Key Points
Nilotinib plus multiagent chemotherapy was feasible and showed a comparable outcome to previous results with imatinib for Ph-pos ALL.
The achievement of deep MR with nilotinib at postremission correlated well with the clinical outcomes for Ph-pos ALL.
Abstract
We investigated the effects of nilotinib plus multiagent chemotherapy, followed by consolidation/maintenance or allogeneic hematopoietic cell transplantation (allo-HCT) for adult patients with newly diagnosed Philadelphia-positive (Ph-pos) acute lymphoblastic leukemia (ALL). Study subjects received induction treatment that comprised concurrent vincristine, daunorubicin, prednisolone, and nilotinib. After achieving complete hematologic remission (HCR), subjects received either 5 courses of consolidation, followed by 2-year maintenance with nilotinib, or allo-HCT. Minimal residual disease (MRD) was assessed at HCR, and every 3 months thereafter. The molecular responses (MRs) were defined as MR3 for BCR-ABL1/G6PDH ratios ≤10−3 and MR5 for ratios <10−5. Ninety evaluable subjects, ages 17 to 71 years, were enrolled in 17 centers. The HCR rate was 91%; 57 subjects received allo-HCT. The cumulative MR5 rate was 94%; the 2-year hematologic relapse-free survival (HRFS) rate was 72% for 82 subjects that achieved HCR, and the 2-year overall survival rate was 72%. Subjects that failed to achieve MR3 or MR5 were 9.1 times (P = .004) or 6.3 times (P = .001) more prone to hematologic relapse, respectively, than those that achieved MR3 or MR5. MRD statuses just before allo-HCT and at 3 months after allo-HCT were predictive of 2-year HRFS. Adverse events occurred mainly during induction, and most were reversible with dose reduction or transient interruption of nilotinib. The combination of nilotinib with high-dose cytotoxic drugs was feasible, and it effectively achieved high cumulative complete molecular remission and HRFS rates. The MRD status at early postremission time was predictive of the HRFS. This trial was registered at www.clinicaltrials.gov as #NCT00844298.
Introduction
BCR-ABL1 tyrosine kinase inhibitors (TKIs) are considered an important component of treatment of Philadelphia-positive (Ph-pos) acute lymphoblastic leukemia (ALL). Recent studies reported that treating Ph-pos ALL with the combination of imatinib and multiagent chemotherapy improved the overall outcome.1,2 Based on improvements in efficacy and tolerability, next-generation TKIs have been widely used in first-line treatments for chronic myeloid leukemia (CML),3,4 and dasatinib is used in second-line treatments for patients with Ph-pos ALL that failed to respond or were intolerant to imatinib5 or another first-line agent.6
Nilotinib is a TKI with a potent binding affinity for BCR-ABL1 tyrosine kinase.7,8 Nilotinib achieved significantly higher molecular response (MR) rates at 24 months9 than imatinib in a phase 3 randomized study for patients with chronic-phase CML. This outcome in CML suggested that nilotinib may show improved clinical efficacy for treating Ph-pos ALL compared with imatinib. The adverse event (AE) profile of nilotinib was different from that of imatinib. Emesis and diarrhea, which may impede drug compliance with oral agents, were relatively low with nilotinib; this feature may prove beneficial when combined with cytotoxic chemotherapeutic agents, which can aggravate AEs. However, serious AEs may be of concern (eg, hepatic abnormalities, pancreatitis, and cardiac corrected QT interval prolongation occurred in previous trials of nilotinib treatment of CML).
The present multicenter, phase 2, single arm, prospective study evaluated the feasibility, efficacy, and safety of combining nilotinib with multiagent chemotherapy for treating adult patients with Ph-pos ALL during chemotherapy and during allogeneic hematopoietic cell transplantation (allo-HCT).
Patients and methods
Patient eligibility
Patients were considered for enrollment when they were aged 15 years or more, were newly diagnosed with ALL or biphenotypic acute leukemia, and carried the Philadelphia chromosome (Ph-pos). The Ph-pos status was detected with the conventional G band by trypsin using the Leishman Giemsa band technique, and/or the BCR-ABL1 fusion transcript was detected with a nested reverse-transcription polymerase chain reaction. For study inclusion, subjects had to demonstrate adequate organ functions (serum creatinine, <1.5 mg/dL; serum bilirubin, <1.5 mg/dL; and serum transaminase, <3 times the upper normal limit or <5 times the upper normal limit for subjects with leukemia with hepatic involvement), and subjects had to demonstrate adequate performance (Eastern Cooperative Oncology Group performance status10 ≤2 or Karnofsky scale11 >60%). The institutional review boards of all participating institutions approved this study. All subjects provided written informed consent before enrollment, according to the guidelines at each institution’s Committee on Human Research. This trial was registered at www.clinicaltrials.gov as #NCT00844298.
Induction and postremission treatment
All subjects received induction chemotherapy, which comprised vincristine, daunorubicin, and prednisolone (Table 1). Nilotinib was started on the eighth day of induction therapy at a dose of 400 mg, twice a day.
Details of induction/consolidation chemotherapy
| Therapy . | Details . |
|---|---|
| Induction | Daunorubicin 90 mg/m2 per day; continuous IV infusion for 24 h, on days 1-3 |
| Vincristine 2 mg, IV push on days 1 and 8 | |
| Prednisolone 60 mg/m2 per day p.o., or 48 mg/m2 per day IV, on days 1-14 | |
| Nilotinib 400 mg p.o. twice daily (from day 8 to the start of conditioning for allo-HCT or until the end of 2 y of maintenance therapy) | |
| Consolidation A (cycle 1) | Daunorubicin 45 mg/m2 per day; continuous IV for 24 h on days 1 and 2 |
| Vincristine 2 mg IV, on days 1 and 8 | |
| Prednisolone 60 mg/m2 per day p.o., on days 1-14 | |
| Nilotinib 400 mg p.o. twice daily | |
| Consolidation B (cycles 2,4) | Cytarabine 2000 mg/m2 per day IV for 2 h on days 1-4 |
| Etoposide 150 mg/m2 per day IV for 3 h on days 1-4 | |
| Nilotinib 400 mg p.o. twice daily | |
| Consolidation C (cycles 3,5) | Methotrexate 220 mg/m2 IV bolus, then 60 mg/m2 per hour for 36 h on days 1-2 and 15-16 |
| Leucovorin, followed immediately by 50 mg/m2 IV every 6 h for 3 doses; then leucovorin p.o. until serum methotrexate <0.05 μmol/L | |
| Nilotinib 400 mg p.o. twice daily | |
| Maintenance | Nilotinib 400 mg p.o. twice daily for 2 y |
| CNS prophylaxis | Up to 10 doses of intrathecal methotrexate (15 mg) with hydrocortisone (50 mg) during or after induction therapy |
| Therapy . | Details . |
|---|---|
| Induction | Daunorubicin 90 mg/m2 per day; continuous IV infusion for 24 h, on days 1-3 |
| Vincristine 2 mg, IV push on days 1 and 8 | |
| Prednisolone 60 mg/m2 per day p.o., or 48 mg/m2 per day IV, on days 1-14 | |
| Nilotinib 400 mg p.o. twice daily (from day 8 to the start of conditioning for allo-HCT or until the end of 2 y of maintenance therapy) | |
| Consolidation A (cycle 1) | Daunorubicin 45 mg/m2 per day; continuous IV for 24 h on days 1 and 2 |
| Vincristine 2 mg IV, on days 1 and 8 | |
| Prednisolone 60 mg/m2 per day p.o., on days 1-14 | |
| Nilotinib 400 mg p.o. twice daily | |
| Consolidation B (cycles 2,4) | Cytarabine 2000 mg/m2 per day IV for 2 h on days 1-4 |
| Etoposide 150 mg/m2 per day IV for 3 h on days 1-4 | |
| Nilotinib 400 mg p.o. twice daily | |
| Consolidation C (cycles 3,5) | Methotrexate 220 mg/m2 IV bolus, then 60 mg/m2 per hour for 36 h on days 1-2 and 15-16 |
| Leucovorin, followed immediately by 50 mg/m2 IV every 6 h for 3 doses; then leucovorin p.o. until serum methotrexate <0.05 μmol/L | |
| Nilotinib 400 mg p.o. twice daily | |
| Maintenance | Nilotinib 400 mg p.o. twice daily for 2 y |
| CNS prophylaxis | Up to 10 doses of intrathecal methotrexate (15 mg) with hydrocortisone (50 mg) during or after induction therapy |
CNS, central nervous system; p.o., by mouth.
After achieving hematologic complete remission (HCR), subjects received 5 cycles of consolidation (Table 1) or were considered for allo-HCT. The criteria were that allo-HCT had to be feasible, a suitable donor was available, and the subject was willing to receive allo-HCT. Acceptable donors included matched sibling donors, fully matched or partially matched unrelated donors (MUDs), and haploidentical familial (familial mismatched) donors. Partially MUDs were acceptable when no more than 2 mismatches in HLA alleles were present between the donor and recipient of the allo-HCT. Conditioning for allo-HCT was at the discretion of each investigator. Stem cells were collected either from BM or from granulocyte colony-stimulating factor–mobilized peripheral blood (PB). Prophylaxis for graft-versus-host disease (GVHD) and prophylactic antibacterial/antifungal/antiviral agents were all given at the discretion of each investigator. Autologous HCT (auto-HCT) was not permitted for postremission therapy in this study. Up to 10 intrathecal injections of methotrexate were given during induction/consolidation therapy as a prophylactic for relapse in the CNS. Whole brain irradiation was not permitted for CNS prophylaxis.
Administration of nilotinib
Nilotinib was introduced concurrent with cytotoxic drugs, starting on the eighth day of induction therapy, and it was continued up to the start of allo-HCT conditioning or until the end of consolidation therapy. Subjects that proceeded to allo-HCT did not receive nilotinib after allo-HCT. Subjects that completed 5 cycles of consolidation received 2 years of nilotinib maintenance. Nilotinib was started at a daily dose of 400 mg twice daily, and it was tapered to 400 mg daily temporarily, when the subject experienced a greater than or equal to grade 3, nonhematologic AE. The low nilotinib dose was continued until the AE improved to grade 1. However, nilotinib was discontinued permanently, at the discretion of each investigator, when severe AEs recurred, despite a dose reduction. These subjects continued to receive other cytotoxic drugs, and when desired, they were allowed to remain in the present study.
Assessment of minimal residual disease
Minimal residual disease (MRD) was monitored at the central laboratory of the Department of Laboratory Medicine, Asan Medical Center, Seoul, Korea. PB (5 mL) was collected from each subject every 3 months from the time of achieving HCR until the end of maintenance therapy. For subjects that received allo-HCT, MRD was evaluated within 1 month prior to the beginning of conditioning and every 3 months thereafter.
BCR-ABL1 expression was measured with real-time quantitative polymerase chain reaction with the LightCycler-t(9;22) Quantification Kit (Roche Diagnostics, Basel, Switzerland). Briefly, total RNA was extracted from leukocytes with the High Pure RNA Isolation Kit (Roche Diagnostics); complementary DNA was prepared from purified messenger RNA according to the manufacturer’s instructions. Glucose-6-phosphate dehydrogenase (G6PDH) served as a control. Amplified BCR-ABL1 and G6PDH products were compared with standard curves to determine relative concentrations. The sensitivity of this assay was 10−5.
Complete molecular remission (MCR), or a negative MRD, was assumed when the BCR-ABL1/G6PDH ratio was <1 × 10−5 (MR5). A major MR (MR3) was defined when the ratio was ≤1 × 10−3. Molecular relapse (MREL) was defined as the reappearance of the BCR-ABL1 transcript after achieving MCR.
Assessment of response and toxicity
BM aspirations and biopsies were performed approximately the 28th day after induction initiation to check whether HCR was achieved. HCR was achieved when the BM mononuclear cells comprised <5% blasts, BM cellularity was ≥25%, the absolute neutrophil count had recovered to ≥1000/mm3, and the PB platelet count was ≥100 000/mm3. Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria (version 2.0).
Statistics and analysis of data
Nonrelapse mortality (NRM) was defined as a patient death without disease progression, including overt hematologic relapse (HREL) and MREL. HREL-free survival (HRFS) times were measured from the date of HCR to the date of NRM or HREL. NRM and HREL were considered competing risks. MREL-free survival (MRFS) times were measured from the date of MCR to the date of NRM, MREL, or HREL. Overall survival (OS) was measured from entry into the present study to the date of last follow-up or death. Subjects were followed up until the end of maintenance or for 2 years after allo-HCT. Subjects were dropped out of the current study when any of the following events occurred: MREL that caused a change in the TKI from nilotinib to any other drug; HREL; withdrawal of consent; major protocol violations, such as auto-HCT administration; or death from any cause. Data were censored for subjects that were dropped out of the current study. Relapse-free survival (RFS) and OS were estimated with the Kaplan-Meier method and Cox-regression method. Cumulative incidences were estimated with the Gray method. All statistical analyses were performed with IBM SPSS 21 (IBM Corporation) or NCSS 2004 (Number Cruncher Statistical Systems, Kaysville, UT). All data were analyzed and finally approved by all the authors.
Results
Patient characteristics
From January 2009 to May 2012, 91 subjects were enrolled in 17 centers. One subject withdrew informed consent before we assessed hematologic response to induction therapy. Therefore, 90 subjects were analyzable for efficacy and safety assessments. Table 2 presents the baseline patient characteristics.
Baseline characteristics of all analyzable subjects
| Characteristics . | Total (n = 90) . | Allo-HCT recipients (n = 57) . | Allo-HCT nonrecipients (n = 25) . | P . |
|---|---|---|---|---|
| Age in years, median (range) | 47.0 (17-71) | 47.0 (19-61) | 57.0 (19-71) | .066 |
| Number of subjects | ||||
| <35 vs ≥35 y | 17 vs 73 | |||
| <45 vs ≥45 y | 38 vs 52 | |||
| <55 vs ≥55 y | 65 vs 25 | |||
| Male:female, n:n (%:%) | 45:45 (50:50) | 28:29 (49:51) | 11:14 (44:56) | .669 |
| Splenomegaly, n (%) | 26 (29) | 17 (30) | 7 (28) | .867 |
| Extramedullary involvement, n (%) | 16 (18) | 12 (21) | 3 (12) | .536 |
| Cerebrospinal fluid, n (%) | 8 (9) | 4 (7) | 3 (12) | |
| Others,* n (%) | 8 (9) | 8 (14) | 0 | |
| Hemogram, PB | ||||
| WBC (×103/μL), median (range) | 25.7 (0.2-547.1) | 20.3 (0.2-379.4) | 51.1 (1.6-547.1) | .134 |
| % Blast, median (range) | 59.0 (0-97.0) | 49.0 (0-97.0) | 73.0 (0-97.0) | .051 |
| Serum laboratory results | ||||
| Creatinine, mg/dL, median (range) | 0.8 (0.3-1.5) | 0.8 (0.4-1.5) | 0.8 (0.5-1.5) | .834 |
| LDH, IU, median (range) | 696.0 (116.0-5468.0) | 716.0 (116.0-5468.0) | 786.0 (269.0-3172.0) | .841 |
| Immunologic phenotype | 1.000 | |||
| ALL, n (%) | 83 (92) | 52 (91) | 23 (92) | |
| Biphenotypic acute leukemia, n (%) | 7 (8) | 5 (9) | 2 (8) | |
| Chromosome type | .652 | |||
| Ph alone, n (%) | 30 (33) | 17 (30) | 10 (40) | |
| Additional type, n (%) | 44 (49) | 28 (49) | 12 (48) | |
| Others, n (%) | 6 (7) | 5 (9) | 1 (4) | |
| No mitosis, n (%) | 7 (8) | 5 (9) | 1 (4) | |
| Unknown, n (%) | 3 (3) | 2 (4) | 1 (4) | |
| BCR-ABL1 transcript type | .492 | |||
| Major, n (%) | 25 (28) | 12 (21) | 7 (28) | |
| Minor, n (%) | 65 (72) | 45 (79) | 18 (72) |
| Characteristics . | Total (n = 90) . | Allo-HCT recipients (n = 57) . | Allo-HCT nonrecipients (n = 25) . | P . |
|---|---|---|---|---|
| Age in years, median (range) | 47.0 (17-71) | 47.0 (19-61) | 57.0 (19-71) | .066 |
| Number of subjects | ||||
| <35 vs ≥35 y | 17 vs 73 | |||
| <45 vs ≥45 y | 38 vs 52 | |||
| <55 vs ≥55 y | 65 vs 25 | |||
| Male:female, n:n (%:%) | 45:45 (50:50) | 28:29 (49:51) | 11:14 (44:56) | .669 |
| Splenomegaly, n (%) | 26 (29) | 17 (30) | 7 (28) | .867 |
| Extramedullary involvement, n (%) | 16 (18) | 12 (21) | 3 (12) | .536 |
| Cerebrospinal fluid, n (%) | 8 (9) | 4 (7) | 3 (12) | |
| Others,* n (%) | 8 (9) | 8 (14) | 0 | |
| Hemogram, PB | ||||
| WBC (×103/μL), median (range) | 25.7 (0.2-547.1) | 20.3 (0.2-379.4) | 51.1 (1.6-547.1) | .134 |
| % Blast, median (range) | 59.0 (0-97.0) | 49.0 (0-97.0) | 73.0 (0-97.0) | .051 |
| Serum laboratory results | ||||
| Creatinine, mg/dL, median (range) | 0.8 (0.3-1.5) | 0.8 (0.4-1.5) | 0.8 (0.5-1.5) | .834 |
| LDH, IU, median (range) | 696.0 (116.0-5468.0) | 716.0 (116.0-5468.0) | 786.0 (269.0-3172.0) | .841 |
| Immunologic phenotype | 1.000 | |||
| ALL, n (%) | 83 (92) | 52 (91) | 23 (92) | |
| Biphenotypic acute leukemia, n (%) | 7 (8) | 5 (9) | 2 (8) | |
| Chromosome type | .652 | |||
| Ph alone, n (%) | 30 (33) | 17 (30) | 10 (40) | |
| Additional type, n (%) | 44 (49) | 28 (49) | 12 (48) | |
| Others, n (%) | 6 (7) | 5 (9) | 1 (4) | |
| No mitosis, n (%) | 7 (8) | 5 (9) | 1 (4) | |
| Unknown, n (%) | 3 (3) | 2 (4) | 1 (4) | |
| BCR-ABL1 transcript type | .492 | |||
| Major, n (%) | 25 (28) | 12 (21) | 7 (28) | |
| Minor, n (%) | 65 (72) | 45 (79) | 18 (72) |
LDH, lactate dehydrogenase; Ph, Philadelphia; WBC, white blood cell.
Skin (n = 1), bone and kidney (n = 1), bone (n = 2), pleura (n = 1), intestine (n = 1), liver (n = 1), and soft tissue mass (n = 1).
Progress and outcomes of induction therapy
The HCR rate was 91% (82 subjects), and the median time to HCR was 27 days (range, 13-72). The remaining 8 subjects did not achieve HCR, because of death in aplasia during induction; all 8 subjects died of febrile neutropenia with septic shock, and in particular, 1 died of invasive fungal pneumonia combined with intracranial hemorrhage. At the time of HCR, the MCR achievement rate was 53% (48 of 90 subjects).
Postremission therapy
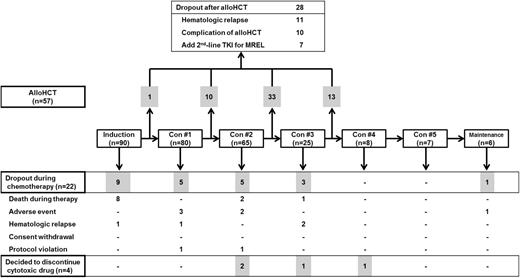
Figure 1 shows a flow diagram of treatment of all subjects. Among 82 subjects that achieved HCR, 57 (70%) received allo-HCT after the first HCR (supplemental Table 1; see the Blood Web site). The other 25 subjects were not eligible for allo-HCT, because of dropout from various causes (n = 8), advanced age (n = 8), patient refusal (n = 4), and no suitable donor (n = 5). Among those without suitable donors, 2 subjects received auto-HCTs; these subjects were excluded from the study at the initiation of conditioning for auto-HCT because of protocol violation.
Flow diagram shows patient treatments and dropouts. The drugs and dosing used for induction and consolidation cycles are itemized in Table 1. Con, consolidation.
Flow diagram shows patient treatments and dropouts. The drugs and dosing used for induction and consolidation cycles are itemized in Table 1. Con, consolidation.
MR and MRD
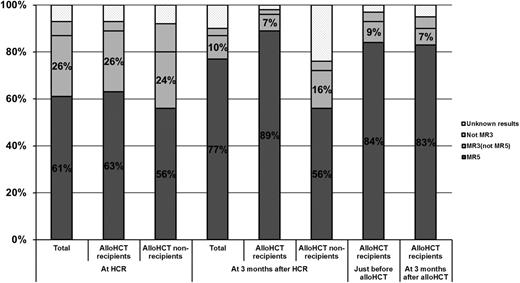
A total of 77 subjects achieved MR5 by the end of the present study. Accordingly, the cumulative rate of MCR was 86% among all 90 subjects and 94% among the 82 subjects that achieved HCR. The median time from treatment initiation to MCR was 1.1 months (range, 0.6-15.8). At the time of HCR, the overall MR5 rate was 56% (61% among the 82 subjects), and the overall MR3 rate was 79% (87% among the 82 subjects). Among the 70 evaluable subjects at 3 months after HCR, the MR5 rate was 77%, and the MR3 rate was 87%. The MR5 rate was 89%, and MR3 rate was 96% among subjects who received allo-HCT, whereas the MR5 rate was 56% and MR3 rate was 72% among subjects who did not receive allo-HCT. Among 57 subjects that received allo-HCT, 48 (84%) achieved MR5 just before conditioning, and 47 (82%) maintained MR5 at 3 months after allo-HCT (Figure 2).
Distribution of MR to treatment. MR3 and MR5, major and complete MRs, respectively.
Distribution of MR to treatment. MR3 and MR5, major and complete MRs, respectively.
Among 25 subjects that did not receive allo-HCT, 7 experienced HREL, and the median time to HREL was 8.8 months (range, 3.1-23.1). Among the other 18 subjects that were in HCR, 3 experienced MREL with MRD levels of 6 × 10−5, 10 × 10−5, and 24 × 10−5. When the administration of nilotinib was continued, MRD negativity was achieved again after 3 months, and it was maintained until the end of follow-up in all 3 subjects.
Survival outcomes
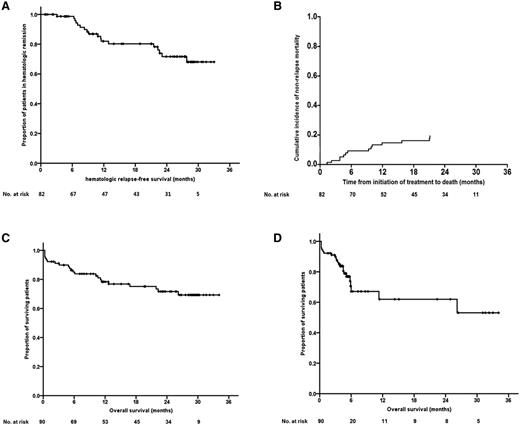
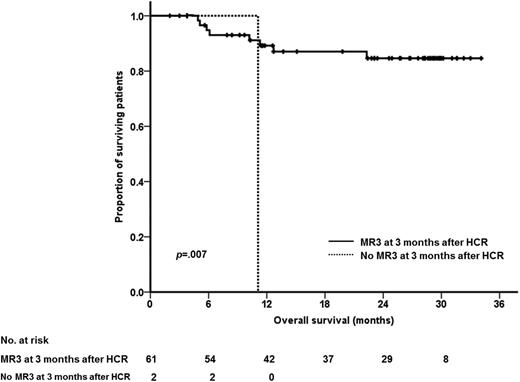
The 2-year HRFS rate was 72% among 82 subjects that achieved HCR, and the 2-year MRFS rate was 63% among 76 subjects that achieved MCR. The cumulative 2-year incidence of NRM was 25% (95% confidence interval, 18% to 37%; standard error, 4.8%). The 2-year OS rate was 72% when survival time was measured to the date of last follow-up or death, or 62% when we censored subjects who received allo-HCT just before conditioning (Figure 3). The OS was similar between allo-HCT recipients and nonrecipients (supplemental Figure 1).
Survival outcomes. (A) HRFS of subjects that achieved HCR. (B) Cumulative incidence of NRM. (C) OS. (D) OS (after censoring subjects who received allo-HCT at the time of conditioning).
Survival outcomes. (A) HRFS of subjects that achieved HCR. (B) Cumulative incidence of NRM. (C) OS. (D) OS (after censoring subjects who received allo-HCT at the time of conditioning).
Details of allo-HCT
Fifty-seven subjects received allo-HCTs at a median of 3.8 months (range, 1.4-8.0) after HCR and after a median of 2 cycles (range, 0-3) of consolidation. Most subjects (n = 54, 95%) achieved neutrophil engraftment, and the median time from stem cell infusion to engraftment was 15.0 days (range, 10-33). Twenty-five subjects experienced acute GVHD (44% of 57), and 12 exhibited greater than or equal to grade 2 acute GVHD (21% of 57). The overall incidence of chronic GVHD was 33% (19 of 57 subjects), and chronic GVHD was extensive in 11%.
Allo-HCT outcomes (HRFS and OS) were not related to any differences in treatment, including donor differences (matched sibling donor vs MUD vs haploidentical familial [familial mismatched] donors), intensity of conditioning (myeloablative vs reduced intensity), total body irradiation (administered vs not administered), and antithymocyte globulin (administered vs not administered). However, subjects that received granulocyte colony-stimulating factor–mobilized PB stem cells showed a superior 2-year HRFS rate compared with those that received BM stem cells (85% vs 61%, P = .028).
Comparison of outcomes with and without allo-HCT
Among the 82 subjects that achieved HCR (Figure 1), the 2-year cumulative HREL incidence was 24%. The HREL incidence was lower in recipients of allo-HCT (n = 57; 19%) than in nonrecipients (n = 25; 41%). Moreover, allo-HCT recipients had significantly higher estimated 2-year HRFS rates than nonrecipients (78% vs 49%, P = .045). However, the 2-year cumulative incidence of NRM (overall 19.3%) was similar between allo-HCT recipients and nonrecipients (19% vs 20%, respectively). Accordingly, the estimated 2-year OS was similar between these groups (80% vs 72%, respectively; P = .227). Among 76 subjects that achieved MCR, the estimated 2-year MRFS rates were not different between nonrecipients and recipients of allo-HCT (65% vs 53%, respectively; P = .783).
Outcomes according to baseline characteristics
The HCR rate was not affected by most characteristics of subjects at diagnosis, including age (<35 vs ≥35 or <45 vs ≥45 or <55 vs ≥55 years), PB WBC count (<30 000/mm3 vs ≥30 000/mm3), immunophenotype (biphenotypic vs ALL), leptomeningeal involvement of leukemic cells (present vs absent), or chromosome aberration (Ph-alone vs an additional type). However, subjects with major BCR-ABL1 transcripts showed superior HCR rates compared with those with the minor transcript (97% vs 76%, P = .005). This effect was mainly because of different frequencies of death in aplasia during induction. The baseline characteristics including the age differences did not affect the 2-year HRFS rates and 2-year OS rates, except that a high WBC count tended to increase the risk of HREL, when analyzed as a continuous variable (hazard ratio [HR], 1.004; P = .025).
HRFS according to MRD status
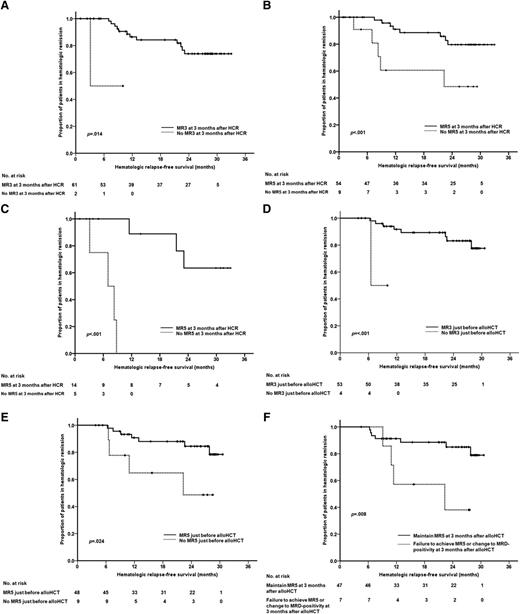
Subjects assessed for MRD were stratified into subgroups of MRD levels at HCR, at 3 months after HCR, at allo-HCT, and at 3 months after allo-HCT. The 2-year HRFS rate was not affected by whether subjects had achieved MR3 or MR5 at HCR. However, subjects that achieved MR3 at 3 months after HCR had significantly higher HRFS rates than those that failed to achieve MR3. In fact, those that failed to achieve MR3 at 3 months after HCR had 9.1-fold higher risk of HREL (P = .004) than those that achieved MR3 (Figure 4A). These outcome differences were more evident when we considered MR5 achievement at 3 months after HCR (Figure 4B). The estimated 2-year HRFS rates were 80% for those that achieved and 33% for those that did not achieve MR5 (P < .001), and the HR for HREL was 6.3 (P = .001). Next, we analyzed the subgroups of subjects that did or did not receive allo-HCT. We found that, among subjects that did not receive allo-HCT, achievement of MR5 at 3 months after HCR significantly affected the estimated 2-year HRFS rates (64% for MR5 achievement vs 0% for MR5 failure, P < .001). However, among subjects that received allo-HCT, achievement of MR5 did not significantly affect the 2-year HRFS rate (Figure 4C).
Comparison of outcomes according to MRD achievement after HCR. (A) HRFS based on whether MR3 was achieved at 3 months after HCR. (B) HRFS based on whether MR5 was achieved at 3 months after HCR. (C) HRFS for subjects that did not receive allo-HCT, based on whether they achieved MR5 at 3 months after HCR. (D) HRFS for allo-HCT recipients, based on whether they achieved MR3 just before allo-HCT. (E) HRFS for allo-HCT recipients, based on whether they achieved MR5 just before allo-HCT. (F) HRFS for allo-HCT recipients, based on whether they had maintained MR5 at 3 months after allo-HCT; the group that failed to maintain MR5 included subjects that had relapsed (MRD positive) and those that had failed to achieve MR5.
Comparison of outcomes according to MRD achievement after HCR. (A) HRFS based on whether MR3 was achieved at 3 months after HCR. (B) HRFS based on whether MR5 was achieved at 3 months after HCR. (C) HRFS for subjects that did not receive allo-HCT, based on whether they achieved MR5 at 3 months after HCR. (D) HRFS for allo-HCT recipients, based on whether they achieved MR3 just before allo-HCT. (E) HRFS for allo-HCT recipients, based on whether they achieved MR5 just before allo-HCT. (F) HRFS for allo-HCT recipients, based on whether they had maintained MR5 at 3 months after allo-HCT; the group that failed to maintain MR5 included subjects that had relapsed (MRD positive) and those that had failed to achieve MR5.
We found that, among 57 subjects that received allo-HCT, significant predictors of the 2-year HRFS were the failure to achieve MR3 (0% vs 83% for MR3 achievers; HR, 19.8; P = < .001) and MR5 (49% vs 85% for MR5 achievers; HR, 3.8; P = .024) just before allo-HCT (Figure 4D-E). The HR for HREL was 4.8 for subjects that either failed to achieve MR5 or experienced MREL at 3 months after allo-HCT (P = .016) compared those that achieved MR5 (Figure 4F).
OS according to MRD status
The OS rates were analyzed according to MRD status. The 2 subjects that failed to achieve MR3 at 3 months after HCR did not survive; however, the 2-year OS rate was 85% for those that achieved MR3 at 3 months after HCR (Figure 5). The HR for death was 10.6 for these groups (P = .031). The failure to achieve MR3 at 3 months after HCR was prognostic of death for the 57 subjects that received allo-HCT. Among these subjects, the HR for death was 13.4 (P = .025) for those that failed compared with those that achieved MR3 at 3 months after HCR.
Multivariate analysis
Cox-regression analyses were performed to identify factors that influenced the HRFS and OS. The analyses included variables that were significant in predicting the outcome in the current study. These variables included the performance of allo-HCT (yes vs no), the MRD status at 3 months after HCR (MR3 vs no MR3), the PB WBC count, and the type of BCR-ABL1 transcript (major vs minor). Both the administration of allo-HCT (HR, 3.3; P = .048) and the achievement of MR3 (HR, 12.3; P = .038) were significant predictors of 2-year HRFS. The results were similar when the achievement of MR5 was included instead of MR3; the administration of allo-HCT had an HR of 3.9 (P = .036), and the achievement of MR5 had an HR of 7.5 (P = .004). None of the variables could significantly predict the 2-year OS.
AEs
The AEs that occurred with incidences of 5% or more during induction and consolidation cycles are shown in Table 3. Episodes of jaundice greater than or equal to grade 3 occurred in 4% to 25% of subjects, but most were reversed when nilotinib was reduced or discontinued transiently. After reversal, most subjects continued to receive nilotinib at the starting dose. The AEs related to the gastrointestinal system were generally tolerable. Although 13% of subjects experienced greater than or equal to grade 3 serum lipase elevations, only 2 subjects (2%) experienced greater than or equal to grade 3 overt pancreatitis during induction. Other AEs thought to be related to nilotinib were also tolerable. Incidence of AEs related to the cardiopulmonary system, including pleural effusion, pulmonary edema, noncardiac chest pain, acute coronary syndrome, paroxysmal atrial tachycardia, and atrial fibrillation, was 1%.
AEs during chemotherapy
| . | Induction (n = 90) . | Con #1 (n = 80) . | Con #2 (n = 65) . | Con #3 (n = 25) . | Con #4 (n = 8) . | Con #5 (n = 7) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total . | G4 . | Total . | G4 . | Total . | G4 . | Total . | G4 . | Total . | G4 . | Total . | G4 . | |
| Hematologic | ||||||||||||
| ANC | — | 98 | — | 42 | — | 98 | — | 39 | — | 100 | — | 43 |
| Platelet | — | 47 | — | 6 | — | 48 | — | 22 | — | 75 | — | 14 |
| Infection | ||||||||||||
| Febrile neutropenia | — | 11 | — | 1 | — | 6 | — | 0 | — | 0 | — | 0 |
| Bacteremia | — | 39 | — | 5 | — | 19 | — | 8 | — | 13 | — | 14 |
| Other infection | 13 | 1 | 4 | 0 | 5 | 0 | 12 | 0 | 13 | 0 | 0 | 0 |
| Lung infection | 6 | 1 | 0 | 0 | 2 | 0 | 12 | 0 | 0 | 0 | 0 | 0 |
| Soft tissue infection | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Others | 7 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 13 | 0 | 0 | 0 |
| . | Induction (n = 90) . | Con #1 (n = 80) . | Con #2 (n = 65) . | Con #3 (n = 25) . | Con #4 (n = 8) . | Con #5 (n = 7) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total . | G4 . | Total . | G4 . | Total . | G4 . | Total . | G4 . | Total . | G4 . | Total . | G4 . | |
| Hematologic | ||||||||||||
| ANC | — | 98 | — | 42 | — | 98 | — | 39 | — | 100 | — | 43 |
| Platelet | — | 47 | — | 6 | — | 48 | — | 22 | — | 75 | — | 14 |
| Infection | ||||||||||||
| Febrile neutropenia | — | 11 | — | 1 | — | 6 | — | 0 | — | 0 | — | 0 |
| Bacteremia | — | 39 | — | 5 | — | 19 | — | 8 | — | 13 | — | 14 |
| Other infection | 13 | 1 | 4 | 0 | 5 | 0 | 12 | 0 | 13 | 0 | 0 | 0 |
| Lung infection | 6 | 1 | 0 | 0 | 2 | 0 | 12 | 0 | 0 | 0 | 0 | 0 |
| Soft tissue infection | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Others | 7 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 13 | 0 | 0 | 0 |
| . | Total . | ≥G3 . | Total . | ≥G3 . | Total . | ≥G3 . | Total . | ≥G3 . | Total . | ≥G3 . | Total . | ≥G3 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonhematologic | ||||||||||||
| Jaundice | 87 | 19 | 81 | 10 | 73 | 14 | 61 | 4 | 63 | 25 | 57 | 0 |
| ALT elevation | 79 | 17 | 67 | 8 | 65 | 5 | 58 | 0 | 38 | 0 | 57 | 0 |
| Azotemia | 12 | 0 | 5 | 0 | 18 | 3 | 29 | 4 | 0 | 0 | 14 | 0 |
| Lipase elevation | 24 | 13 | 15 | 7 | 17 | 9 | 0 | 0 | 33 | 0 | 0 | 0 |
| Anorexia | 2 | 0 | 4 | 0 | 8 | 0 | 4 | 0 | 0 | 0 | 0 | 0 |
| Nausea/vomiting | 55 | 5 | 24 | 1 | 40 | 5 | 42 | 0 | 25 | 0 | 29 | 0 |
| Dyspepsia | 36 | 1 | 9 | 1 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oral mucositis | 38 | 5 | 23 | 5 | 39 | 8 | 32 | 4 | 25 | 0 | 29 | 14 |
| Abdominal pain | 9 | 2 | 6 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 14 | 0 |
| Diarrhea | 30 | 5 | 10 | 0 | 23 | 3 | 4 | 0 | 0 | 0 | 0 | 0 |
| Fecal incontinence | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0 |
| Constipation | 12 | 0 | 11 | 0 | 6 | 0 | 8 | 0 | 13 | 0 | 14 | 0 |
| Ileus | 8 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pancreatitis | 24 | 2 | 15 | 0 | 17 | 0 | 0 | 0 | 33 | 0 | 0 | 0 |
| Hemorrhage | 17 | 2 | 5 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fever | 2 | 0 | 4 | 0 | 8 | 0 | 4 | 0 | 0 | 0 | 0 | 0 |
| Myalgia | 26 | 2 | 2 | 3 | 0 | 0 | 13 | 0 | 0 | 0 | 14 | 0 |
| Headache | 4 | 1 | 6 | 0 | 5 | 0 | 8 | 0 | 13 | 0 | 14 | 0 |
| Seizure | 0 | 0 | 0 | 0 | 2 | 0 | 4 | 0 | 0 | 0 | 0 | 0 |
| Peripheral neuropathy | 20 | 1 | 25 | 0 | 23 | 2 | 8 | 0 | 0 | 0 | 0 | 0 |
| Pruritus | 0 | 0 | 3 | 0 | 5 | 0 | 8 | 0 | 0 | 0 | 0 | 0 |
| Skin rash | 16 | 4 | 6 | 1 | 32 | 3 | 16 | 0 | 13 | 0 | 14 | 0 |
| Insomnia | 7 | 0 | 1 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| . | Total . | ≥G3 . | Total . | ≥G3 . | Total . | ≥G3 . | Total . | ≥G3 . | Total . | ≥G3 . | Total . | ≥G3 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonhematologic | ||||||||||||
| Jaundice | 87 | 19 | 81 | 10 | 73 | 14 | 61 | 4 | 63 | 25 | 57 | 0 |
| ALT elevation | 79 | 17 | 67 | 8 | 65 | 5 | 58 | 0 | 38 | 0 | 57 | 0 |
| Azotemia | 12 | 0 | 5 | 0 | 18 | 3 | 29 | 4 | 0 | 0 | 14 | 0 |
| Lipase elevation | 24 | 13 | 15 | 7 | 17 | 9 | 0 | 0 | 33 | 0 | 0 | 0 |
| Anorexia | 2 | 0 | 4 | 0 | 8 | 0 | 4 | 0 | 0 | 0 | 0 | 0 |
| Nausea/vomiting | 55 | 5 | 24 | 1 | 40 | 5 | 42 | 0 | 25 | 0 | 29 | 0 |
| Dyspepsia | 36 | 1 | 9 | 1 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oral mucositis | 38 | 5 | 23 | 5 | 39 | 8 | 32 | 4 | 25 | 0 | 29 | 14 |
| Abdominal pain | 9 | 2 | 6 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 14 | 0 |
| Diarrhea | 30 | 5 | 10 | 0 | 23 | 3 | 4 | 0 | 0 | 0 | 0 | 0 |
| Fecal incontinence | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0 |
| Constipation | 12 | 0 | 11 | 0 | 6 | 0 | 8 | 0 | 13 | 0 | 14 | 0 |
| Ileus | 8 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pancreatitis | 24 | 2 | 15 | 0 | 17 | 0 | 0 | 0 | 33 | 0 | 0 | 0 |
| Hemorrhage | 17 | 2 | 5 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fever | 2 | 0 | 4 | 0 | 8 | 0 | 4 | 0 | 0 | 0 | 0 | 0 |
| Myalgia | 26 | 2 | 2 | 3 | 0 | 0 | 13 | 0 | 0 | 0 | 14 | 0 |
| Headache | 4 | 1 | 6 | 0 | 5 | 0 | 8 | 0 | 13 | 0 | 14 | 0 |
| Seizure | 0 | 0 | 0 | 0 | 2 | 0 | 4 | 0 | 0 | 0 | 0 | 0 |
| Peripheral neuropathy | 20 | 1 | 25 | 0 | 23 | 2 | 8 | 0 | 0 | 0 | 0 | 0 |
| Pruritus | 0 | 0 | 3 | 0 | 5 | 0 | 8 | 0 | 0 | 0 | 0 | 0 |
| Skin rash | 16 | 4 | 6 | 1 | 32 | 3 | 16 | 0 | 13 | 0 | 14 | 0 |
| Insomnia | 7 | 0 | 1 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
All values are percentiles. Dashes indicate that the number was omitted because specifying the exact number was not relevant in the table.
ANC, absolute neutrophil count; ALT, alanine aminotransferase; G4, grade 4; G3, grade 3.
All grades of QTc prolongation occurred among 66 cycles (38%) of the total 172 evaluable induction/consolidation cycles. Only 3 episodes (2%) were categorized as grade 3.
Administration of cytotoxic drugs and nilotinib
Table 4 shows the number of subjects that experienced AEs that caused dose reductions or interruptions and the dose intensity of nilotinib during chemotherapy. During induction, 45 subjects (50%) experienced 55 episodes that required dose reduction or transient interruption of nilotinib. The causative AEs were mainly febrile neutropenia (n = 11), jaundice (n = 8), nausea/vomiting (n = 6), and lung infiltration/infection (n = 5). Nilotinib was not discontinued permanently because of AEs, apart from subjects that died during induction. During induction, the median daily dose of nilotinib was 669 mg (range, 133-800), and the median dose intensity per day was 84% (range, 17% to 100%). Both the number of subjects that experienced nilotinib dose reductions/transient interruptions and the number of episodes that caused nilotinib dose reductions/transient interruptions decreased during subsequent consolidations. However, these events did not affect the overall outcome, including the HRFS and OS rates, regardless of whether subjects experienced dose reductions or interruptions in nilotinib.
Details of cytotoxic drugs and nilotinib during chemotherapy
| . | Induction (n = 90) . | Con #1 (n = 80) . | Con #2 (n = 65) . | Con #3 (n = 25) . | Con #4 (n = 8) . | Con #5 (n = 7) . |
|---|---|---|---|---|---|---|
| Cytotoxic drugs | ||||||
| Subjects with episode(s),* n (%) | 5 (6) | 15 (19) | 0 | 10 (40) | 1 (1) | 3 (43) |
| Cause of episode(s),* n | ||||||
| AE | 3 | 15 | 0 | 9 | 0 | 1 |
| Comorbidity or age | 0 | 0 | 0 | 1 | 1 | 2 |
| Others | 2 | 0 | 0 | 0 | 0 | 0 |
| Nilotinib | ||||||
| Median dose per day (mg) | 669 | 800 | 800 | 800 | 800 | 800 |
| Median dose intensity (%) | 84 | 100 | 100 | 100 | 100 | 100 |
| Subjects with episode(s),* n (%) | 45 (50) | 21 (26) | 18 (28) | 7 (28) | 1 (13) | 1(14) |
| No. of episode(s),* n | 55 | 24 | 26 | 8 | 1 | 1 |
| Cause of episode(s),* n | ||||||
| Febrile neutropenia | 11 | 3 | 2 | 0 | 0 | 0 |
| Fever | 1 | 1 | 1 | 0 | 0 | 0 |
| Myalgia | 3 | 0 | 1 | 0 | 0 | 0 |
| Fatigue | 1 | 0 | 0 | 0 | 0 | 0 |
| Headache | 1 | 1 | 0 | 0 | 0 | 0 |
| Cognitive dysfunction | 0 | 1 | 0 | 0 | 0 | 0 |
| Lung infiltration/infection | 5 | 2 | 2 | 0 | 0 | 0 |
| Jaundice | 8 | 5 | 3 | 1 | 1 | 0 |
| Serum ALT elevation | 2 | 2 | 2 | 0 | 0 | 0 |
| Serum lipase elevation | 2 | 0 | 1 | 0 | 0 | 0 |
| Oral mucositis | 0 | 0 | 0 | 1 | 0 | 0 |
| Nausea/vomiting | 6 | 2 | 1 | 0 | 0 | 0 |
| Dyspepsia | 0 | 1 | 0 | 0 | 0 | 0 |
| Pancreatitis | 0 | 0 | 0 | 0 | 0 | 1 |
| Abdominal pain | 3 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 1 | 0 | 3 | 0 | 0 | 0 |
| Ileus | 1 | 2 | 0 | 0 | 0 | 0 |
| Skin rash/itching | 2 | 1 | 2 | 2 | 0 | 0 |
| Edema | 1 | 1 | 0 | 1 | 0 | 0 |
| PSVT/arrhythmia | 1 | 0 | 0 | 0 | 0 | 0 |
| QTc prolongation | 2 | 1 | 0 | 0 | 0 | 0 |
| Palpitation | 0 | 0 | 1 | 0 | 0 | 0 |
| Angina | 0 | 1 | 0 | 0 | 0 | 0 |
| Azotemia | 0 | 0 | 3 | 1 | 0 | 0 |
| Peripheral neuropathy | 0 | 0 | 1 | 0 | 0 | 0 |
| Tremor | 0 | 0 | 1 | 0 | 0 | 0 |
| Cytopenia | 2 | 0 | 1 | 2 | 0 | 0 |
| Bleeding | 1 | 0 | 0 | 0 | 0 | 0 |
| Infection | 0 | 0 | 1 | 0 | 0 | 0 |
| Unknown | 1 | 0 | 0 | 0 | 0 | 0 |
| . | Induction (n = 90) . | Con #1 (n = 80) . | Con #2 (n = 65) . | Con #3 (n = 25) . | Con #4 (n = 8) . | Con #5 (n = 7) . |
|---|---|---|---|---|---|---|
| Cytotoxic drugs | ||||||
| Subjects with episode(s),* n (%) | 5 (6) | 15 (19) | 0 | 10 (40) | 1 (1) | 3 (43) |
| Cause of episode(s),* n | ||||||
| AE | 3 | 15 | 0 | 9 | 0 | 1 |
| Comorbidity or age | 0 | 0 | 0 | 1 | 1 | 2 |
| Others | 2 | 0 | 0 | 0 | 0 | 0 |
| Nilotinib | ||||||
| Median dose per day (mg) | 669 | 800 | 800 | 800 | 800 | 800 |
| Median dose intensity (%) | 84 | 100 | 100 | 100 | 100 | 100 |
| Subjects with episode(s),* n (%) | 45 (50) | 21 (26) | 18 (28) | 7 (28) | 1 (13) | 1(14) |
| No. of episode(s),* n | 55 | 24 | 26 | 8 | 1 | 1 |
| Cause of episode(s),* n | ||||||
| Febrile neutropenia | 11 | 3 | 2 | 0 | 0 | 0 |
| Fever | 1 | 1 | 1 | 0 | 0 | 0 |
| Myalgia | 3 | 0 | 1 | 0 | 0 | 0 |
| Fatigue | 1 | 0 | 0 | 0 | 0 | 0 |
| Headache | 1 | 1 | 0 | 0 | 0 | 0 |
| Cognitive dysfunction | 0 | 1 | 0 | 0 | 0 | 0 |
| Lung infiltration/infection | 5 | 2 | 2 | 0 | 0 | 0 |
| Jaundice | 8 | 5 | 3 | 1 | 1 | 0 |
| Serum ALT elevation | 2 | 2 | 2 | 0 | 0 | 0 |
| Serum lipase elevation | 2 | 0 | 1 | 0 | 0 | 0 |
| Oral mucositis | 0 | 0 | 0 | 1 | 0 | 0 |
| Nausea/vomiting | 6 | 2 | 1 | 0 | 0 | 0 |
| Dyspepsia | 0 | 1 | 0 | 0 | 0 | 0 |
| Pancreatitis | 0 | 0 | 0 | 0 | 0 | 1 |
| Abdominal pain | 3 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 1 | 0 | 3 | 0 | 0 | 0 |
| Ileus | 1 | 2 | 0 | 0 | 0 | 0 |
| Skin rash/itching | 2 | 1 | 2 | 2 | 0 | 0 |
| Edema | 1 | 1 | 0 | 1 | 0 | 0 |
| PSVT/arrhythmia | 1 | 0 | 0 | 0 | 0 | 0 |
| QTc prolongation | 2 | 1 | 0 | 0 | 0 | 0 |
| Palpitation | 0 | 0 | 1 | 0 | 0 | 0 |
| Angina | 0 | 1 | 0 | 0 | 0 | 0 |
| Azotemia | 0 | 0 | 3 | 1 | 0 | 0 |
| Peripheral neuropathy | 0 | 0 | 1 | 0 | 0 | 0 |
| Tremor | 0 | 0 | 1 | 0 | 0 | 0 |
| Cytopenia | 2 | 0 | 1 | 2 | 0 | 0 |
| Bleeding | 1 | 0 | 0 | 0 | 0 | 0 |
| Infection | 0 | 0 | 1 | 0 | 0 | 0 |
| Unknown | 1 | 0 | 0 | 0 | 0 | 0 |
PSVT, paroxysmal supraventricular tachycardia.
Episodes that caused dose reduction or transient interruption of drug.
Discussion
Before the era of imatinib, the HCR rate for Ph-pos ALL was 64% to 83% with high-dose multiagent chemotherapy,12 and the median survival time was only ∼1 year.13 The introduction of imatinib into the treatment of Ph-pos ALL has increased the HCR rate to 95% to 100%14-20 and has achieved meaningful improvement in OS.1,2,21 Despite its overall benefits, imatinib has some limitations as a treatment of Ph-pos ALL. First, the gastrointestinal AEs of imatinib may be aggravated when combined with cytotoxic drugs; this aggravation results in reduced drug compliance and, consequently, reduced dose intensity. Second, among the patients ineligible for allo-HCT that received consolidation followed by maintenance, many relapsed after discontinuing imatinib.22 That result suggested that even when imatinib was included in the treatment, allo-HCT remained important for overcoming the poor prognosis of Ph-pos ALL. The advantages of nilotinib include its high in vitro affinity to BCR-ABL1 tyrosine kinase,7,8 its improved MR in the treatment of chronic-phase CML,9 and its tolerability. Nilotinib’s low AE incidence improves drug compliance, which makes it more attractive than imatinib in the treatment of Ph-pos ALL. Moreover, the high incidence of ABL1 kinase mutations in Ph-pos ALL diagnoses23,24 justifies the use of nilotinib because nilotinib can effectively inhibit various mutated BCR-ABL1 tyrosine kinases.25
The present study showed that, other than the HCR rate, many outcomes with nilotinib were superior to those with imatinib and comparable to those with dasatinib.6,26 However, direct comparisons with previous studies were difficult because of the heterogeneity of inclusion criteria and end points (supplemental Table 2). Nilotinib was associated with AE types and severity similar to those of imatinib or dasatinib, when combined with cytotoxic drugs. During induction, we found 13% to 19% incidences of grades 3 or 4 jaundice, ALT elevations, and lipase elevations. However, most of these AEs were reversible after dose reduction or transient interruption of nilotinib. Most subjects continued with nilotinib for the intended period at a full or reduced dose. Grade 3 or 4 QTc prolongation occurred in only 2% of subjects. The AEs related to allo-HCT, particularly the incidence and severity of acute and chronic GVHD, were not significantly different from those typically found in clinical practice.
We found that MRD levels at 3 months after HCR were correlated with the overall outcomes, consistent with previous reports.27-30 Interestingly, the achievement of MR5 significantly affected the 2-year HRFS rate of subjects that did not receive allo-HCT, but not that of subjects that received allo-HCT. It was encouraging to find that, among subjects that achieved MR5, the 2-year HRFS rate was similar between those that did not receive allo-HCT (64%) and those that received allo-HCT (78%). A recent study by Wetzler et al31 also showed that the OS and HRFS were similar between those who underwent auto-HCT and those who underwent allo-HCT. These results suggested that administering TKI plus chemotherapy followed by TKI maintenance with or without auto-HCT may provide similar outcomes to those achieved by administering allo-HCT, if the patient achieves deep MR during the early postremission period.
It should be emphasized that in the current study, a significant portion of patients that failed to achieve a good MR (MR3 or MR5) at 3 months after HCR could be rescued successfully with allo-HCT. The estimated 2-year HRFS rate was 33% for those that did not achieve MR5. Also, 1 of 2 patients that failed to achieve even MR3 remained in HCR for 2 years after allo-HCT. The multivariate analysis showed that both the administration of allo-HCT and the achievement of deep MR at early postremission times were important. These results suggested that allo-HCT continued to play a significant role in treating Ph-pos ALL, despite the advent of potent TKIs, such as nilotinib. Therefore, in future MRD-based strategies for Ph-pos ALL, active administration of allo-HCT should be considered for patients that show suboptimal early MRD responses to a TKI plus multiagent chemotherapy.
Patients that failed to achieve a good MR (MR3 or MR5) just before allo-HCT had a significantly higher risk of HREL than those that achieved good MRs. Patients that failed to achieve MR5 or showed early MREL at 3 months after allo-HCT were at high risk of HREL. Future studies should consider early interventions, including post–allo-HCT empirical TKI administration, donor lymphocyte infusion, and/or a second-line TKI, to reduce the risk of post–allo-HCT HREL.
The limitation of the current study was that this study was not performed in a randomized comparative way, and the advantages of nilotinib over other TKIs were not definitely demonstrated. Although the combination of nilotinib and chemotherapy was feasible, the AEs cannot be overlooked. Moreover, the HCR rate was relatively lower than those reported for imatinib or dasatinib, which reported the high remission rates with low induction mortality using more minimal cytotoxic chemotherapeutic agents.6,26 The relatively low HCR rate was mainly because of the high NRM rate we observed during induction in the current study, and future studies should investigate whether a combination of nilotinib and less intensive cytotoxic chemotherapeutic agents might achieve improved outcomes and decrease NRM rates in a randomized comparative way.
To the best of our knowledge, the present study was the first phase 2 prospective trial of nilotinib plus multiagent chemotherapy for adult patients with Ph-pos ALL. Although the NRM rate was relatively high, because of high doses of anthracycline and nilotinib during induction, we found a high cumulative incidence of MCR (negative MRD) and HRFS rates comparable to those reported previously with imatinib or dasatinib. Furthermore, nilotinib was well-tolerated during consolidation with cytotoxic drugs. The MRD status at 3 months after HCR was closely related to the HRFS. Moreover, the MRD levels just before and at 3 months after allo-HCT were predictive of the HREL after allo-HCT. We recommend that future studies evaluate whether, compared with imatinib or dasatinib, nilotinib may improve the outcome of Ph-pos ALL by decreasing the HREL rate and decreasing the proportion of patients that require allo-HCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staffs of the participating institutes in the Adult Acute Lymphoblastic Leukemia Working Party of the Korean Society of Hematology, especially Hun Mo Ryoo, Sung Hwa Bae, Min Kyoung Kim, Moo-Rim Park, Hyeok Shim, Joon Seoung Park, Seong Hyun Jeong, Byung Soo Kim, Yong Park, and Hong Ghi Lee for participating in the study group and advising the study as members of the steering committee.
This work was supported by research funding from Novartis, which provided nilotinib and laboratory costs for monitoring MRD (CAMN107A2403), and by a grant from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea (2010-0668).
Authorship
Contribution: K.-H.L. contributed to the study design; D.-Y.K. and K.-H.L. contributed to the literature search, data interpretation, and writing the manuscript; D.-Y.K., Y.-D.J., and S.-N.L. conducted the study; Y.-U.C., S.J., C.-J.P., and H.-S.C. collected the blood samples for MRD evaluation, performed the laboratory process, and analyzed the results; and all the authors contributed to the data collection, data analysis, and final approval of the manuscript.
Conflict-of-interest disclosure: D.-Y.K. has received personal fees from Bristol Myers Squibb, Novartis, Otsuka, and Merck. K.-H.L. has received research grant support from Novartis Korea. The remaining authors declare no competing financial interests.
A complete list of the members of the Adult Acute Lymphoblastic Leukemia Working Party of the Korean Society of Hematology appears in “Appendix: study group members.”
Correspondence: Kyoo-Hyung Lee, Department of Hematology, Asan Medical Center, University of Ulsan College of Medicine, 88, Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea; e-mail: khlee2@amc.seoul.kr.
Appendix: study group members
The members of the Adult Acute Lymphoblastic Leukemia Working Party of the Korean Society of Hematology are: Kyoo-Hyung Lee, Young-Don Joo, Byoung Kook Kim, Jae Hoo Park, Hyun-Sook Chi, Whi-Hoong Yoon, Seonyang Park, Kyung Sam Cho, Yoo Hong Min, Hong Ghi Lee, Chan-Jeoung Park, Chul-Soo Kim, Jong-Ho Won, Hyeoung Joon Kim, Byung Soo Kim, Sung-Soo Yoon, Chul Won Jung, Je-Hwan Lee, Jae Hoon Lee, Sang Kyun Sohn, Yang Soo Kim, Je-Jung Lee, Deog-Yeon Jo, Joo-Seop Chung, Seok Lee, Jae-Young Kwak, Joon Seoung Park, Kihyun Kim, Inho Kim, Myung Soo Hyun, Jung Lim Lee, Hun Mo Ryoo, Moo-Rim Park, Hyeon-Seok Eom, Jun Ho Jang, Chul Won Choi, Jinny Park, Ho Young Kim, Hyo Jung Kim, Dae Young Zang, Ho-Jin Shin, Hyeok Shim, Seongsoo Jang, Dong Hwan (Dennis) Kim, Jung-Hee Lee, June-Won Cheong, Jin Seok Kim, Sung-Hyun Kim, Seok Jin Kim, Hawk Kim, Sung Hwa Bae, Won Sik Lee, Yeung-Chul Mun, Chan-Kyu Kim, Deok-Hwan Yang, Seong Hyun Jeong, Sang Min Lee, Gyeong Won Lee, Young-Uk Cho, Min Kyoung Kim, Dae-Young Kim, Joon Ho Moon, Ho-Sup Lee, Sung-Nam Lim, Sung-Doo Kim, Se Hyung Kim, Jae-Sook Ahn, Yong Park, Hyewon Lee, Kyoung Ha Kim, and Jae-Cheol Jo.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal