 groups
| . | Use of ATG . | P* . | |
|---|---|---|---|
| No . | Yes . | ||
| Relapse or progression, n (%) | 158 (27) | 20 (24) | .58 |
| Cause of NRM, n (%) | 148 (25) | 37 (45) | .0002 |
| Infections | 76 (13.1) | 23 (28) | .0013 |
| Bacterial | 26 (4.5) | 5 (6.1) | |
| Viral | 18 (3.1) | 6 (7.3) | |
| Parasitical | 4 (0.7) | 3 (3.7) | |
| Fungal | 12 (2.1) | 2 (2.4) | |
| Unknown | 16 (2.8) | 7 (8.5) | .016 |
| PTLD | 3 (0.5) | 5 (6.1) | .001 |
| Pulmonary toxicity | 2 (0.3) | 2 (2.4) | |
| CNS toxicity | 9 (6.1) | 1 | |
| GVHD | 25 (16.9) | 2 | .56 |
| Hemorrhage | 4 | — | |
| Multiorgan failure | 5 | — | |
| Renal toxicity | 3 | — | |
| . | Use of ATG . | P* . | |
|---|---|---|---|
| No . | Yes . | ||
| Relapse or progression, n (%) | 158 (27) | 20 (24) | .58 |
| Cause of NRM, n (%) | 148 (25) | 37 (45) | .0002 |
| Infections | 76 (13.1) | 23 (28) | .0013 |
| Bacterial | 26 (4.5) | 5 (6.1) | |
| Viral | 18 (3.1) | 6 (7.3) | |
| Parasitical | 4 (0.7) | 3 (3.7) | |
| Fungal | 12 (2.1) | 2 (2.4) | |
| Unknown | 16 (2.8) | 7 (8.5) | .016 |
| PTLD | 3 (0.5) | 5 (6.1) | .001 |
| Pulmonary toxicity | 2 (0.3) | 2 (2.4) | |
| CNS toxicity | 9 (6.1) | 1 | |
| GVHD | 25 (16.9) | 2 | .56 |
| Hemorrhage | 4 | — | |
| Multiorgan failure | 5 | — | |
| Renal toxicity | 3 | — | |
CNS: central nervous system; PTLD, posttransplant lymphoproliferative disorder.
The P value for overall causes of death is <.001 and was determined by Fisher’s exact test.
Of note, 23 (28%) and 76 (13%) patients died of infections of ATG group and non-ATG group, respectively (P = .0013). In addition, 5 (6%) and 3 (0.5%) patients died of PTLDs in ATG group and non-ATG group, respectively (P = .001).
Discussion
In adults undergoing UCBT following a RIC for hematologic malignancies, this study shows that adding rabbit ATG to the conditioning leads to less satisfactory outcomes.
To our knowledge, this is the first report regarding the role of ATG incorporated within RIC regimens in the setting of UCBT. Several studies have previously reported on the ATG in pretransplantation setting.26 However, none of them has specifically addressed the impact of ATG on patients’ outcome.
It has been previously reported that patients without recent chemotherapy before transplant, often including MDS, presented a higher risk of graft failure,4 and for this reason there would be a justification to use ATG for those patients. Indeed, we found that there was a higher frequency of patients with MDS/MPN receiving ATG in the conditioning regimen when compared with other diseases. Moreover, we found that patients with lymphoproliferative diseases presented a higher rate of neutrophil engraftment when compared with patients with acute leukemias or MDS/MPN. As expected, a significantly higher percentage of patients with lymphoproliferative diseases were treated with previous ASCT, and a detailed analysis showed that previous ASCT and not diagnosis, was associated with greater neutrophil recovery. Nevertheless, when analyzing separately the subgroup of patients not having previous ASCT as well as those with MDS/MPN, we still do not find an impact of ATG in engraftment (data not shown). Therefore, based on our results, the use of ATG had no impact on neutrophil engraftment or rejection, and we do not recommend using ATG in patients with MDS for this purpose.
According to other reports,27,28 we found that the absence of ATG had a detrimental impact on acute GVHD development. However, the absence of clear improvement on survival despite a beneficial effect of ATG in reducing acute GVHD could be explained by an overlapping impact of several complex variables on the final outcome. Indeed, the higher NRM observed in ATG group might outweigh the lower incidence of GVHD in the same group and could explain the detrimental impact of ATG on OS. In addition, patients of ATG group experienced more often fatal infections than did those of non-ATG group. As reported by Brunstein et al,21 we observed that patients of ATG group died more often of infections and PTLD than did those of non-ATG group. Indeed, delayed immune reconstitution following UCBT has been reported to be associated with high incidence of infection.3,19-21
The impact of dose of ATG on patients’ outcome was not evaluable because the type of ATG used varied among centers (Fresenius or Genzyme), and their doses are not equivalent. As reported by Lindemans et al,27 in this study the time elapsed from the last day of ATG administration and graft infusion seems to have impact on survival and NRM. Despite the relatively small size of the cohort that could be analyzed for the effect of timing of ATG administration, we performed an analysis using different groups of ATG timing with similar results: the 17 patients that received ATG before D-2 had increased OS and LFS and decreased NRM when compared with the patients that received ATG closer to CB cells infusion. Although we acknowledge that is difficult to draw definitive conclusions of timing of ATG because of low number of patients and missing information, we do believe in the adverse effect of ATG and its timing. Recently, it has been described in children that the timing of ATG administration may influence UCBT outcomes and this study corroborates our findings.19 Moreover, the importance of ATG pharmacokinetics to determine the better therapeutic window to provide improved outcomes on UCBT is under study by other groups, highlighting the importance of timing of administration.19 Still, one could argue that other factors may be playing a role in the decision process on ATG or no ATG, like the centers’ policies. To better evaluate that, we looked in our data and observed that only 7 of the 79 centers participating in this study did not follow a “pattern” of including or not ATG in the conditioning. Thus, 631 out of the 661 UCBT analyzed were performed in centers that used (or not) ATG in a homogeneous fashion for the patients included in our study.
We are aware of the retrospective nature of our study and we recognize its limitations. However, we do believe that our findings are important and potentially practice changing for transplanters. Our results suggest that the use of ATG in patients receiving UCBT following Cy/Flu/TBI200 regimen, especially if given close to graft infusion, should be avoided.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
L.P. and I.Y.-A. would like to thank the “Association Capucine” for their generous and continuous support for their clinical and basic research work.
Authorship
Contribution: L.P. and L.T. designed research, performed research, analyzed data, and wrote the manuscript; A.R. analyzed data; D.B., P. Ceballos, P. Chevallier, J.C., N.M., R.T., E.P., W.L., H.S., C.K., A.P., E.H., and H.E. performed research; and E.G., V.R., and I.Y.-A. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: I.Y.-A. received research support and honoria from Genzyme/Sanofi, whose product is discussed in this study. The remaining authors declare no competing financial interests.
A complete list of the members of the Eurocord and EBMT Group appears in the supplemental Appendix.
Correspondence: Ibrahim Yakoub-Agha, UAM allogreffes de CSH, CHRU de Lille, F-59037 Lille CEDEX, France; e-mail: ibrahim.yakoubagha@chru-lille.fr.
References
Author notes
L.P. and L.T. contributed equally to this study.
V.R. and I.Y.-A. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal