Key Points
Ribosome deficiency in zebrafish leads to defects in erythroid maturation and is reversed by RAP-011 treatment.
Identification of lefty1 as a key mediator of erythropoiesis.
Abstract
Diamond-Blackfan Anemia (DBA) is a bone marrow failure disorder characterized by low red blood cell count. Mutations in ribosomal protein genes have been identified in approximately half of all DBA cases. Corticosteriod therapy and bone marrow transplantation are common treatment options for patients; however, significant risks and complications are associated with these treatment options. Therefore, novel therapeutic approaches are needed for treating DBA. Sotatercept (ACE-011, and its murine ortholog RAP-011) acts as an activin receptor type IIA ligand trap, increasing hemoglobin and hematocrit in pharmacologic models, in healthy volunteers, and in patients with β-thalassemia, by expanding late-stage erythroblasts through a mechanism distinct from erythropoietin. Here, we evaluated the effects of RAP-011 in zebrafish models of RPL11 ribosome deficiency. Treatment with RAP-011 dramatically restored hemoglobin levels caused by ribosome stress. In zebrafish embryos, RAP-011 likely stimulates erythropoietic activity by sequestering lefty1 from erythroid cells. These findings identify lefty1 as a signaling component in the development of erythroid cells and rationalize the use of sotatercept in DBA patients.
Introduction
Diamond-Blackfan Anemia (DBA) is a bone marrow failure disorder where the predominant defect is in the development of erythroid precursors into mature erythrocytes. Although macrocytic anemia is the primary characteristic, physical abnormalities and a predisposition to certain cancers are also observed.1,2 Although the exact mechanism of red blood cell failure is unknown, a defect in ribosome biogenesis or function is believed to be the primary cause of DBA; thus, DBA is classified as part of a group of disorders characterized by malfunctioning ribosomes, also referred to as ribosomopathies. Approximately half of all DBA patients carry a mutation in a gene coding for a ribosomal protein, and it is predicted that several unidentified mutations in DBA are involved with ribosome function or biogenesis. A mutation in the ribosomal protein RPS19 was the first identified ribosomal protein aberration discovered in DBA patients.3-5 This gene is the most commonly mutated ribosomal protein found in DBA patients, occurring in ∼25% of cases.6 Since then, the repertoire of ribosomal proteins found mutated in DBA patients has expanded and includes mutation in RPS24, RPS26, RPS10, RPS35A, RPL5, and RPL11.7
Defects in ribosomal proteins are expected to have a profound impact on the whole animal and affect various cell types; therefore, the predominantly tissue-specific defect of DBA, and other ribosomopathies, remains an interesting question. Several hypotheses have been proposed to explain the specificity of the disease.7,8 One widely accepted model is that the affected tissues have an increase in p53 activity upon ribosome stress.9 Elevation in the p53-pathway leads to cell-cycle arrest and apoptosis, whereas suppression of the p53-pathway upon ribosome stress has been shown to alleviate both the phenotypic and erythropoietic defects in zebrafish and other models of DBA.10-12
Although targeting the p53-signaling pathway has shown promising results in cellular and animal models, caution must be taken into account because of the potential for cancer development in patients.13 The p53-pathway is abnormally regulated in many cases of cancer; therefore, targeting this pathway may further increase a patient’s risk for cancer development. Recent works using cellular and animal models of ribosome deficiency have also demonstrated p53-independent pathways involved in the erythroid defect.14-16 Identification of such pathways has created promising new avenues into the treatment of this disease.
Sotatercept (ACE-011) is a ligand trap drug generated from the fusion of the activin receptor type IIA (ActRIIA) extracellular domain to the Fc domain of human IgG1.17-19 This molecule antagonizes signaling downstream of ActRIIA through sequestering distinct members within the transforming growth factor-β (TGF-β) family, including bone morphogenetic proteins (BMPs), growth differentiation factors (GDFs), and Activin molecules, from binding to the endogenous receptor. Injection of RAP-011 (the murine form of ACE-011) into murine models of anemia can restore red blood cell parameters.17,20 Furthermore, a study using sotatercept in healthy volunteers demonstrated that it can increase hemoglobin levels.18 Currently, sotatercept is being evaluated for efficacy in anemia associated with diseases such as β-thalassemia, myelodyspastic syndrome and renal disease. Also, there is an ongoing clinical trial to test the efficacy of sotatercept in the treatment of DBA.
We aim to test the ability of RAP-011 to restore erythroid levels in zebrafish models of ribosome deficiency and to elucidate the role of TGF-β signaling molecules during ribosome stress. Our work demonstrates that RAP-011 can elevate erythroid counts under normal physiological conditions and during ribosome deficiency in zebrafish. The rescue in erythropoiesis occurred at early stages of development, when the erythroid arrest appears to be p53-independent. Finally, we provide evidence to show that RAP-011 can block the function of lefty1 (Lft1), an activin/nodal signaling antagonist; thus providing a model where RAP-011 can activate or inhibit activin/nodal signaling in a context-dependent manner.
Methods
Zebrafish stains and husbandry
Zebrafish protocol and maintenance were performed using methods approved by the University of California, Los Angeles Institutional Animal Care and Use Committee.
Zebrafish injections
Rpl11 was targeted in embryos by injecting a translational start codon morpholino (5′-CTTCTTCTCGCTCTGGTCCGCCATG-3′) into the cell at the one-cell stage. Unless noted, morpholino was used at ∼1.0 ng per embryo. mRNA (20 pg) injection was also done at the one-cell stage into the cell of the developing embryo. RAP-011 and mouse IgG was injected at 4 ng per embryo at 4.0 hpf.
Quantitative polymerase chain reaction
Total RNA was extracted in Trizol Reagent (Life Technologies) according to the manufacturer’s protocol. cDNA was synthesized using Superscript III (Life Technologies) and oligoDt (Life Technologies) following the manufacturer’s protocol. Quantitative real-time polymerase chain reaction was performed using FastStart SYBR Green Master (Roche). Primer sequences can be found in supporting documents (supplemental Table 1) available on the Blood Web site.
Whole-mount in situ hybridization
Embryos were fixed overnight at 4°C using 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS). After fixation, PFA was washed using 4 washes (5 minutes each) in PBS followed by 2 washes (5 minutes each) with 100% methanol. Embryos were stored at −20°C. Whole-mount in situ hybridization was performed using DIG-labeled probed as previously described.21
o-Dianisidine staining
Embryos were fixed at room temperature for 2 hours using 4% PFA in PBS followed by 3 washes (5 minutes each) in PBS. Embryos were incubated in o-Dianisidine staining solution (40% ethanol, 0.65% hydrogen peroxide, 10 mM Na-acetate, and 0.6 mg/mL o-Dianisidine [Sigma]) in the dark for 30 minutes. After incubation, embryos were washed 4 times in PBS and then placed into bleach solution (1% potassium hydroxide, 3% hydrogen peroxide) for 20 minutes to remove pigmentation. Embryos were then washed in PBS and immediately imaged.
Flow cytometry
Embryos were removed from the chorion, and yolk protein was removed mechanically in 4°C calcium-free Ringers solution. After deyolking, embryos were dispersed into a single cell using a 0.5% trypsin solution. Trypsinization was stopped using Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum. Cells were then pelleted, resuspended in PBS with 1% bovine serum antigen, and filtered with a 40-μ mesh.
Results
Rpl11 deficiency causes erythroid-specific defect
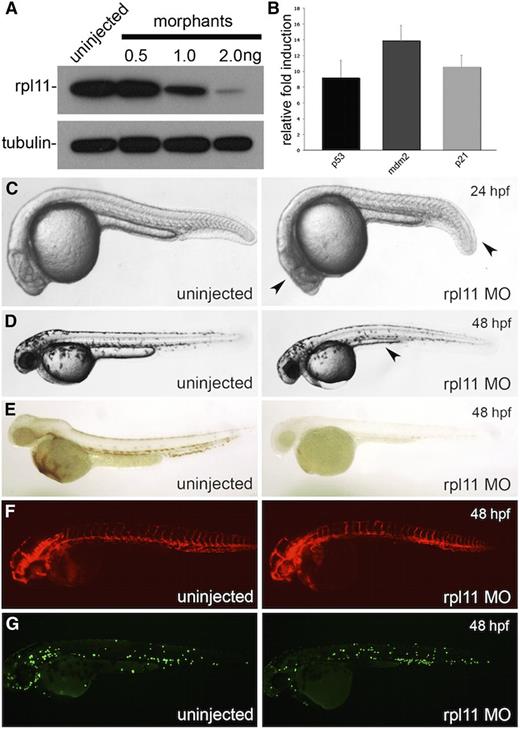
Although the rpl11 genetic mutant is available in zebrafish,12 it is less ideal for use in experiments for this study, because only one-fourth of the offspring will carry a homozygous mutation and show a phenotype; furthermore, mutations in ribosomal protein genes lead to insufficient protein levels in patients as opposed to a complete absence of the protein. We therefore model rpl11 deficiency in zebrafish embryos by using a translational start codon morpholino to knockdown rpl11 protein levels by ∼50% (Figure 1A). This level is comparable with levels seen in DBA patients carrying mutations in RPL11.22 Similar to previous observations, we detected an increase in levels of p53 and p53-target genes such as p21 and mdm2 at our level of rpl11 knockdown (Figure 1B).12,23 A mild developmental delay is also observed in embryos by 24 hours post-fertilization (hpf). At this stage, a shortened trunk and aplasia in the head can be seen (Figure 1C). By 48 hpf, knockdown of rpl11 results in mild edema, a defective yolk extension, and a smaller head (Figure 1D). o-Dianisidine staining for mature erythroid cells reveals that morphants had significantly reduced levels of hemoglobin (Figure 1E). Because of the close developmental association between vascular and myeloid lineages in the developing embryo, we injected morpholino into kdrl:dsRED and mpo:EGFP transgenic embryos to observe the effects of ribosome deficiency on endothelial and neutrophil cell development, respectively. Analysis of these embryos reveals no significant changes in the developing vasculature or neutrophil cells, suggesting that development of these lineages is normal (Figure 1F-G).
Knockdown of Rpl11 in zebrafish embryos model the erythroid and developmental defect in DBA. Rpl11 in embryos were targeted using a translational start codon morpholino. (A) Western blot against Rpl11 in 24 hpf embryos injected with various doses of morpholino. (B) Transcript quantification of p53, mdm2, and p21 at 24 hpf. (C-D) Morphologic features of morphants at 24 hpf (C) and 48 hpf (D). Arrowheads point to the defective head development and shorted tail extension at 24 hpf. At 48 hpf, a smaller head, edema, and defective yolk extension were observed (arrowhead) in morphants. (E) o-Dianisidine staining of zebrafish embryos at 48 hpf. (F) Flk:dsred transgenic zebrafish at 48 hpf. (G) Mpo:EGFP transgenic zebrafish at 48 hpf.
Knockdown of Rpl11 in zebrafish embryos model the erythroid and developmental defect in DBA. Rpl11 in embryos were targeted using a translational start codon morpholino. (A) Western blot against Rpl11 in 24 hpf embryos injected with various doses of morpholino. (B) Transcript quantification of p53, mdm2, and p21 at 24 hpf. (C-D) Morphologic features of morphants at 24 hpf (C) and 48 hpf (D). Arrowheads point to the defective head development and shorted tail extension at 24 hpf. At 48 hpf, a smaller head, edema, and defective yolk extension were observed (arrowhead) in morphants. (E) o-Dianisidine staining of zebrafish embryos at 48 hpf. (F) Flk:dsred transgenic zebrafish at 48 hpf. (G) Mpo:EGFP transgenic zebrafish at 48 hpf.
Rag1 and c-myb are two markers typically used to label cells of the definitive lineage of hematopoiesis in zebrafish. The expression pattern of rag1 was reduced in morphants compared with control embryos (supplemental Figure 1). In addition, work by previous groups showed that knockdown of rpl11 led to lower levels of c-myb.24 These data suggest that knockdown of rpl11 in zebrafish embryos also affects definitive hematopoiesis. Furthermore, staining by acridine orange showed an elevation of apoptosis upon knockdown of rpl11 (supplemental Figure 2). Overall, insufficient levels of RPL11 in zebrafish embryos led to mild physical abnormalities, apoptosis, hematopoietic defects, and elevated p53 activity, phenotypes similar to those observed in DBA patients.
Maturation and proliferation of erythroid cells is defective in Rpl11 DBA model
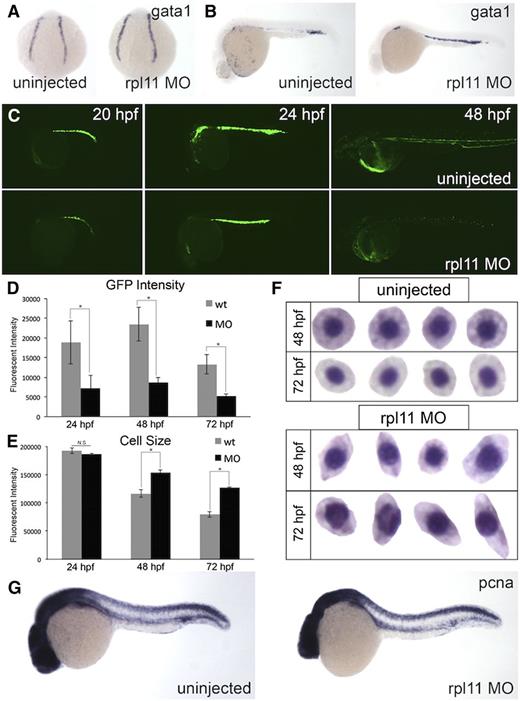
Next, we wanted to determine whether the defect in erythroid development is caused by improper specification and/or differentiation of the erythroid cells. To address specification, we looked at the expression pattern of gata1, one of the earliest markers for erythroid development.25 When the expression of gata1 was studied by whole-mount in situ hybridization, we did not notice observable differences between control or morphants at the 4 somites stage, suggesting that hematopoietic cells can be specified into the erythroid lineage (Figure 2A). We also looked at the expression of gata1 at 30 hpf, when circulation is well established. Again, morphants had equivalent levels of gata1 expression compared with control, although there was a delay in the number of cells entering circulation (Figure 2B). This was further confirmed by quantification of gata1 transcript levels (supplemental Figure 3). To further characterize the development of erythroid cells, we performed a time-course analysis on LCR2:EGFP transgenic embryos (which have enhanced green fluorescent protein [EGFP]-labeled globin-positive cells).26 When morpholino to rpl11 was injected into LCR2:EGFP embryos, EGFP-positive cells were observed in morphants at 20 hpf, and an increase in EGFP can be seen in embryos by 24 hpf. Interestingly, by 48 hpf, EGFP was also observed but was significantly less compared with control (Figure 2C). The remaining EGFP-positive cells can be seen circulating throughout the embryo (supplemental Video 1), further demonstrating the presence of an intact vasculature. Across all time points analyzed, the average EGFP intensity in morphants was significantly lower than in controls (Figure 2D). The lower EGFP intensity and lack of o-Dianisidine–positive staining suggest a defect in globin production and/or maturation in the erythroid cells. Furthermore, analysis of the size of EGFP-positive cells reveal that the cells in morphants are larger compared with control (Figure 2E). The circulating erythroid cells are also irregularly shaped (Figure 2F). Together, these findings indicate that a late-stage erythropoietic defect may be involved in rpl11 deficiency.
Terminal differentiation and proliferation, but not specification, are defective in RPL11 deficiency. Erythroid cells at various stages of development were analyzed. (A-B) In situ hybridization for gata1 expression. (A) Four somites stage. (B) 30 hpf. (C) Time course of morphants in LCR2:EGFP transgenic zebrafish. (D-E) Flow cytometry analysis of EGFP-positive cells. (D) EGFP intensity. (E) Cell size, measured by forward scatter; *P < .05. (F) Wright-Giemsa staining of isolated erythroid cells collected from zebrafish embryos. (G) In situ hybridization for PCNA in uninjected or morpholino-injected embryos. Observed phenotype (G): uninjected = 24/24 morphants = 23/25.
Terminal differentiation and proliferation, but not specification, are defective in RPL11 deficiency. Erythroid cells at various stages of development were analyzed. (A-B) In situ hybridization for gata1 expression. (A) Four somites stage. (B) 30 hpf. (C) Time course of morphants in LCR2:EGFP transgenic zebrafish. (D-E) Flow cytometry analysis of EGFP-positive cells. (D) EGFP intensity. (E) Cell size, measured by forward scatter; *P < .05. (F) Wright-Giemsa staining of isolated erythroid cells collected from zebrafish embryos. (G) In situ hybridization for PCNA in uninjected or morpholino-injected embryos. Observed phenotype (G): uninjected = 24/24 morphants = 23/25.
The decrease in EGFP-positive cells between 24 and 48 hpf raises the possibility that proliferation may be affected at this stage of development. In zebrafish embryos, proliferating cell nuclear antigen (PCNA) was previously reported to label actively dividing cells in the intermediate cell mass (ICM), a site of hematopoietic development in the developing embryo.27,28 Compared with control, morphants had a slight reduction of PCNA in the ICM, suggesting that proliferation of cells in the ICM is affected upon ribosome stress (Figure 2G). Collectively, these results suggest that it is not specification of erythrocytes that is affected in rpl11 deficiency, but proliferation and terminal maturation that are dysregulated.
Delay in erythroid maturation is independent of p53
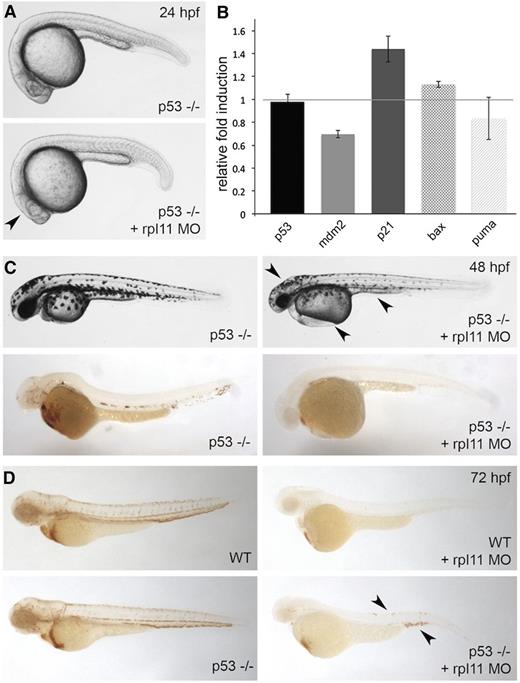
The association between ribosome stress and p53 is well-established.9,10,23 To determine whether the defects in terminal maturation are p53-dependent, we injected morpholino against rpl11 into p53-null zebrafish embryos.29 As previously demonstrated, inhibition of p53 partially prevents the physical abnormalities associated with ribosome stress at 24 hpf (Figure 3A).23 Transcript levels of p53 and p53-target genes were analyzed by real-time polymerase chain reaction to confirm the decrease in p53 activity (Figure 3B). Interestingly, a slight increase in p21 levels was still observed when rpl11 was targeted in the p53 mutant embryos, but the downstream targets mdm2, bax, and puma remain unchanged. At 48 hpf, the developmental abnormalities and lack of globin-positive cells were still apparent when rpl11 was targeted in p53-null zebrafish embryos (Figure 3C). However, inhibition of p53 partially restored the erythroid defect at 72 hpf (Figure 3D). Collectively, these results suggest that the erythroid defect is p53-independent at early stages of development and become p53-dependent later on.
Inhibition of p53 partially restored erythroid levels at later stages of development. Rpl11 morpholino was injected in p53-null zebrafish embryos. (A) Morphology of p53-null zebrafish embryos at 24 hpf. The arrowhead indicates the recovery of aplasia in the head. (B) Transcript quantification for p53 and genes downstream of p53: mdm2, p21, bax, and puma at 24 hpf. (C, top) Morphology of p53-null zebrafish embryos at 48 hpf. The arrowhead points to the presence of a smaller head, edema, and defective yolk extension. (C, bottom) o-Dianisidine staining of embryos reveals lack of hemoglobin-positive cells. (D) o-Dianisidine staining of wild-type (WT) (top) or p53-null (bottom) embryos at 72 hpf. The arrowhead points to the recovery of erythroid cells in p53-null embryos. Observed phenotype (D): WT + rpl11 MO = 40/40 p53(−/−) + rpl11 MO = 30/40.
Inhibition of p53 partially restored erythroid levels at later stages of development. Rpl11 morpholino was injected in p53-null zebrafish embryos. (A) Morphology of p53-null zebrafish embryos at 24 hpf. The arrowhead indicates the recovery of aplasia in the head. (B) Transcript quantification for p53 and genes downstream of p53: mdm2, p21, bax, and puma at 24 hpf. (C, top) Morphology of p53-null zebrafish embryos at 48 hpf. The arrowhead points to the presence of a smaller head, edema, and defective yolk extension. (C, bottom) o-Dianisidine staining of embryos reveals lack of hemoglobin-positive cells. (D) o-Dianisidine staining of wild-type (WT) (top) or p53-null (bottom) embryos at 72 hpf. The arrowhead points to the recovery of erythroid cells in p53-null embryos. Observed phenotype (D): WT + rpl11 MO = 40/40 p53(−/−) + rpl11 MO = 30/40.
Activin ligand trap increases proliferation of erythroid cells in zebrafish embryos
Because RAP-011 was successfully used to treat various models of anemia,20,30 we wanted to see whether RAP-011 could also be used to restore erythroid levels in our model. Alignment of the activin receptor among zebrafish, mice, and humans showed high similarity (supplemental Figure 4). Therefore, we hypothesize that RAP-011 would also elevate erythroid levels in zebrafish and potentially restore erythroid levels in our rpl11 DBA model.
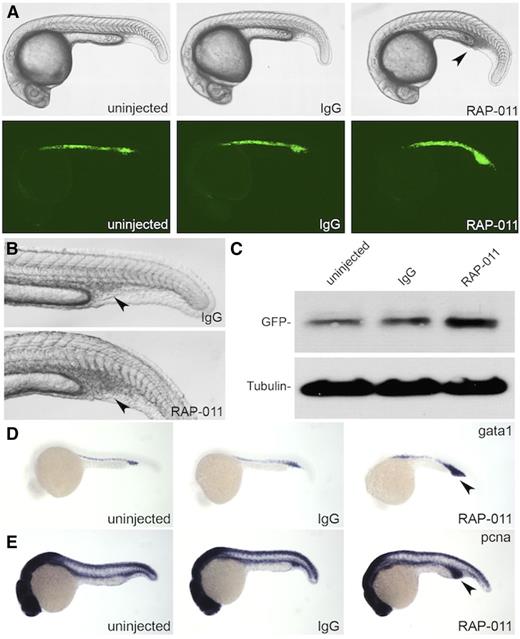
To test whether RAP-011 can function in zebrafish, we injected it into the embryo at the shield stage of development (∼4.0 hpf) between individual cells (extracellularly) to ubiquitously distribute the drug in the embryos (supplemental Figure 5). Staining against the IgG domain using fluorescent secondary antibodies reveals that, at 24 hpf, IgG and RAP-011 are present throughout the embryo (supplemental Figure 5). Interestingly, at 24 hpf, an expansion of the ICM was apparent in RAP-011–injected embryos, but not in IgG-injected embryos (Figure 4A-B). Furthermore, this expansion occurred dose dependently (supplemental Figure 6). We found that 4 ng of RAP-011 injected into the embryos expanded the ICM with minimal developmental defects. Consistent with the notion that RAP-011 functions as a ligand trap, injection of RAP-011 into the developing embryo did not cause an ICM expansion (data not shown).
RAP-011 expands erythroid cells in zebrafish embryos. (A-B) Expansion of ICM observed in RAP-011–treated embryos but not in IgG control. The arrow points to the ICM region. (C) Western blot of EGFP from LCR2:EGFP embryos treated with IgG or RAP-011. (D) In situ hybridization for gata1 expression in IgG or RAP-011–treated embryos at 24 hpf. (E) In situ hybridization for PCNA expression in IgG or RAP-011–treated embryos at 24 hpf. The arrows in (D-E) point to the expanded ICM region. Observed phenotype (D): WT + IgG = 30/30 WT + RAP-011 = 26/30; (E) WT + IgG = 29/30 WT + RAP-011 = 27/29.
RAP-011 expands erythroid cells in zebrafish embryos. (A-B) Expansion of ICM observed in RAP-011–treated embryos but not in IgG control. The arrow points to the ICM region. (C) Western blot of EGFP from LCR2:EGFP embryos treated with IgG or RAP-011. (D) In situ hybridization for gata1 expression in IgG or RAP-011–treated embryos at 24 hpf. (E) In situ hybridization for PCNA expression in IgG or RAP-011–treated embryos at 24 hpf. The arrows in (D-E) point to the expanded ICM region. Observed phenotype (D): WT + IgG = 30/30 WT + RAP-011 = 26/30; (E) WT + IgG = 29/30 WT + RAP-011 = 27/29.
The expanded cells within the ICM were also EGFP-positive, as revealed by injecting RAP-011 into LCR2:EGFP transgenic embryos (Figure 4A, bottom panel, Figure 4C, and supplemental Figure 5). Probing for gata1 via in situ hybridization further demonstrates that cells within the expanded ICM are indeed of the erythroid lineage (Figure 4D). Injection of RAP-011 into mpo:EGFP transgenic fish reveal that cells of the myeloid lineage are also expanded (supplemental Figure 7).
Next, we wanted to determine whether the expansion of cells within the ICM is caused by an increase in cell proliferation. Higher levels of PCNA were observed in RAP-011–injected embryos compared with IgG or uninjected controls (Figure 4E). These experiments suggest that RAP-011 can function to increase erythroid levels in zebrafish embryos by increasing cell proliferation, similar to observations made in cell culture systems.17,31
Erythroid levels can be recovered using RAP-011
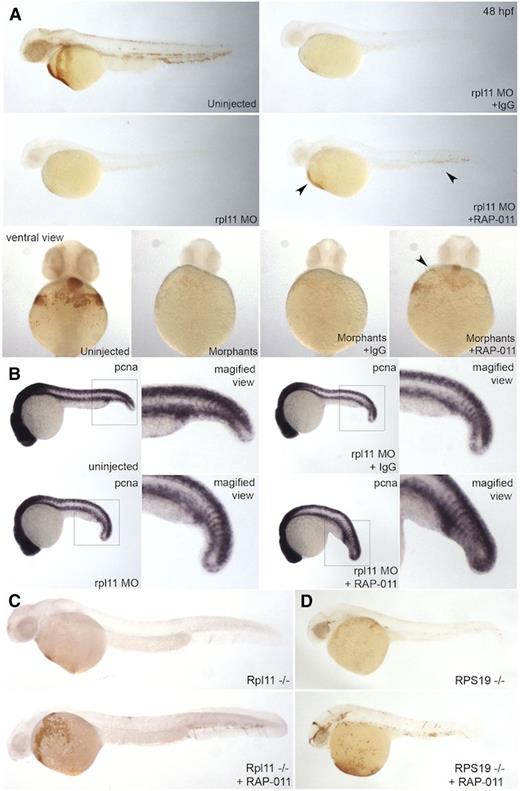
To test whether RAP-011 can be used to recover the erythroid cells in zebrafish deficient for rpl11, we injected RAP-011 into rpl11 morphant embryos. Treatment with RAP-011 partially restored erythroid cells, as shown by the presence of positively stained cells with o-Dianisidine. Furthermore, no phenotypic rescue was observed, because the defective yolk extension and head development were still present. No rescue of erythroid cells was observed upon treatment with IgG (Figure 5A). Staining for PCNA suggests that RAP-011 restores erythroid count by promoting the proliferation of cells upon ribosome stress (Figure 5B). Gene expression analysis of p53, p21, and mdm2 indicate no changes in the expression levels of these genes, suggesting that RAP-011 works independently of the p53-pathway (supplemental Figure 8).
RAP-011 can restore erythroid levels in ribosome-deficient embryos. (A) Rpl11 morpholino-injected embryos were treated with IgG or RAP-011. Staining of 48 hpf embryos with o-Dianisidine shows a partial recovery (seen in ∼87.0% of embryos; P < .05) of globin-positive cells (arrows). (B) In situ hybridization for PCNA expression in morpholino-injected embryos, 24 hpf, treated with IgG or RAP-011. Rescue by PCNA staining seen in ∼87.2% of embryos; P < .05. (C-D) o-Dianisidine staining of RPL11 or RPS19-mutant embryos treated with RAP-011. Observed phenotype (A): Rpl11 MO + IgG = 32/32 Rpl11 MO + RAP-011 = 54/62; (B): Rpl11 MO + IgG = 58/58 Rpl11 MO + RAP-011 = 41/47; (C): Rpl11(−/−) = 15/15 Rpl11(−/−) + RAP-011 = 10/10; (D): Rps19(−/−) = 8/8 Rps19(−/−) + RAP-011 = 12/12.
RAP-011 can restore erythroid levels in ribosome-deficient embryos. (A) Rpl11 morpholino-injected embryos were treated with IgG or RAP-011. Staining of 48 hpf embryos with o-Dianisidine shows a partial recovery (seen in ∼87.0% of embryos; P < .05) of globin-positive cells (arrows). (B) In situ hybridization for PCNA expression in morpholino-injected embryos, 24 hpf, treated with IgG or RAP-011. Rescue by PCNA staining seen in ∼87.2% of embryos; P < .05. (C-D) o-Dianisidine staining of RPL11 or RPS19-mutant embryos treated with RAP-011. Observed phenotype (A): Rpl11 MO + IgG = 32/32 Rpl11 MO + RAP-011 = 54/62; (B): Rpl11 MO + IgG = 58/58 Rpl11 MO + RAP-011 = 41/47; (C): Rpl11(−/−) = 15/15 Rpl11(−/−) + RAP-011 = 10/10; (D): Rps19(−/−) = 8/8 Rps19(−/−) + RAP-011 = 12/12.
We further tested this drug on the previously described rpl11 and rps19 genetic models of ribosome deficiency in zebrafish.12,32 Injection of RAP-011 into both rpl11 and rps19-mutant embryos restored hemoglobin levels (Figure 5C-D). Together, these data suggest that RAP-011 treatment promoted erythroid maturation and proliferation in our models.
Next, we wanted to test whether RAP-011 could be used in combination with other treatments. Dexamethasone has been shown to restore erythropoiesis in cellular and zebrafish models of DBA.12,33 When rpl11-deficient embryos were treated with both dexamethasone and RAP-011, an additive effect on erythroid levels was observed compared with either treatment alone (supplemental Figure 9).
Lefty1 is expressed within tissues surrounding the ICM and upregulated upon Rpl11 deficiency
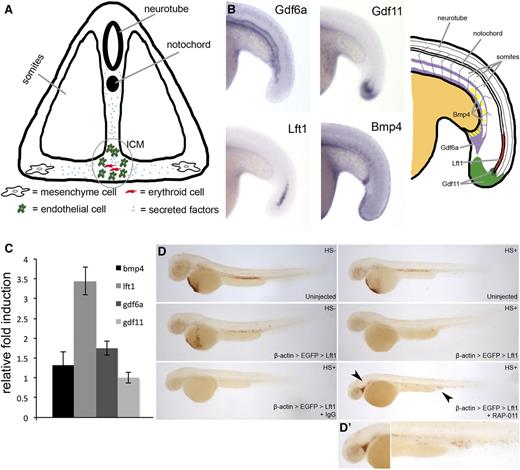
Previous work showed that RAP-011 influences the hematopoietic niche within the bone marrow to elicit the increase in erythropoiesis.31 RAP-011 sequesters extracellular ligands from binding to receptors on the cell surface. RAP-011 might also function in a similar manner in the developing embryo (Figure 6A). Because the expanded ICM can first be observed between 18 and 20 hpf, we looked at the expression of various members of the TGF-β family in tissues surrounding the ICM at this stage. BMP4, LFT1, GDF6a, and GDF11 were detected in the tissues surrounding the ICM (Figure 6B and supplemental Figure 10). To determine which factor may be involved in the delayed maturation of erythroid cells upon ribosome deficiency, we measured transcript levels of these factors in rpl11 morphants. Interestingly, Lft1 was upregulated in morphants compared with control (Figure 6C). Elevated Lft1 expression was also observed in rpl11 and rps19-mutant zebrafish (supplemental Figure 11). This raises the possibility that Lft1 can inhibit globin production in erythroid cells.
RAP-011 antagonizes the function of Lft1. (A) Illustrated cross-section of ICM region. Secreted factors from somites, neurotube, notochord, mesenchyme, and endothelial cells can affect erythroid development. (B, left) In situ hybridization for GDF6a, GDF11, Lft1, and BMP4 at 18 hpf. (B, right) Illustration of the expression pattern of various TGF-β family members. (C) Transcript quantification of TGF-β family members in rpl11 morphants compared with control embryos at 48 hpf. (D) hsp:Cre-dsRed transgenic embryos injected with plasmid for the temporal expression of Lft1. Embryos were also treated with IgG or RAP-011. o-Dianisidine staining of embryos at 48 hpf. The arrows point to elevated erythroid cells seen in the caudal vein and head region (D’). Observed phenotype (D): Uninjected (no heatshock) = 30/30, Uninjected (heatshock) = 26/26, Injected (no heatshock) = 26/28, Injected (heatshock) = 25/30, Injected (heatshock) + IgG = 23/25, Injected (heatshock) + RAP-011 = 23/30.
RAP-011 antagonizes the function of Lft1. (A) Illustrated cross-section of ICM region. Secreted factors from somites, neurotube, notochord, mesenchyme, and endothelial cells can affect erythroid development. (B, left) In situ hybridization for GDF6a, GDF11, Lft1, and BMP4 at 18 hpf. (B, right) Illustration of the expression pattern of various TGF-β family members. (C) Transcript quantification of TGF-β family members in rpl11 morphants compared with control embryos at 48 hpf. (D) hsp:Cre-dsRed transgenic embryos injected with plasmid for the temporal expression of Lft1. Embryos were also treated with IgG or RAP-011. o-Dianisidine staining of embryos at 48 hpf. The arrows point to elevated erythroid cells seen in the caudal vein and head region (D’). Observed phenotype (D): Uninjected (no heatshock) = 30/30, Uninjected (heatshock) = 26/26, Injected (no heatshock) = 26/28, Injected (heatshock) = 25/30, Injected (heatshock) + IgG = 23/25, Injected (heatshock) + RAP-011 = 23/30.
RAP-011 antagonizes the effects of lefty1 overexpression
To test whether overexpression of Lft1 can delay the maturation of erythroid cells, we overexpressed Lft1 in zebrafish embryos. We used a construct to facilitate the temporal expression of Lft1, thereby allowing us to overcome the gastrulation defects associated with Lft1 overexpression by mRNA injection, and to overexpress Lft1 when globin expression occurs (supplemental Figure 12). This plasmid was injected into transgenic zebrafish embryos with Cre-recombinase being driven under the control of the heat shock promoter. When Lft1 is overexpressed by heat shocking the embryos at approximately 14-18 hpf, reduced globin levels in erythroid cells was observed, demonstrating the inhibitory effects of Lft1 on globin production (Figure 6D). We then injected RAP-011 into the Lft1 temporally overexpressing embryos, and saw a recovery of o-Dianisidine–positive staining (Figure 6D).
Previous work showed that overexpression of the extracellular domain of the activin receptor can antagonize the effects of Lft1.34 We therefore tested whether RAP-011, which has the extracellular domain of the activin receptor type IIA, can also antagonize the effects of Lft1 overexpression in vivo. We injected in vitro transcribed, capped mRNA coding for Lft1 into the developing embryos and saw that overexpression of Lft1 by mRNA injection caused developmental defects and a reduction in mesoderm-derived tissues (supplemental Figure 13).35 As expected from a reduction in mesoderm levels, there is also a reduction in the development of erythroid cells. When RAP-011 or IgG was injected into zebrafish embryos overexpressing Lft1, RAP-011–treated embryos showed recovery of erythroid cells (supplemental Figure 13). Overall, these finding indicate that RAP-011 can function in zebrafish embryos and antagonize the effects of Lft1.
Discussion
We demonstrated that haploid insufficiency of ribosomal proteins caused an erythroid-specific defect in zebrafish embryos. Rpl11 deficiency causes an erythroid-specific defect though both a p53-dependent and p53-independent pathway, although this defect appears to be p53-independent at the earlier stages of development. Both p53-dependent and p53-independent mechanism in the pathogenesis of ribosome deficiency has been previously described.12,14,16,23
Through analysis of gata1 expression, the specification of erythroid cells appears to be unaffected. Injection of morpholino into LCR2:EGFP embryos and analysis of PCNA expression reveal that terminal maturation and proliferation are defective. Using the activin ligand trap RAP-011, we demonstrated that treatment of zebrafish embryos with RAP-011 could increase erythropoiesis and partially restore hemoglobin levels in embryos deficient for ribosomal proteins, rationalizing the use of sotatercept in DBA patients. The incomplete rescue in erythroid levels may be caused by several factors: (1) Not all RAP-011–expanded cells can mature into erythroid cells and (2) protein translation is not fully rescued. Interestingly, l-Leucine was shown to overcome defects in zebrafish models of ribosome deficiency and restore erythroid levels.32,36 This raises the possibility of using sotatercept in combination with l-Leucine treatment in DBA patients.
Lft1, an antagonist to activin/nodal signaling, was upregulated in our RPL11-deficient zebrafish embryos, as well as in the RPL11 and RPS19-mutant embryos, albeit to a lower degree. We believe that differences in gene expression level between the knockout and knockdown may be a result of the presence of maternal transcripts in the genetic model. Differences between the two RPL11 zebrafish models have been previously described.24 We demonstrated that this elevation of Lft1 could block the maturation of erythroid cells, potentially through antagonizing activin signaling. Past work in cell culture demonstrated the importance of activin signaling for the induction of globin during erythroid development.37-42 Using RAP-011, we can antagonize the effects of Lft1 overexpression. Others have demonstrated that Lft1 is a member of the TGF-β family of signaling molecules, and provided evidence that suggest Lft1 inhibits activin receptor signaling by binding to the activin receptors without activating it.35 RAP-011 may therefore enhance activin receptor signaling through sequestering Lft1. Biochemical assays to identify whether Lft1 can interact with RAP-011 in vitro proved technically challenging. However, our rescue experiments, as well as work from other groups, support an in vivo interaction between Lft1 and the activin receptor.34,35 We cannot rule out that the effects of Lft1 on erythroid development in our model are specific to the primitive stages of erythropoiesis. The exact role of Lft1 in hematopoiesis, especially definitive hematopoiesis, remains an interesting question.
Other members of the TGF-β family cannot be ruled out as having a role in contributing to the erythroid defect. In some instances, members of the TGF-β family are not regulated at the transcriptional level, but rather at the posttranslational level, such as through the cleavage of a repression domain by matrix metalloproteinases (MMPs).43,44 Upon increased MMP activity, latent TGF-β molecules are converted to active TGF-β. An increase in MMP9 was previously observed in response to rpl11 deficiency.12,24 In our model, an increase in MMP9 is also observed (data not shown). The precise role of this elevated MMP9 has yet to be determined, but we speculate that this elevation in MMP9 may activate latent members of the TGF-β family in the ICM. This increase in TGF-β activity may explain the elevation of p21 independent of p53.45 Interestingly, MMP9 is expressed within a subset of myeloid cells within the ICM46 ; therefore, it may be possible that these myeloid cells secret MMP9, thereby activating the latent TGF-β family molecules, which in turn effect the development of erythroid cells. Such a mechanism would be analogous to the regulation of erythroid cells by macrophages in the bone marrow.47
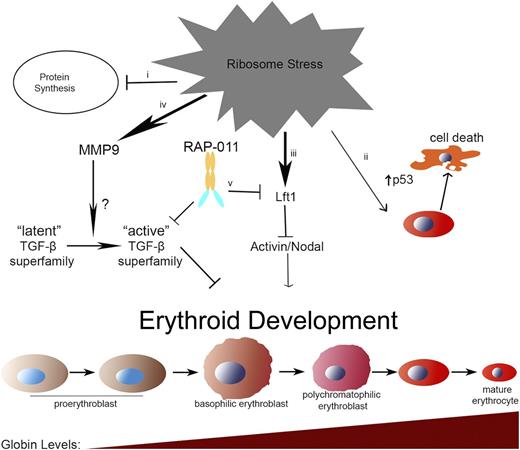
In the current study, we have provided a model in which members of the TGF-β family of signaling molecules are misregulated during ribosome stress (Figure 7). These molecules affect the development of erythroid cells by blocking proliferation, delaying globin production, and/or inducing cell-cycle arrest and apoptosis. RAP-011 binds to and sequesters several of these molecules, thus restoring erythroid development. Understanding how an activin ligand trap such as RAP-011 can promote erythropoiesis remains difficult to explain, given the contribution of activin molecules to erythroid development.17,48 Our work provides evidence to suggest RAP-011 can function to enhance activin signaling by antagonizing activin inhibitors such as Lft1. Therefore, RAP-011 can promote an increase in erythroid counts by maintaining homeostasis.
Model of erythroid failure during ribosome stress in zebrafish embryos and rescue with RAP-011. Multiple defects lead to the erythroid failure in zebrafish embryos upon ribosome deficiency. (i) Ribosome stress leads to lower rates of protein synthesis. (ii) Increase in p53 activity in erythroid cells lead to cell-cycle arrest and cell-death after the delay in maturation. (iii) Lft1 blocks the erythroid-promoting activity of activin/nodal signaling. (iv) Elevated MMP9 activity converts latent TGF-β to its active form. Activated TGF-β/TGF-β superfamily members induce cell-cycle arrest in erythroid cells. (v) RAP-011 sequesters and blocks active TGF-β superfamily members and Lft1 from binding to the endogenous receptor.
Model of erythroid failure during ribosome stress in zebrafish embryos and rescue with RAP-011. Multiple defects lead to the erythroid failure in zebrafish embryos upon ribosome deficiency. (i) Ribosome stress leads to lower rates of protein synthesis. (ii) Increase in p53 activity in erythroid cells lead to cell-cycle arrest and cell-death after the delay in maturation. (iii) Lft1 blocks the erythroid-promoting activity of activin/nodal signaling. (iv) Elevated MMP9 activity converts latent TGF-β to its active form. Activated TGF-β/TGF-β superfamily members induce cell-cycle arrest in erythroid cells. (v) RAP-011 sequesters and blocks active TGF-β superfamily members and Lft1 from binding to the endogenous receptor.
In humans, Lft1 (also called EBAF or TGF-β4) is highly expressed in women with abnormal endometrial bleeding and has been correlated with infertility.49-53 Furthermore, expression of Lft1 has been observed in colonic, duodenal, and ovarian adenocarcinomas.54 The antagonistic relationship between Lft1 and RAP-011 raises the interesting possibility of using sotatercept to treat these diseases where the expression of Lft1 is misregulated.
Finally, our studies demonstrate that protein drugs can be effectively evaluated in zebrafish disease models, offering a unique tool to study their mechanisms of action.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Matt Veldmen and Dr Nadia Danilova for their insightful discussions throughout the course of the work and Jessica Hsueh for providing invaluable technical assistance. They acknowledge Linda Dong for maintenance of the zebrafish strains used in the experiments.
This work was supported by Celgene.
Authorship
Contribution: J.E., H.H., Z.T., A. Lindgren., and T.W. performed the experiments; J.E., S.L., V.S., T.O.D., A. Laadem, and R.C. designed experiments; and J.E. and S.L. wrote the manuscript.
Conflict-of-interest disclosure: V.S., R.L., T.D., and R.C. are employees of Celgene. The remaining authors declare no competing financial interests.
Correspondence: Shuo Lin, University of California at Los Angeles, Department of Molecular, Cell, and Developmental Biology, 615 Charles E. Young Drive South, BSRB 490B, Los Angeles, CA 90095-1606; e-mail: shuolin@ucla.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal