Abstract
T-cell immunophenotype of acute lymphoblastic leukemia (T-ALL) is an uncommon aggressive leukemia that can present with leukemic and/or lymphomatous manifestations. Molecular studies are enhancing our understanding of the pathogenesis of T-ALL, and the discovery of activating mutations of NOTCH1 and FBXW7 in a majority of patients has been a seminal observation. The use of pediatric intensive combination chemotherapy regimens in adolescents and young adults has significantly improved the outcome of patients with T-ALL. The use of nelarabine for relapsed and refractory T-ALL results in responses in a substantial minority of patients. Allogeneic hematopoietic cell transplantation (HCT) still plays a key role in patients with high-risk or relapsed/refractory disease. γ-Secretase inhibitors hold promise for the treatment of patients with NOTCH1 mutations, and the results of clinical trials with these agents are eagerly awaited. It is recommended that younger patients receive a pediatric-intensive regimen. Older and unfit patients can receive suitable multiagent chemotherapy and be allocated to HCT based on their response, risk factors, and comorbidities. Although advances in the treatment of T-ALL have lagged behind those of B-cell ALL, it is hoped that the molecular revolution will enhance our understanding of the pathogenesis and treatment of this aggressive lymphoid malignancy.
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive malignant neoplasm of the bone marrow. It accounts for ∼20% of all cases of ALL and is somewhat more common in adults than children, although the incidence diminishes with older age.1 Its clinical presentation can include hyperleukocytosis with extramedullary involvement of lymph nodes and other organs, including frequent central nervous system infiltration and the presence of a mediastinal mass, arising from the thymus. T-ALL is a precursor lymphoid neoplasm according to the World Health Organization (WHO) classification and is a distinct entity from adult T-cell leukemia/lymphoma, which is a malignancy of mature T cells caused by human T-cell lymphotropic virus type I.2
Biology and pathogenesis
The WHO defines lymphoblasts in T-ALL as TdT positive with variable expression of CD1a, CD2, CD3, CD4, CD5, CD7, and CD8. Cytoplasmic CD3 and CD7 are often positive. T-ALL can be subdivided into different stages by intrathymic differentiation, including pro-T, pre-T, cortical T, and medullary T.3,4 The immunophenotypes of these subtypes are listed in Table 1.
Immunologic classification of T-ALL
| . | cCD3 . | CD7 . | CD28 . | CD1a . | CD34 . | CD4 . | CD8 . |
|---|---|---|---|---|---|---|---|
| Pro-T | + | + | − | − | +/− | − | − |
| Pre-T | + | + | + | − | +/− | − | − |
| Cortical T | + | + | + | + | − | + | + |
| Mature T* (medullary) | + | + | + | − | − | +/− | +/− |
| . | cCD3 . | CD7 . | CD28 . | CD1a . | CD34 . | CD4 . | CD8 . |
|---|---|---|---|---|---|---|---|
| Pro-T | + | + | − | − | +/− | − | − |
| Pre-T | + | + | + | − | +/− | − | − |
| Cortical T | + | + | + | + | − | + | + |
| Mature T* (medullary) | + | + | + | − | − | +/− | +/− |
cCD3, cytoplasmic CD3.
Also surface (membrane) CD3+.
Microarray gene expression studies have established a close association between differentiation arrest gene expression signatures, immunophenotype, and activation of oncogenic pathways, enriching our understanding of T-ALL heterogeneity from that gained from immunophenotype-only–based classifications.5,6 Supervised analysis of microarray data based on expression of T-ALL transcription factor oncogenes activated by chromosomal translocations distinguished different major groups of T-ALL: early T-cell precursor (ETP) ALL or immature T-ALLs (related to pro-T and pre-T immunophenotypic groups), TLX tumors with expression of high levels of CD1 genes and transcriptionally related to early cortical thymocytes (related to cortical T ALLs), and TAL1-associated leukemias, which showed a gene expression signature, reflecting an arrest in the late stages of thymocyte differentiation characterized by upregulation of CD3 and TCR genes (related to the medullary-T immunophenotypic group).5,6 In this context, the poorly understood ETP group has received much attention lately.
In children, ETP ALL accounts for 15% of all T-ALL, whereas in adults, the incidence has been variably reported but seems to be significantly higher than in the pediatric population.7-9 The transcriptional profile of ETP ALL shows similarities to immature myeloid progenitors and hematopoietic stem cells, suggesting these tumors may be part of a spectrum of stem cell–like leukemias.7 ETP ALL was originally defined by the absence of expression of CD1a, CD8, and CD5 and aberrant expression of myeloid and stem cell markers.9,10 ETP ALL may be immunophenotypically,11 genetically,8 and biologically heterogeneous.12 Of note, gene expression profiling studies suggest that early immature leukemias transcriptionally related to ETPs may account for up to 50% of T-ALLs in some series.8
The WHO classifies T-ALL and T-cell lymphoblastic lymphoma (T-LL) together despite differences in clinical presentation.3 Lymphoblastic lymphoma accounts for 1% to 2% of non-Hodgkin lymphoma and is of T-cell origin in 90% of cases with frequent presence of a large mediastinal mass. It is distinguished from T-ALL somewhat arbitrarily by the presence of <20% marrow blasts. Studies of gene expression profiling have shown differential expression of genes involved in chemotactic responses and angiogenesis, suggesting a role in tumor cell localization, which may explain the differences in clinical expression.13,14
T-cell transformation involves the cooperative effect of oncogenes and tumor suppressors, which deregulate the mechanisms controlling normal T-cell proliferation, differentiation, and survival during thymopoiesis, as recently reviewed by Van Vlierberghe et al15 (Figure 1). Among these, constitutive activation of the NOTCH1 gene plays a key role. NOTCH1 was first discovered as a partner gene in the t(7;9)(q34;q34) chromosomal translocation16 and was later implicated in the pathogenesis of up to 60% of T-ALL, harboring activating mutations, involving negative regulatory domains responsible for the control of initiation and termination of NOTCH signaling.17,18 Additionally, it was found that mutations in the FBXW7 gene are present in 15% of T-ALL cases, and these interfere with the proteasomal degradation of activated NOTCH1 protein.18-21 In addition to NOTCH1 mutations, T-ALLs frequently show T-cell receptor gene chromosomal translocations, resulting in aberrant expression of transcription factor oncogenes, such as TAL1, LMO1, LMO2, TLX1, and TLX3.15 Furthermore, mutations in the IL7R, JAK1, and JAK3 genes activate the IL7R/JAK-STAT pathway, and loss of the PTEN tumor suppressor gene drives aberrant phosphatidylinositol 3-kinase (PI3K)-AKT signaling.15 The genetic landscape of T-ALL also includes loss of transcription factors (eg, WT1, LEF1, RUNX1, ETV6, and BCL11B), epigenetic (eg, EZH2, SUZ12, and PHF6) tumor suppressors, cell-cycle inhibitors (eg, CDKN2A, RB, and CDKN1B), gains of oncogenes (eg, MYB), and chromosomal rearrangements that can result in fusion products, including CALM-AF10, MLL1-ENL, and NUP214-ABL1.15 Of note, the genetics of ETP ALL are closely related to that of myeloid leukemias with a low frequency of NOTCH-activating mutations and deletions in the CDKN2A/B loci, otherwise present in 70% of T-ALLs, and a higher prevalence of mutations activating cytokine receptor and RAS signaling, including N-RAS, K-RAS, FLT3, IL7R, JAK3, JAK1, SH2B3, and BRAF, and inactivating hematopoietic transcription factors, such as GATA3, ETV6, RUNX1, IKZF1, and epigenetic factors, such as EZH2, EED, SUZ12, SETD2, and EP300.7,22,23
The landscape of genetic alterations in T-ALL. Schematic representation of the most common genes targeted by chromosomal translocations, deletions, and mutations in T-ALL. Font size is indicative of the relative prevalence of these alterations, with highly prevalent targeted genes shown in in larger font sizes and less frequently altered loci shown in smaller font size.
The landscape of genetic alterations in T-ALL. Schematic representation of the most common genes targeted by chromosomal translocations, deletions, and mutations in T-ALL. Font size is indicative of the relative prevalence of these alterations, with highly prevalent targeted genes shown in in larger font sizes and less frequently altered loci shown in smaller font size.
What is the best therapy for patients with newly diagnosed T-ALL?
Chemotherapy
The remarkable success of pediatric ALL treatment has not been achieved in adults, although outcomes in pediatric T-ALL have been inferior to pediatric B-cell ALL (B-ALL).24,25 For the last several decades, the treatment of adults with ALL has resulted in survivals of ∼40%. However, multiple studies have now demonstrated that adolescents and young adults (AYAs) treated with pediatric-intensive chemotherapy regimens fare better than AYAs treated with adult-intensive chemotherapy regimens. This was first reported in a retrospective comparison of 321 AYAs who were treated in several trials, either by the Children’s Cancer Group (CCG) or the Cancer and Leukemia Group B (CALGB), from 1988 to 2001.26 The complete remission (CR) rates between the pediatric and adult cohorts were the same, but the AYAs treated by the CCG had a 67% overall survival (OS) at 7 years in contrast to 46% for the AYAs in the CALGB trials. No significant differences in outcome were noted between the B-ALL and T-ALL subsets in either cohort.26 Comparisons from pediatric and adult groups in other countries have shown similar results.27 These studies have generally focused on patients under the age of 21 years. In T-ALL, a randomized pediatric study showed that the addition of high-dose methotrexate improved event-free survival and OS.28
Based on these retrospective comparisons, several prospective studies using pediatric-intensive regimens in AYAs in different countries have uniformly shown favorable outcomes, with OS in the 60% to 70% range.29-31 These studies included patients with B-ALL and T-ALL. However, a Canadian study has reported their retrospective experience with pediatric-intensive regimens in patients with T-ALL and has reported similar results, with a 75% survival at 5 years.32 Similarly, the French Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) assessed the results of young adults treated with T-ALL and has seen favorable outcomes, with an OS at 3 years of 58%.33 The upper age limit for administration of a pediatric-intensive regimen is not well defined. Some studies have used these regimens in patients up to age 50 to 60 years and shown feasibility, though toxicity and treatment-related mortality (TRM) are higher in older adults.29,34,35
On the basis of these studies, it is our recommendation that AYA patients with T-ALL be treated with a pediatric-intensive regimen at diagnosis to achieve remission and potentially cure them.
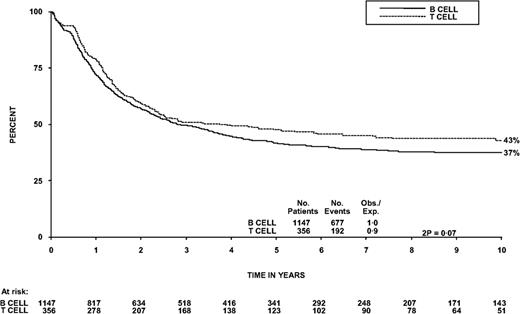
More challenging is the optimal approach to the treatment of older patients with T-ALL. Studies have demonstrated that these patients do not tolerate pediatric-intensive regimens as well as AYAs.29 The large combined trial of adults with ALL from the Medical Research Council (MRC) in Great Britain and the Eastern Cooperative Oncology Group (ECOG) in the United States, UKALLXII/E2993, has reported the outcomes of a subset of 356 patients with T-ALL up to age 60 years. This trial showed that the outcome of T-ALL is equivalent to or superior to that of B-ALL1 (Figure 2).
OS from the diagnosis of patients with B- vs T-ALL in the UKALLXII/E2993 trial.
There are few data on the role of positron emission tomography in the diagnosis and follow-up of patients with T-ALL. In one series, the use of positron emission tomography did not predict the risk of relapse.36 In this study, however, the addition of mediastinal irradiation appeared to result in prolonged time to progression compared with patients who only received chemotherapy. However, the role of mediastinal radiation in patients with T-ALL is not well defined, and we do not recommend its routine use.
Another regimen to consider is the hyper-CVAD (cyclophosphamide, vincristine, Adriamycin, and dexamethasone alternating with high dose methotrexate and cytarabine) regimen. Although intensive, this regimen is reasonably well tolerated in fit older individuals. However, in a small series of T-ALL patients, although a high CR rate was seen with the hyper-CVAD regimen, and it was safely administered, there was a high risk of relapse after achievement of remission.37
In 2009, the German ALL Group (GMALL) reported their experience with 744 T-ALL patients between the ages of 15 and 55 years. The CR rate was 86%, with OS at 10 years of 47%. OS at 5 years improved from 44% to 56% with the addition of pegaspargase in induction and high-dose methotrexate with pegaspargase in consolidation in latter trials.38 The treatment of older adults with ALL has also been reviewed in this “How I Treat” series in 2013.39
An important therapeutic agent in the management of T-ALL is nelarabine, a prodrug of guanine arabinoside, with increased solubility.40
Nelarabine has been combined with chemotherapy in the front-line setting in both pediatric and adult patients.41 In adults, nelarabine has been combined with hyper-CVAD as initial therapy. Of 40 patients with T-ALL or T-LL, the CR rate was 89% in T-ALL and 94% in T-LL. OS at 3 years was 63%.42 Nelarabine is being further tested in a phase 2 randomized trial in Great Britain, comparing its addition as consolidation therapy in patients with T- ALL (NCT01085617).
The management of T-ALL has evolved from the use of standard lymphoma regimens to the use of ALL regimens, incorporating induction, consolidation, delayed intensification, and maintenance with CNS prophylaxis with high-dose chemotherapy and intrathecal therapy. Although no randomized trials have been done, outcomes with ALL-type regimens appear superior to the use of lymphoma regimens, as has been recently reviewed.43
Hematopoietic cell transplantation
Given the poor outcome of older adults with ALL, the question has been raised as to whether they could benefit from an allogeneic hematopoietic cell transplantation (HCT) with a reduced-intensity conditioning (RIC) regimen. A retrospective analysis from the European Society for Blood and Marrow Transplant (EBMT) assessed the outcome of 576 adult ALL patients over the age of 45 years who were transplanted and received either an RIC (n = 127) or a myeloablative conditioning regimen (n = 449) from HLA-identical siblings in first or second remission. The number of patients with T-ALL was not specified; therefore, results from this study should be interpreted with caution. Patients receiving the RIC regimen had a median age of 56 years (range 45-73), with a TRM of 21% and OS of 48% vs the myeloablative conditioning regimen patients, who had median age of 50 years (45-68, P < .0004), and TRM was 29% (P = .03) and OS 45% (P = .56). Patients over the age of 60 years had a survival rate of 32% with an RIC transplant.44 Similar results were seen in a study by the Center for International Blood and Marrow Transplant Research (CIBMTR).45 Thus, serious consideration should be given to RIC allogeneic HCT for older adults with ALL.
The role of autologous HCT in T-ALL is limited. The MRC/ECOG trial showed that 5-year OS of the 99 patients randomized between autologous HCT and chemotherapy was 51% in both arms (P = .09).1 A retrospective report from Russia showed that, in a group of 72 patients, 18 patients proceeded to autologous HCT with carmustine (BCNU), etoposide, cytarabine (ara-C), and methotrexate (BEAM) conditioning, followed by prolonged maintenance, and had 100% disease-free survival with no relapses, compared with 53% disease-free survival for patients who received chemotherapy only.46 Based on this small study, it is difficult to recommend autologous HCT as standard practice in light of the MRC/ECOG trial results, but one wonders what role prolonged maintenance therapy played in lessening relapse in the Russian study.
Prognosis
Prognostic factors are less clear in patients with T-ALL than in patients with B-ALL. In the series of T-ALL patients treated in the MRC/ECOG trial, the traditional prognostic factor of a leukocyte count >100 × 109/L resulted in a poorer OS at 5 years, compared with patients with a leukocyte count <100 × 109/L. Patients with a complex cytogenetic karyotype (≥5 chromosomal abnormalities) had a significantly lower OS at 5 years, compared with patients with simple or normal karyotypes (19% vs 51%, P = .006), and this impact was not affected by a higher leukocyte count or age. Patients with activating mutations of NOTCH1 and/or FBXW7 had a higher event-free survival of 51% compared with 27% without these abnormalities, although this difference did not reach statistical significance.1 Multiple studies in both pediatric and adult patients have shown that mutations in NOTCH1 and FBXW7 are associated with an improved early treatment response and increased sensitivity to corticosteroid therapy.47-51 However, not all these studies show that the early benefits seen translate into improved survival. Activated NOTCH1 expression can impair glucocorticoid-induced cell death in thymocytes,52 and it has been shown that blocking NOTCH1 signaling with γ-secretase inhibitors can reverse glucocorticoid resistance in some T-ALLs.53 Further studies of patients with NOTCH1/FBXW7 mutations have shown that patients within these subgroups who have K-RAS, N-RAS, and PTEN mutations or deletions have a poorer prognosis than patients who are NOTCH1 and/or FBXW7 mutated without mutations in these other genes.54 This refined classification may explain why some patients with NOTCH1/FBXW7 mutations do poorly.
By immunophenotype, patients who were CD1a positive in the MRC/ECOG trial had an OS at 5 years of 64% (95% CI, 48%-80%) vs 39% (26%-52%) in CD1a-negative patients (P = .01). This appeared to be caused by a higher risk of relapse in CD1a-negative patients (50%; 36%-65%) at 5 years compared with 23% (8%-38%) in CD1a-positive patients (P = .02).1 The GMALL study of 744 patients identified patients with early T-ALL and mature T-ALL as high risk as defined by their criteria noted in the previous section above.38
ETP ALL were originally described as a high-risk group associated with poor response to therapy and dismal prognosis10,55,56 ; however, this group seems to be heterogeneous and not always associated with poor prognosis.12 In adult T-ALL, a comprehensive analysis integrating immunophenotype, gene expression profiling, chromosomal alterations, and mutation profiling of 53 patients treated in the UKALLXII/E2993 trial identified an ETP gene expression signature as a marker of poor prognosis.7,8,15 In addition, the absence of biallelic TCRG deletion,57 CD13 surface expression, heterozygous deletions of the short arm of chromosome 17, and mutations in IDH1 or IDH2 and DNMT3A genes were also associated with poor prognosis, whereas homozygous deletion of CDKN2A/CDKN2B, NOTCH1 and/or FBXW7 mutations, and mutations or deletions in the BCL11B tumor suppressor gene were associated with favorable outcomes.8 Looking at recent data from a large trial of 1144 children with T-ALL, the Children’s Oncology Group has shown a higher rate of induction failure of patients with ETP ALL but similar survivals.58 Thus, we would not currently recommend different management of adult patients with ETP ALL.
In a donor/no-donor analysis in the MRC/ECOG trial, there was less relapse in those with a donor at 25% vs 51% relapse in those without a donor (P < .001). Nonrelapse mortality was increased in the donor group (22% vs 12% at 5 years, P = .006). Survival was 57% with a donor compared with 42% without a donor (P = .07).1
The strongest prognostic factor emerging for patients with ALL is the assessment of minimal residual disease (MRD) by flow cytometry, clonal immunoglobulin, or T-cell receptor gene rearrangements. Despite the high remission rates, many patients still relapse, indicating that many patients harbor residual disease. In adult ALL, the GMALL has shown that patients with standard-risk ALL (T-ALL in 33% of patients) who have a rapid decline in measurement of MRD within the first month of therapy had no relapses at 3 years.59 A more recent GMALL study of 1648 newly diagnosed patients (T-ALL 35%), of whom 580 (T-ALL 34%) were evaluable for molecular response, showed that T-ALL patients reached a significantly higher rate of molecular negativity by day 71 of therapy compared with B-ALL (79% vs 66%, P = .001). Patients who achieved molecular negativity had improved OS (80% vs 42%, P = .0001), and molecular response was the only variable with significant prognostic impact in multivariate analysis.60
A recent study from the GRAALL group has elegantly combined MRD assessment with genetic markers to assess prognosis in 423 of the 860 patients who achieved CR and had MRD analysis done. Of these, 260 were B-ALL and 163 T-ALL patients. A higher risk of relapse was seen in the T-ALL patients who had MRD level ≥10−4, if they lacked NOTCH1/FBXW7 or had N/K-RAS mutation and/or PTEN gene alteration. A high-risk group containing either high MRD levels and/or adverse genetics represented 59% of the T-ALL patients, and the hazard ratio for their cumulative incidence of relapse was 5.31 (95% CI, 2.00-14.07; P = .001) with OS at 5 years from CR of 91% for standard-risk patients and 62% for high-risk patients61 (Figure 3).
Relapse-free survival and OS from CR according to the new risk classification of Beldjord et al. (A) Relapse-free survival in T-ALL patients (86% vs 52% at 5 years; hazard ratio [HR], 4.20; 95% CI, 1.85-9.51; P = .001). (B) OS from CR in T-ALL patients (91% vs 62% at 5 years; HR, 4.14; 95% CI, 1.58-10.83; P = .004). High risk includes MRD-positive and or adverse genetics including no NOTCH1/FBXW7 mutation or presence of N/K-RAS mutation and/or PTEN gene alteration.
Relapse-free survival and OS from CR according to the new risk classification of Beldjord et al. (A) Relapse-free survival in T-ALL patients (86% vs 52% at 5 years; hazard ratio [HR], 4.20; 95% CI, 1.85-9.51; P = .001). (B) OS from CR in T-ALL patients (91% vs 62% at 5 years; HR, 4.14; 95% CI, 1.58-10.83; P = .004). High risk includes MRD-positive and or adverse genetics including no NOTCH1/FBXW7 mutation or presence of N/K-RAS mutation and/or PTEN gene alteration.
Decision-making with T-ALL requires an assessment of prognostic factors to determine whether to continue consolidation and maintenance chemotherapy or to consider allogeneic HCT. The presence of MRD postinduction would be a strong indication to consider HCT. Other adverse prognostic factors, predominantly the genetic markers mentioned in the previous paragraph (if available), should be assessed in the context of the patient’s physiologic status and comorbidities along with discussions with the patient regarding the risks and benefits of chemotherapy vs allogeneic HCT. It is recognized that these genetic markers are not yet widely available for use, but likely will be in the near future.
How should patients with relapsed T-ALL be managed?
Case presentation
A 25-year-old male presented with a mediastinal mass. A bone marrow biopsy showed 31% blasts that expressed CD2, CD5, CD17, cytoplasmic CD3, CD10, weak CD4, and TdT. Cytogenetics were normal, and cerebrospinal fluid examination was negative. He was treated with hyper-CVAD and completed 5 cycles of therapy. He achieved a complete remission, but with his sixth cycle of therapy, he developed Escherichia coli septic shock and went into multiorgan failure with development of ischemic skin necrosis in all four extremities. He required bilateral above-the-elbow and below-the-knee amputations. No further chemotherapy was given.
A year later, he presented with a leukocyte count of 356 × 109/L and was treated with high-dose cytarabine and idarubicin without response. He was subsequently salvaged with 2 cycles of nelarabine with achievement of a CR. He then had a myeloablative matched sibling-related allogeneic HCT with a conditioning regimen of cyclophosphamide and total body irradiation and subsequent development of chronic graft-versus-host disease. Four years later, he relapsed with an ischial mass. Fluorescent in situ hybridization from the biopsy specimen revealed a t(5;14) (TLX3; BCL11B) in 96% of nuclei and a +22q11.2 in 73% of nuclei. Cytogenetics showed a complex monosomal karyotype with monosomies 7 and 21. Bone marrow biopsy showed <5% atypical blasts. Bone marrow fluorescent in situ hybridization analysis showed a TLX3-BCL11B rearrangement.62 He received radiation therapy to the left ischial area and then 4 cycles of nelarabine followed by 2 donor lymphocyte infusions 2 months apart, which resulted in oral chronic graft-versus-host disease. The patient has remained in remission and is currently attending undergraduate school.
Nelarabine for relapsed/refractory T-ALL
Patients who relapse have a poor prognosis. In the MRC/ECOG trial, 123 of 356 patients (37%) relapsed. Twenty-seven underwent an allogeneic HCT, but only 8 survived at a median of 5.2 years, confirming the dismal outcome of these patients.1
Nelarabine was initially studied in 93 patients with refractory hematologic malignancies in a daily 1-hour IV infusion for 5 days. The maximum tolerated dose based on neurotoxicity was 40 mg/kg per day in adults and 60 mg/kg per day in children.63
In adults with relapsed/refractory T-cell ALL, a regimen of 1.5 g/m2 per day on days 1, 3, and 5 was administered in multiple cycles every 22 days. The complete remission rate was 31%, overall response rate 41%, and OS at 1 year was 28%.64 GMALL reported on 126 patients with the same schedule. Forty-five patients achieved a complete remission (36%) and 12 a partial remission. Of the CR patients, 80% were able to proceed to a stem cell transplant (SCT). The OS was 24% at 1 year and 11% at 6 years, with SCT patients achieving an OS of 31% at 3 years.65
HCT
The use of allogeneic HCT in T-cell ALL in both first CR and beyond has been reported. A study from Saudi Arabia reported 53 patients, 32 (60%) in first CR, 18 (34%) in second or later CR, and 3 (6%) in relapse. The TRM at 5 years was 22.5%, and relapse was 36%. OS and disease-free survival at 5 years were 44% and 42%, respectively.66
The EBMT has reported the outcome of 886 patients with T-cell ALL who had undergone allogeneic HCT. Four-year OS and leukemia-free survival were 58% and 55%, respectively, whereas 4-year TRM and relapse incidence were 19% and 26%, respectively. The use of total body irradiation in the conditioning regimen was associated with improved outcomes.67
There is an emerging experience with the use of umbilical cord blood HCT. A report from the CIBMTR of 116 mismatched single- or double-cord HCT compared with 546 unrelated donor peripheral blood HCT and 140 bone marrow HCT in B- and T-ALL patients in the first or second remission showed no difference in 3-year probabilities of survival between recipients of cord blood, matched adult donors, and mismatched adult donors at 44%, 44%, and 43%, respectively.68 Thus, donor choice in the allogeneic HCT setting is similar to other hematologic malignancies, where a matched sibling donor is the first choice and an unrelated donor is the second choice, depending on the degree of matching, cell dose, and urgency of proceeding to transplant. Umbilical cord blood also represents a viable donor option for transplantation for T-ALL, but experience is more limited with this donor source.
New and investigational agents for relapsed disease
The development of new agents for the treatment of T-ALL has lagged behind developments in B-ALL, where multiple monoclonal antibody constructs have shown significant success.69 Other conventional chemotherapy drugs for the treatment of ALL can be considered for patients with relapsed T-ALL. Although not tested exclusively in T-ALL, clofarabine with or without other agents demonstrated modest responses in T-ALL.70
The high frequency of NOTCH1/FBXW7 mutations in T-ALL suggests the potential for therapeutic targeting. The NOTCH1 receptor is a class I transmembrane protein. Its activation is mediated by a transmembrane proteolytic cleavage catalyzed by the γ-secretase complex, which is involved in the deposition of amyloid fibrils in the brains of patients with Alzheimer disease and has been the focus of research, resulting in the development of highly active small-molecule γ-secretase inhibitor (GSI) drugs. In preclinical models, inhibition by GSI of NOTCH1 receptor activation resulted in G0/G1 cell-cycle arrest and decreased proliferation.17 Several GSIs are in clinical development for the treatment of T-ALL. One such agent, MK-0752, disappointingly showed significant gastrointestinal toxicity with only 1 transient clinical response.71 However, other GSIs show promising clinical activity. Recently, data from a phase 1 trial of a novel GSI inhibitor, BMS-906024, were presented. Eight of 25 evaluable patients showed a 50% or more reduction in bone marrow blasts, with 1 partial remission, 3 patients with 98% to 100% clearance of blasts, and 1 CR. Minimal diarrhea was noted.72 Studies in cell lines and patient samples showed that combining GSIs and glucocorticoids can induce apoptotic cell death in glucocorticoid-resistant T-ALL cells while simultaneously abrogating the gastrointestinal toxicity seen in experimental mice and rats.73-76
The NUP214-ABL1 rearrangement, present in ∼5% of T-ALL, may serve as a biomarker for cases that may benefit from ABL1-directed tyrosine kinase inhibitor therapies.77,78 Moreover, targeted inhibition of JAK-STAT signaling with tyrosine kinase inhibitor, such as tofacitinib, has been proposed for the treatment of T-ALL with activating JAK3 mutations79 and could also benefit cases harboring IL7R-activating lesions. Also, inhibition of the PI3K-signaling pathway, using inhibitors of PI3K,80 AKT,81,82 mTOR,83 or dual PI3K-mTOR inhibitors,84 offers an attractive therapeutic avenue with or without glucocorticoids85,86 for the treatment of high-risk PTEN-null T-ALL cases.
Finally, preclinical evaluation of a BCL2 inhibitor, ABT-199, in a cell-line model of ETP ALL showed strong antileukemic effects and synergism with glucocorticoids, doxorubicin, as well as l-asparaginase, and analysis of primary patient samples identified highly sensitive T-ALL cases, primarily among ETP leukemias, which are characterized by higher levels of BCL2 expression.87,88
Conclusion
The diagnosis and treatment of T-ALL remains a challenge with its uncommon and aggressive presentation. Further characterization of subtypes of T-ALL, such as ETP ALL, is reframing our ability to prognosticate in these patients. The discovery of the activating mutations of NOTCH1 and FBXW7 in a majority of patients with T-ALL has been a seminal observation that will hopefully result in the development of effective targeted therapies.
The use of pediatric, intensive chemotherapy regimens in AYAs is showing improved outcomes. Nelarabine for relapsed and refractory disease can result in significant responses. Allogeneic SCT remains an important component of the treatment of T-ALL for patients with high-risk or relapsed/refractory disease. A suggested approach to the management of T-ALL is outlined in Figure 4 and summarized below:
Suitable patients should receive a pediatric-intensive regimen of multiagent chemotherapy and, if they have no adverse genetic factors and are MRD negative, can continue treatment on this regimen. Patients with adverse genetic factors and/or who are MRD positive should be considered for allogeneic HCT from a matched-related, unrelated, or cord blood donor(s).
Patients not suited for a pediatric-intensive regimen should receive a multiagent chemotherapy regimen of the clinician’s choice, as the ideal regimen has not been determined. Based on response, MRD status, adverse genetic features, and suitability for HCT, a determination can be made as to whether to treat the patient with further chemotherapy or HCT.
Algorithm for management of newly diagnosed T-ALL. MA, myeloablative. *Adverse genetics include no NOTCH1/FBXW7 mutation or presence of N/K-RAS mutation and/or PTEN gene alteration. #Assess comorbidities and determine suitability for HCT.
Algorithm for management of newly diagnosed T-ALL. MA, myeloablative. *Adverse genetics include no NOTCH1/FBXW7 mutation or presence of N/K-RAS mutation and/or PTEN gene alteration. #Assess comorbidities and determine suitability for HCT.
Acknowledgments
The authors thank Ms Denise Chase for transcription of the manuscript.
Authorship
Contribution: M.R.L. and A.A.F. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark R. Litzow, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: litzow.mark@mayo.edu.


![Figure 3. Relapse-free survival and OS from CR according to the new risk classification of Beldjord et al. (A) Relapse-free survival in T-ALL patients (86% vs 52% at 5 years; hazard ratio [HR], 4.20; 95% CI, 1.85-9.51; P = .001). (B) OS from CR in T-ALL patients (91% vs 62% at 5 years; HR, 4.14; 95% CI, 1.58-10.83; P = .004). High risk includes MRD-positive and or adverse genetics including no NOTCH1/FBXW7 mutation or presence of N/K-RAS mutation and/or PTEN gene alteration.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/7/10.1182_blood-2014-10-551895/4/m_833f3.jpeg?Expires=1769797197&Signature=QEdBqO9PbogLa-Ct4d9ZgHU95-Fz3CtWYtVBYOOyaedmgzAnFRINYkkzwstfVok01VOs1cAHJvql8ftvwnZ6DV2SnvP9YYVaQ-uvnXyfckox0wPyIPHMZSo7vHxpeMM9QTJ9NvzFHWPZl~2X~aELROBO8naQoBTTACKKuMgZacUMq7d3awo23BGfcoo7eGd9kRXAa00XsgSNf~fo1jRJ8Q73aAogRx6iIG2QdUzZJHxryUre9EogaV-yWwvUB9v7NwAjdRjlhxYqvHja5oe7mWN2aZkMR-2OQTvQ3rnFTfVbqYnjZnNoiagjNpr3uczmffYdMeDhuf~Cee07WfJZzQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
