Key Points
Plasma free hemoglobin is associated with abnormal systemic and pulmonary vascular function markers.
Red cell storage time and physical changes in blood are associated with acute transfusional changes in endothelial function.
Abstract
Tricuspid regurgitant (TR) jet velocity and its relationship to pulmonary hypertension has been controversial in sickle cell disease (SCD). Plasma free hemoglobin is elevated in SCD patients and acutely impairs systemic vascular reactivity. We postulated that plasma free hemoglobin would be negatively associated with both systemic and pulmonary endothelial function, assessed by flow-mediated dilation (FMD) of the brachial artery and TR jet velocity, respectively. Whole blood viscosity, plasma free hemoglobin, TR jet, and FMD were measured in chronically transfused SCD pre- and posttransfusion (N = 25), in nontransfused SCD (N = 26), and in ethnicity-matched control subjects (N = 10). We found increased TR jet velocity and decreased FMD in nontransfused SCD patients compared with the other 2 groups. TR jet velocity was inversely correlated with FMD. There was a striking nonlinear relationship between plasma free hemoglobin and both TR jet velocity and FMD. A single transfusion in the chronically transfused cohort improved FMD. In our patient sample, TR jet velocity and FMD were most strongly associated with plasma free hemoglobin and transfusion status (transfusions being protective), and thus consistent with the hypothesis that intravascular hemolysis and increased endogenous erythropoiesis damage vascular endothelia.
Introduction
Sickle cell disease (SCD) is a single gene defect resulting from a point mutation in the β-globin gene, causing hemoglobin (Hb) to polymerize when deoxygenated.1,2 Polymerization in the microvasculature may cause vasoocclusion, leading to painful crisis and end-organ damage, particularly in the kidney, lungs, and bone.3,4 Repeated Hb polymerization also damages the red blood cell (RBC) structural integrity by mechanical and oxidative stress, lowering RBC deformability and increasing RBC adhesion properties.5 Abnormal mechanical properties of RBCs, coupled with increased blood flow as a compensation for decreased oxygen-carrying capacity, affect shear forces on the vascular endothelium.6 The vascular endothelium is also exposed to chemical stressors from intravascular release of Hb and chronic inflammation.7,8 Together, mechanical and chemical endothelial stressors are thought to impair endothelial release of, and response to, nitric oxide, thus impairing vasodilatory reserve.9 Intravascular hemolysis and plasma free Hb as a link between tricuspid regurgitation velocity, pulmonary hypertension, and systemic vascular dysfunction has been controversial.10
Flow-mediated dilation (FMD) of the brachial artery in response to transient vasoocclusion is a safe and reproducible metric for assessing endothelial health.11,12 Specifically, it is a test of shear-mediated endothelial nitric oxide release resulting in conduit artery vasodilation. FMD is strongly correlated with tricuspid regurgitant (TR) jet velocity, cardiac index, and myocardial performance index in children with primary pulmonary hypertension, reinforcing its role in pulmonary vascular disease.13 We do not know whether FMD is correlated with TR jet velocity in patients with SCD and whether chronic transfusions ameliorate endothelial dysfunction. We postulated that patients on chronic transfusion therapy would have higher FMD and that FMD would correlate with TR jet velocity in patients with SCD; furthermore, we postulated that increased cell free plasma Hb would be negatively associated with FMD.
Materials and methods
Patients
Twenty-six chronically transfused (CT) SCD patients, 26 patients with SCD not on a chronic transfusion protocol (NTN) and 11 age- and ethnicity-matched control subjects were enrolled in a prospective cross-sectional study to evaluate whether there are differences in FMD and TR jet velocity in NTN vs CT patients. CT patients were studied immediately before transfusion (CT-pre) as well as 12 to 120 hours posttransfusion (CT-post) to evaluate the effects of transfusion after volume-shifting effects have subsided. The NTN cohort included 23 patients on simple transfusion protocol and 3 patients on exchange transfusions. We did not limit the CT cohort to patients on simple transfusion because there was not enough evidence to support that cardiovascular physiologic differences exist, particularly with regard to plasma free Hb and vascular function. One patient was excluded from the transfusion arm because of a lack of posttransfusion data and 1 control subject was excluded because of an acute viral illness at the time of the study. Our institution’s simple CT protocol involves a target HbS% of 30% with a goal Hb level of 11 g/dL. The amount of blood and storage age of blood was recorded in all transfused patients. CT patients were identified in the outpatient transfusion clinic at Children’s Hospital Los Angeles. NTN patients were identified in the hematology clinic at Children’s Hospital Los Angeles. Control subjects were identified through family members of patients and hospital staff.
CT group exclusion criteria were age <10 years old, transfusions <1 year, or sickle crisis within the previous 4 weeks, with crisis defined as requiring admission or an emergency room visit with an increase in pain medications higher than their baseline usage for longer than 2 days. A total of 38 patients on chronic transfusion met our inclusion criteria, 6 patients declined to participate, and 6 were not approached at the request of the patient’s primary hematologist because of social or logistical barriers. Thus, we approached 81% and studied 68% of all eligible patients at our institution. The NTN group exclusion criteria were age <10 years old, >4 weeks’ posttransfusion, and no history of sickle crisis within the previous 4 weeks. All patients provided written informed consent, or parental consent was obtained according to the protocol approved by the Committee for Clinical Investigation at Children’s Hospital Los Angeles.
All subjects underwent history and physical examination, vascular ultrasound, echocardiography, and laboratory analysis on the morning of the study. They were in a fasting state except for fluids to prevent dehydration before the study; caffeinated drinks and fruit juices with high sugar content were not allowed. For the CT group, all vascular ultrasound, echocardiography, and laboratory testing was done before receiving any blood products. Vascular testing occurred in an isolated room with the patient recumbent at 45°, and completed within 30 to 45 minutes after the echocardiogram. CT subjects were seen again in the morning, in a fasting state except clear fluids, at 12 to 120 hours posttransfusion (mean 2.3 days, SD = 1.4 days) when peritransfusional changes in fluid, hormonal, and immune axes should have dissipated. The control and NTN groups were not transfused as part of the study and were seen only once. Approximately 10% of the nontransfused subjects were studied in the afternoon because of school scheduling. They were also required to maintain a fasting state (water allowed).
Hematologic analysis
A venous blood sample of ∼15 to 20 mL was drawn before transfusion and at the posttransfusion study visit to evaluate hematocrit, Hb, white blood cell (WBC) count, platelets, and reticulocyte count. Markers of hemolysis were measured, including lactic acid dehydrogenase (LDH), cell free Hb, and arginine:ornithine (Arg:Orn) ratio. High-sensitivity C-reactive protein (hsCRP) was measured as a marker of inflammation. The control group underwent Hb electrophoresis to ensure that they did not have sickle cell trait.
Rheology
Blood viscosity measurements were made on venous blood samples, collected in EDTA (1.8 mg/mL) tubes, over a shear rate range of 1 to 1000 seconds−1 using an automatic tube viscometer (Rheolog, Health Vector Co., Pennsauken, NJ).14 Blood was equilibrated with room air/2.5% CO2 via gas exchanger (Living Systems Instrumentation, Burlington, VT). The pH was 7.15 ± 0.02 as measured by an i-STAT-1 Analyzer (i-STAT Corporation, East Windsor, NJ).15 In the CT group, 16 had viscosity measurements, whereas 23 NTN subjects had viscosity measurements. All control patients had viscosity testing.
Echocardiography
Either the principal investigator (J.D.) or a trained technologist (H.G.) performed all echocardiograms using a Philips IE 33 ultrasound machine, version 3.0.2.711 (Philips Healthcare, Andover, MA). Standard 2-dimensional, M-mode, and tissue Doppler–derived function measurements were made according to published standards.16 Briefly, at least 3 averaged pulsed-wave Doppler or spectral Doppler signals were analyzed for cardiac index and TR jet velocity measurements. Peak and mean of all measurements were recorded.
FMD
FMD was performed using published guidelines for the ultrasound assessment of flow-mediated vasodilation of the brachial artery.17-19 All studies were performed by 1 of 2 physicians, J.D. or M.R. Baseline brachial artery images were obtained after a resting period 2 to 3 minutes before forearm cuff occlusion. The blood pressure cuff was inflated to >50 mm Hg above systolic blood pressure for 3 minutes, then released. The 3-minute protocol was based on previous studies of FMD in patients with SCD.6 Brachial artery diameter was recorded continuously for 7 minutes following occlusion. FMD was calculated as: (peak systolic diameter – baseline diameter)/baseline diameter) × 100, expressed as percent change.
Data analysis
Data were analyzed using 2 approaches: (1) Comparisons among groups were assessed using analysis of variance (ANOVA) or Kruskal-Wallis test when data were not normally distributed; (2) pre-CT and post-CT comparisons were evaluated by 2-sided, paired Student t test. The α for individual variables was adjusted using Bonferroni correction; for laboratory variables, we used α < 0.005 as significant and for our 2 primary vascular function measures α < 0.025 was considered significant. Variables measured in the transfusion group for pre-post differences were weighted by 50% and pooled for regression analysis. Chi-square analysis was used for categorical data and relative risk was determined for exposure (plasma free Hb >10) and the disease outcomes (TR jet >2.5 m/s, FMD <6%). These cutoffs were determined using control group values and are consistent with published normal values.20 Candidate variables determined by univariate linear regression analysis, using P < .10 as a cutoff for inclusion, were placed first into stepwise multivariate analysis and then into multivariate linear regression. Plasma free Hb had a nonlinear relationship with FMD, the primary variable for our study, and therefore it was log-transformed to satisfy the assumptions of linear regression analysis. JMP Pro (version 9.0.0, SAS Institute Inc., Cary, NC) was used for all statistical analyses.
Results
Patient characteristics
The sample groups were well-balanced for age, gender, ethnicity, and anthropomorphic data including height, weight, body mass index (BMI), and body surface area (Table 1). There was a trend toward increased mean blood pressure in the control group (ANOVA P = .06). Sixty-nine percent of nontransfused SCD patients were on hydroxyurea vs 16% of transfused patients. The most common reasons for starting transfusion therapy were history of stroke and high transcranial Doppler (data not shown). There were no episodes of pain crisis attributable to the forearm occlusion.
Demographics
| . | Nontransfused (n = 26) . | Transfused (n = 25) . | Control (n = 10) . | ANOVA . |
|---|---|---|---|---|
| Age | 22.7 ± 6.8 | 19.3 ± 4.4 | 22.9 ± 8.0 | 0.06 |
| % Male | 35% | 48% | 50% | 0.54 |
| % Hispanic | 12% | 16% | 10% | 0.85 |
| % On hydroxyurea | 75% | 16% | 0% | <0.0001 |
| % On folic acid | 27% | 28% | 0% | 0.16 |
| % SS | 92% | 100% | 0% | <0.0001 |
| % S-bη thalassemia | 8% | 0% | 0% | <0.0001 |
| Height (cm) | 167.3 ± 9.5 | 162.2 ± 10.4 | 171.6 ± 8.9 | 0.07 |
| Weight (kg) | 64.2 ± 2.2 | 58.8 ± 2.3 | 63.0 ± 3.6 | 0.24 |
| Systolic BP (mm Hg) | 113.2 ± 9.2 | 115.6 ± 6.9 | 116.7 ± 5.9 | 0.25 |
| Diastolic BP (mm Hg) | 61.8 ± 10.0 | 63.3 ± 5.7 | 66.1 ± 8.9 | 0.32 |
| Mean arterial pressure (mm Hg) | 79.3 ± 1.3 | 80.7 ± 1.3 | 85.1 ± 5.2 | 0.06 |
| Body surface area (m2) | 1.72 ± 0.21 | 1.62 ± 0.20 | 1.73 ± 0.16 | 0.15 |
| Body mass index (kg/m2) | 22.8 ± 3.6 | 22.0 ± 2.6 | 21.4 ± 2.6 | 0.45 |
| . | Nontransfused (n = 26) . | Transfused (n = 25) . | Control (n = 10) . | ANOVA . |
|---|---|---|---|---|
| Age | 22.7 ± 6.8 | 19.3 ± 4.4 | 22.9 ± 8.0 | 0.06 |
| % Male | 35% | 48% | 50% | 0.54 |
| % Hispanic | 12% | 16% | 10% | 0.85 |
| % On hydroxyurea | 75% | 16% | 0% | <0.0001 |
| % On folic acid | 27% | 28% | 0% | 0.16 |
| % SS | 92% | 100% | 0% | <0.0001 |
| % S-bη thalassemia | 8% | 0% | 0% | <0.0001 |
| Height (cm) | 167.3 ± 9.5 | 162.2 ± 10.4 | 171.6 ± 8.9 | 0.07 |
| Weight (kg) | 64.2 ± 2.2 | 58.8 ± 2.3 | 63.0 ± 3.6 | 0.24 |
| Systolic BP (mm Hg) | 113.2 ± 9.2 | 115.6 ± 6.9 | 116.7 ± 5.9 | 0.25 |
| Diastolic BP (mm Hg) | 61.8 ± 10.0 | 63.3 ± 5.7 | 66.1 ± 8.9 | 0.32 |
| Mean arterial pressure (mm Hg) | 79.3 ± 1.3 | 80.7 ± 1.3 | 85.1 ± 5.2 | 0.06 |
| Body surface area (m2) | 1.72 ± 0.21 | 1.62 ± 0.20 | 1.73 ± 0.16 | 0.15 |
| Body mass index (kg/m2) | 22.8 ± 3.6 | 22.0 ± 2.6 | 21.4 ± 2.6 | 0.45 |
ANOVA was performed to assess for differences in demographic and baseline vital signs. Bold P values indicate significant differences, P < 0.05.
SS, initial sickle cell genotype.
Acute and groupwise effect of transfusion on laboratory characteristics
The values for hematocrit and Hb increased significantly after transfusion, whereas HbS% and reticulocyte count decreased (P < .001). There were no significant changes in markers of hemolysis or inflammation after transfusion: WBC count, hsCRP, plasma free Hb, LDH, or Arg:Orn ratio. There was an increase in whole blood viscosity after transfusion at all shear rates (P < .001), suggesting that increased hematocrit had a greater effect on whole blood viscosity than the decrease in HbS% and reticulocyte count.
Groupwise comparisons of laboratory variables were all significant because of the striking differences between nontransfused and control subjects; therefore, we used the Tukey test for multiple comparisons to evaluate differences between CT-pre, CT-post, and NTN. The WBC count for both CT-pre and CT-post was 47% and 41% higher than NTN patients. The CT-pre Hb and hematocrit levels were similar to the NTN patients, whereas the CT-post Hb and hematocrit were 26% higher than in NTN patients. HbS% was significantly lower than NTN; the nontransfused HbS% was not 100% because of 2 S-bη thalassemia0 patients and patients on hydroxyurea with elevated Hb F. Six patients had >10% HbA (not HbA2), possibly from a simple transfusion >4 weeks before our study. There were no significant differences in markers of hemolysis between CT and NTN. Across all shear rates, CT-pre subjects had a similar whole blood viscosity when compared with NTN subjects, whereas CT-post subjects had similar viscosity measurements to control.
Acute and groupwise effect of transfusion on cardiac function
Cardiac index decreased following transfusion concomitant with the increase in oxygen-carrying capacity. There was no change in left ventricular shortening fraction, a marker of systolic function, nor mitral E-wave to A-wave ratio, a marker of diastolic function (Table 2).
Laboratory and cardiovascular function between groups
| Parameter . | Pretransfusion . | Posttransfusion . | Paired t test P value . | Nontransfused . | Control . |
|---|---|---|---|---|---|
| Laboratory | |||||
| WBC (×103/μL) | 14.0 ± 4.7 | 13.4 ± 4.9 | .22 | 9.5 ± 3.9 | 4.6 ± 1.3 |
| Hematocrit (%) | 28.4 ± 2.6 | 34.2 ± 3.1 | <.0001 | 26.8 ± 4.0 | 40.9 ± 3.1 |
| Hb (g/dL) | 9.5 ± 0.9 | 11.7 ± 1.1 | <.001 | 9.3 ± 1.4 | 13.8 ± 1.2 |
| Platelet (×103/μL) | 321.6 ± 110.0 | 303.7 ± 97.0 | .08 | 385.3 ± 139.3 | 238.4 ± 45.2 |
| HbS (%) | 34.8 ± 18.7 | 29.5 ± 15.7 | <.001 | 78.3 ± 8.5 | 0 |
| RC (×106/μL) | 11.8 ± 6.3 | 8.3 ± 4.8 | <.0001 | 9.3 ± 4.9 | 0.9 ± 0.3 |
| LDH (U/L) | 1196 ± 543 | 1163 ± 536 | .19 | 1134 ± 447 | 484 ± 54 |
| hsCRP (mg/L) | 5.7 ± 5.6 | 4.1 ± 4.3 | .40 | 4.7 ± 3.9 | 0.5 ± 0.8 |
| Arg:Orn | 0.27 ± 0.09 | 0.30 ± 0.15 | .78 | 0.23 ± 0.08 | 0.32 ± 0.05 |
| Plasma free Hb (mg/dL) | 20.7 ± 14.6 | 18.6 ± 13.6 | .71 | 18.7 ± 12.0 | 4.7 ± 2.9 |
| Cardiovascular | |||||
| LV shortening fraction (%) | 38.8 ± 5.5 | 36.9 ± 7.0 | .17 | 37.4 ± 5.2 | 39.2 ± 4.2 |
| Mitral E/A | 2 ± 0.43 | 2.1 ± 0.51 | .04 | 2.2 ± 0.64 | 2.3 ± 0.56 |
| FMD (%) | 5.7 ± 2.8 | 7.2 ± 2.8 | .01 | 5.5 ± 2.5 | 7.9 ± 1.6 |
| TR jet maximum (cm/s) | 219 ± 43 | 215 ± 31 | .51 | 243 ± 31 | 208 ± 12 |
| Cardiac index (L/min per square meter) | 3.3 ± 0.7 | 2.8 ± 0.8 | .004 | 3.6 ± 1.1 | 2.5 ± 0.7 |
| Rheology | |||||
| Viscosity 1 second−1 (n = 16) | 6.4 ± 1.0 | 8.1 ± 1.3 | .0003 | 6.3 ± 1.5 | 9.3 ± 2.3 |
| Viscosity 1000 second−1 (n = 16) | 3.2 ± 0.3 | 3.8 ± 0.4 | .0006 | 3.3 ± 0.5 | 4.0 ± 0.8 |
| Parameter . | Pretransfusion . | Posttransfusion . | Paired t test P value . | Nontransfused . | Control . |
|---|---|---|---|---|---|
| Laboratory | |||||
| WBC (×103/μL) | 14.0 ± 4.7 | 13.4 ± 4.9 | .22 | 9.5 ± 3.9 | 4.6 ± 1.3 |
| Hematocrit (%) | 28.4 ± 2.6 | 34.2 ± 3.1 | <.0001 | 26.8 ± 4.0 | 40.9 ± 3.1 |
| Hb (g/dL) | 9.5 ± 0.9 | 11.7 ± 1.1 | <.001 | 9.3 ± 1.4 | 13.8 ± 1.2 |
| Platelet (×103/μL) | 321.6 ± 110.0 | 303.7 ± 97.0 | .08 | 385.3 ± 139.3 | 238.4 ± 45.2 |
| HbS (%) | 34.8 ± 18.7 | 29.5 ± 15.7 | <.001 | 78.3 ± 8.5 | 0 |
| RC (×106/μL) | 11.8 ± 6.3 | 8.3 ± 4.8 | <.0001 | 9.3 ± 4.9 | 0.9 ± 0.3 |
| LDH (U/L) | 1196 ± 543 | 1163 ± 536 | .19 | 1134 ± 447 | 484 ± 54 |
| hsCRP (mg/L) | 5.7 ± 5.6 | 4.1 ± 4.3 | .40 | 4.7 ± 3.9 | 0.5 ± 0.8 |
| Arg:Orn | 0.27 ± 0.09 | 0.30 ± 0.15 | .78 | 0.23 ± 0.08 | 0.32 ± 0.05 |
| Plasma free Hb (mg/dL) | 20.7 ± 14.6 | 18.6 ± 13.6 | .71 | 18.7 ± 12.0 | 4.7 ± 2.9 |
| Cardiovascular | |||||
| LV shortening fraction (%) | 38.8 ± 5.5 | 36.9 ± 7.0 | .17 | 37.4 ± 5.2 | 39.2 ± 4.2 |
| Mitral E/A | 2 ± 0.43 | 2.1 ± 0.51 | .04 | 2.2 ± 0.64 | 2.3 ± 0.56 |
| FMD (%) | 5.7 ± 2.8 | 7.2 ± 2.8 | .01 | 5.5 ± 2.5 | 7.9 ± 1.6 |
| TR jet maximum (cm/s) | 219 ± 43 | 215 ± 31 | .51 | 243 ± 31 | 208 ± 12 |
| Cardiac index (L/min per square meter) | 3.3 ± 0.7 | 2.8 ± 0.8 | .004 | 3.6 ± 1.1 | 2.5 ± 0.7 |
| Rheology | |||||
| Viscosity 1 second−1 (n = 16) | 6.4 ± 1.0 | 8.1 ± 1.3 | .0003 | 6.3 ± 1.5 | 9.3 ± 2.3 |
| Viscosity 1000 second−1 (n = 16) | 3.2 ± 0.3 | 3.8 ± 0.4 | .0006 | 3.3 ± 0.5 | 4.0 ± 0.8 |
ANOVA for multiple group comparisons was performed to assess differences in hematologic, rheologic, and cardiovascular function parameters. Tukey post hoc comparisons were then performed to determine individual group differences; last, paired Student t tests were performed to evaluate pre- to posttransfusion changes. Values expressed as mean ± standard deviation. ANOVA was significant for almost every parameter (only LV shortening fraction and mitral E/A were not significant); the P value denotes the significance for a paired Student t test (pre- to posttransfusion).
LV, left ventricular; RC, reticulocyte count.
All groups had normal systolic and diastolic function by echocardiography and there were no differences in these variables among the 3 groups, except cardiac index was elevated in nontransfused and in CT SCD patients pretransfusion. Cardiac index normalized following transfusion (Table 2).
Acute and groupwise effect of transfusion on vascular function
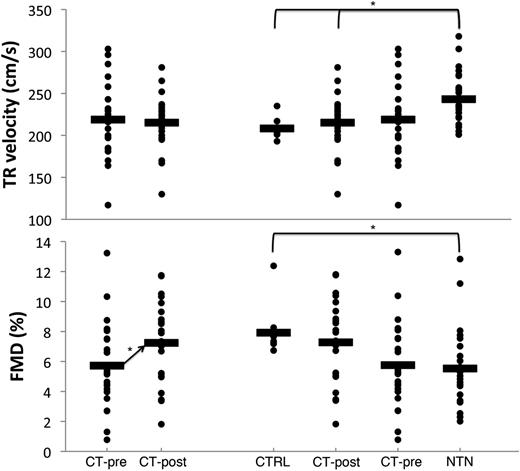
There was no acute change in TR jet velocity following transfusion (Figure 1A). There were 5 CT-pre subjects with a TR jet velocity >2.5 m/s and 3 CT-post subjects with a TR jet velocity >2.5 m/s (P = .44). There were no CT-pre patients with a TR jet velocity <2.5 m/s who reached a velocity >2.5 m/s after transfusion. FMD increased by 26% following transfusion (Figure 1B).
Acute change with transfusion and groupwise comparisons are shown for TR jet velocity and FMD. There was no significant change in TR jet velocity from pre- to posttransfusion. The distribution between control subjects, nontransfused SCD, pretransfusion SCD, and posttransfusion SCD is shown next to the acute change with transfusion. The TR jet velocity in the nontransfused subjects was significantly higher than the control and posttransfusion subjects, but not different than the pretransfusion subjects (*P < .05). There was a wide distribution of FMD among SCD patients, similar to TR jet velocity. However, there was a significant increase in FMD acutely after transfusion (P < .05), and FMD was significantly higher in control subjects vs nontransfused SCD patients (P < .05).
Acute change with transfusion and groupwise comparisons are shown for TR jet velocity and FMD. There was no significant change in TR jet velocity from pre- to posttransfusion. The distribution between control subjects, nontransfused SCD, pretransfusion SCD, and posttransfusion SCD is shown next to the acute change with transfusion. The TR jet velocity in the nontransfused subjects was significantly higher than the control and posttransfusion subjects, but not different than the pretransfusion subjects (*P < .05). There was a wide distribution of FMD among SCD patients, similar to TR jet velocity. However, there was a significant increase in FMD acutely after transfusion (P < .05), and FMD was significantly higher in control subjects vs nontransfused SCD patients (P < .05).
Across groups, significant differences in TR and FMD were observed only between controls and NTN (Figure 1C-D). CT patients exhibited intermediate values, with pretransfusion measurement closer to NTN values and posttransfusion closer to control values. Variability was high in the SCD patients. In general, males had higher TR jet velocities and lower FMDs than females (data not shown). FMD and TR velocity were not different between patients on hydroxyurea (data not shown).
Univariate analysis of improvement in FMD posttransfusion
Univariate analysis indicated that only 3 variables were associated with the increase in FMD after transfusion: higher BMI, lower blood storage age, and a decrease in low shear rate hematocrit to viscosity ratio. BMI had a strong positive association with the increase in FMD (r2 = 0.39, P = .002), whereas age of the stored blood had a weaker negative association (r2 = 0.23, P = .02). However, BMI and blood age were confounded because newer blood was given to the larger patients in our cohort. The hematocrit:viscosity ratio, a marker of oxygen transport effectiveness used in the Framingham Heart Study, demonstrates a weak association with the change in FMD. Neither blood viscosity nor hematocrit alone was associated with the change in FMD.
Association between endothelial function and TR jet velocity, and the effect of plasma free Hb on both vascular markers
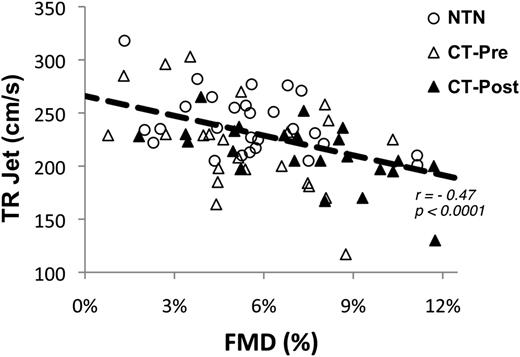
TR jet velocity was negatively correlated with FMD in both the NTN and CT groups (P < .0001) (Figure 2) with significant associations in both groups individually (data not shown: P = .001 for CT and P = .06 for NTN). Most SCD patients with a TR jet velocity >270 cm/s had endothelial dysfunction, FMD <6% (chi-square P = .04); this relationship was not significant for a cutoff TR jet velocity of 250 cm/s (chi-square P = .11).
TR jet velocity is negatively associated with FMD. The inverse relationship between FMD and TR jet velocity for all 3 groups studied: nontransfused, pretransfusion, and posttransfusion HbSS patients. At lower (worse) FMD values, the TR jet velocity is higher, suggesting that a component of elevated TR jet velocity is likely endothelial dysfunction.
TR jet velocity is negatively associated with FMD. The inverse relationship between FMD and TR jet velocity for all 3 groups studied: nontransfused, pretransfusion, and posttransfusion HbSS patients. At lower (worse) FMD values, the TR jet velocity is higher, suggesting that a component of elevated TR jet velocity is likely endothelial dysfunction.
Figure 3A demonstrates a curvilinear relationship for TR jet velocity vs plasma free Hb. Figure 4A demonstrates a striking curvilinear association between FMD and plasma free Hb. This curvilinear relationship was preserved across CT and NTN SCD patients. In addition to stable levels of plasma free Hb after transfusion, there was no significant difference between levels of plasma free Hb in CT and NTN groups. Patients with elevated plasma free Hb levels defined as >10 mg/dL, which is >2 standard deviations above the mean for controls, had elevated TR jet velocity >250 cm/s (P = .03, relative risk 3.47 (95% confidence interval [CI]: 0.88-13.65) and decreased FMD <6%, (P = .003, relative risk 2.51, 95% CI: 1.15-5.47) (Figures 3B and 4B).
Relationship between TR jet velocity and cell free hemoglobin. (A) The nonlinear relationship between tricuspid regurgitation velocity (TR jet velocity) and cell free hemoglobin for all subjects; at higher cell free hemoglobin levels, TR jet velocity is higher across all 3 groups. (B) A higher proportion of patients with cell free hemoglobin levels >10 mg/dL (elevated) has a TR jet velocity >250 cm/s, above which portends increased risk of mortality in SCD. (B inset) A 2 × 2 table showing relative risk assessment of plasma free hemoglobin >10 mg/dL for TR jet >250 cm/s.
Relationship between TR jet velocity and cell free hemoglobin. (A) The nonlinear relationship between tricuspid regurgitation velocity (TR jet velocity) and cell free hemoglobin for all subjects; at higher cell free hemoglobin levels, TR jet velocity is higher across all 3 groups. (B) A higher proportion of patients with cell free hemoglobin levels >10 mg/dL (elevated) has a TR jet velocity >250 cm/s, above which portends increased risk of mortality in SCD. (B inset) A 2 × 2 table showing relative risk assessment of plasma free hemoglobin >10 mg/dL for TR jet >250 cm/s.
Relationship between FMD and cell free hemoglobin. (A) The nonlinear relationship between FMD of the brachial artery and cell free hemoglobin for all subjects; at higher cell free hemoglobin levels, FMD is lower across all 3 groups. (B) A higher proportion of patients with cell free hemoglobin levels >10 mg/dL (elevated) have an FMD <6%, the lower limit of our control population. (B inset) A 2 × 2 table showing relative risk assessment of plasma free hemoglobin >10 mg/dL for FMD<6%.
Relationship between FMD and cell free hemoglobin. (A) The nonlinear relationship between FMD of the brachial artery and cell free hemoglobin for all subjects; at higher cell free hemoglobin levels, FMD is lower across all 3 groups. (B) A higher proportion of patients with cell free hemoglobin levels >10 mg/dL (elevated) have an FMD <6%, the lower limit of our control population. (B inset) A 2 × 2 table showing relative risk assessment of plasma free hemoglobin >10 mg/dL for FMD<6%.
Predictors of TR and FMD in CT and NTN patients: multivariate analysis of transfusion, hematologic, and hemolytic variables on systemic and pulmonary vascular function
Lower TR jet velocity was associated with chronic transfusion therapy, improvement in markers of anemia (reticulocyte count, Hct, Hb, and HgS%), increasing viscosity (Table 3), and decreased surrogates for hemolysis (LDH, higher Arg:Orn, and lower plasma free Hb).
Univariate analysis for TR jet velocity and FMD
| Parameter . | Parameter estimate . | 95% CI . | R2 . | P value . |
|---|---|---|---|---|
| Univariate TR jet velocity (cm/s) vs (X) | ||||
| Transfusion status (transfused) | −13.07 | −20.84 to −5.28 | 0.13 | .001 |
| FMD (%) | −5.94 | −8.74 to −3.14 | 0.2 | <.0001 |
| Sex (female) | −8.8 | −16.4 to −1.3 | 0.06 | .02 |
| Height (cm) | 0.74 | 0.07 to 1.40 | 0.06 | .03 |
| Hct (%) | −3.35 | −5.01 to −1.68 | 0.18 | .0001 |
| Hb (g/dL) | −8.67 | −13.67 to −3.67 | 0.14 | .0009 |
| HgS (%) | 0.52 | 0.22 to 0.82 | 0.16 | .001 |
| Reticulocyte count (×106/μL) | 2.27 | 0.78 to 3.77 | 0.12 | .003 |
| LDH (U/L) | 0.02 | 0.006 to 0.04 | 0.09 | .01 |
| Arg:Orn | −98.3 | −175.5 to −21.2 | 0.08 | .01 |
| ln(plasma free Hb) | 22.06 | 10.9 to 33.2 | 0.19 | .0002 |
| Viscosity at 300 s−1 | −24.73 | −42.44 to −7.03 | 0.13 | .007 |
| Univariate FMD (%) vs (X) | ||||
| Transfusion status (nontransfused) | −0.65 | −1.55 to 0.25 | 0.08 | .15 |
| Transfusion status (pretransfusion) | −0.43 | −1.33 to 0.47 | .34 | |
| Sex (female) | 0.94 | 0.34 to 1.56 | 0.12 | .003 |
| Height (cm) | −0.07 | −0.12 to −0.03 | 0.12 | .003 |
| Body surface area (m2) | −2.67 | −5.13 to −0.19 | 0.06 | .04 |
| Cardiac stroke volume (mL) | −0.02 | −0.05 to 0.001 | 0.06 | .04 |
| Hct (%) | 0.14 | 0.005 to 0.28 | 0.06 | .04 |
| Reticulocyte count (×106/μL) | −0.14 | −0.26 to −0.03 | 0.09 | .01 |
| HgS (%) | −0.03 | −0.05 to −0.0005 | 0.07 | .05 |
| LDH (U/L) | −0.001 | −0.002 to 0.0001 | 0.05 | .07 |
| ln(plasma free Hb) | −1.98 | −2.79 to −1.16 | 0.27 | <.0001 |
| Parameter . | Parameter estimate . | 95% CI . | R2 . | P value . |
|---|---|---|---|---|
| Univariate TR jet velocity (cm/s) vs (X) | ||||
| Transfusion status (transfused) | −13.07 | −20.84 to −5.28 | 0.13 | .001 |
| FMD (%) | −5.94 | −8.74 to −3.14 | 0.2 | <.0001 |
| Sex (female) | −8.8 | −16.4 to −1.3 | 0.06 | .02 |
| Height (cm) | 0.74 | 0.07 to 1.40 | 0.06 | .03 |
| Hct (%) | −3.35 | −5.01 to −1.68 | 0.18 | .0001 |
| Hb (g/dL) | −8.67 | −13.67 to −3.67 | 0.14 | .0009 |
| HgS (%) | 0.52 | 0.22 to 0.82 | 0.16 | .001 |
| Reticulocyte count (×106/μL) | 2.27 | 0.78 to 3.77 | 0.12 | .003 |
| LDH (U/L) | 0.02 | 0.006 to 0.04 | 0.09 | .01 |
| Arg:Orn | −98.3 | −175.5 to −21.2 | 0.08 | .01 |
| ln(plasma free Hb) | 22.06 | 10.9 to 33.2 | 0.19 | .0002 |
| Viscosity at 300 s−1 | −24.73 | −42.44 to −7.03 | 0.13 | .007 |
| Univariate FMD (%) vs (X) | ||||
| Transfusion status (nontransfused) | −0.65 | −1.55 to 0.25 | 0.08 | .15 |
| Transfusion status (pretransfusion) | −0.43 | −1.33 to 0.47 | .34 | |
| Sex (female) | 0.94 | 0.34 to 1.56 | 0.12 | .003 |
| Height (cm) | −0.07 | −0.12 to −0.03 | 0.12 | .003 |
| Body surface area (m2) | −2.67 | −5.13 to −0.19 | 0.06 | .04 |
| Cardiac stroke volume (mL) | −0.02 | −0.05 to 0.001 | 0.06 | .04 |
| Hct (%) | 0.14 | 0.005 to 0.28 | 0.06 | .04 |
| Reticulocyte count (×106/μL) | −0.14 | −0.26 to −0.03 | 0.09 | .01 |
| HgS (%) | −0.03 | −0.05 to −0.0005 | 0.07 | .05 |
| LDH (U/L) | −0.001 | −0.002 to 0.0001 | 0.05 | .07 |
| ln(plasma free Hb) | −1.98 | −2.79 to −1.16 | 0.27 | <.0001 |
Using the method of least squares, univariate linear regression was performed using either TR jet velocity or flow-mediated dilation of the brachial artery as the dependent variable, with multiple hematologic, rheologic, and vital sign/demographic data as the independent predictors.
On multivariate analysis, plasma free Hb, chronic transfusion, and height were the strongest predictors of TR jet velocity (Table 4). LDH, another marker of hemolysis, also maintains a positive association with TR jet velocity, whereas hematocrit was no longer statistically significant.
Multivariate regression analysis for FMD and TR jet velocity
| . | Parameter estimate . | SE . | 95% CI . | P value . |
|---|---|---|---|---|
| Multivariate linear regression analysis TR jet velocity (cm/s) (n = 73) | ||||
| ln(plasma free Hb) | 13.9 | 6.1 | 1.8 to 26.1 | .025 |
| Transfusion status (CT) | −12.5 | 5.8 | −24.1 to −0.8 | .037 |
| Height | 0.55 | 0.27 | 0.01 to 1.10 | .045 |
| LDH | 0.01 | 0.01 | −0.002 to 0.03 | .102 |
| Hct | −1.36 | 0.98 | −3.3 to 0.6 | .167 |
| Multivariate linear regression analysis FMD (%) (n = 61) | ||||
| ln(plasma free Hb) | −1.21 | 0.43 | −2.1 to −0.4 | .007 |
| Height | −0.05 | 0.02 | −0.1 to −0.01 | .03 |
| Hematocrit | 0.11 | 0.07 | −0.03 to 0.26 | .12 |
| Sex (female) | 0.53 | 0.33 | −0.13 to 1.18 | .11 |
| HbS% | 0.001 | 0.01 | −0.02 to 0.03 | .88 |
| . | Parameter estimate . | SE . | 95% CI . | P value . |
|---|---|---|---|---|
| Multivariate linear regression analysis TR jet velocity (cm/s) (n = 73) | ||||
| ln(plasma free Hb) | 13.9 | 6.1 | 1.8 to 26.1 | .025 |
| Transfusion status (CT) | −12.5 | 5.8 | −24.1 to −0.8 | .037 |
| Height | 0.55 | 0.27 | 0.01 to 1.10 | .045 |
| LDH | 0.01 | 0.01 | −0.002 to 0.03 | .102 |
| Hct | −1.36 | 0.98 | −3.3 to 0.6 | .167 |
| Multivariate linear regression analysis FMD (%) (n = 61) | ||||
| ln(plasma free Hb) | −1.21 | 0.43 | −2.1 to −0.4 | .007 |
| Height | −0.05 | 0.02 | −0.1 to −0.01 | .03 |
| Hematocrit | 0.11 | 0.07 | −0.03 to 0.26 | .12 |
| Sex (female) | 0.53 | 0.33 | −0.13 to 1.18 | .11 |
| HbS% | 0.001 | 0.01 | −0.02 to 0.03 | .88 |
Univariate candidate variables were input to multivariate linear regression first by stepwise analysis and second using the method of least squares analysis. The results provide insight into the most significant variables that predict TR jet velocity and flow-mediated dilation of the brachial artery. Bold P values indicate significant differences, P < 0.05.
FMD was most strongly associated with cell free Hb (r2 = 0.27, P < .0001), as well as weaker contributions from other hemolytic markers (Table 3). FMD also had a positive association with sex (female) and a negative association with height and body surface area. Only cell free Hb and height were retained in the multivariate analysis (Table 4).
Discussion
The importance and potential mechanisms of intravascular hemolysis and decellularized Hb in sickle cell–related vascular disease is controversial. We found a strong nonlinear association between cell free Hb and markers of both systemic and pulmonary vascular function, even after controlling for common modulators of blood flow and vascular function. This provides strong evidence for plasma free Hb as a causative agent in systemic and pulmonary vascular disease. The nonlinear response of FMD and TR jet velocity to cell free Hb has a striking resemblance to the forearm blood flow response to nitroprusside and cell free Hb demonstrated by Reiter and colleagues.21 In that study, cell free Hb levels >6 μM attenuated vascular reactivity by 80%. Also, cell free Hb was linearly correlated with the rate of nitric oxide scavenging on in vitro assays. Cell free Hb potentially produces vascular disease by Hb interactions with nitric oxide,22,23 heme-mediated oxidative damage,24 arginase consumption of endothelial nitric oxide synthase (eNOS) substrates,25 and adenosine-mediated shifts in Hb dissociation curve.26 Reiter and colleagues also demonstrated an increase in endothelial vascular cell adhesion molecule, which may lead to adverse, chronic vascular remodeling. Our observations are consistent with work done by Belhassen and colleagues.6 They demonstrated that SCD patients exhibited blunted vasodilatory response to both acute and chronic increases in wall shear stress. They postulated 2 potential points of disruption in flow-vasodilation coupling: (1) disruption of eNOS signal transduction from the endothelial cell surface or (2) abnormal nitric oxide transit to the smooth muscle after it is produced by eNOS in the endothelium. Increased cell free Hb may contribute to both of these pathways by serving as a powerful nitric oxide sink and by direct oxidative damage to the vascular endothelium, particularly if decellularized heme deposits on the vascular surface.
It is not surprising that SCD patients demonstrated a broad range of FMD values, including some patients that exhibited very high FMD values. Cell free Hb accumulation may change the endothelial set point and responsiveness to acute changes in mechanical properties of the blood, potentially explaining the wide range, both super- and subnormal FMD responses in our patient sample. Kaul and colleagues, using the Berkley SCD mouse model, demonstrated an increase in both eNOS expression and cyclooxygenase-2 expression in cremaster muscle lysate. Despite the increase in eNOS and cyclooxygenase-2 expression, there was a blunted response to a nitric oxide donor, single nucleotide polymorphism, and an endothelial dependent vasodilator, Ach, that acts through both eNOS and cyclooxygenase-2.27
It is intriguing that cell free Hb was the strongest predictor of both FMD and TR jet velocity in cross-sectional analysis, but it could not account for the improvement in FMD acutely after a single transfusion; neither the initial plasma free Hb level nor the change in plasma free Hb were associated with the change in FMD after a single transfusion. The only variables associated with improvement in FMD after a transfusion were BMI, age of stored blood, and hematocrit:viscosity ratio (HVR). Because the transfusion volume is dosed on a per-kilogram basis, the BMI relationship may reflect the change in red cell mass. Transfusion acutely alters the physical interaction among red cell mass, plasma, and the endothelial surface. Viscosity increases but cardiac output and blood velocity decrease, producing competing effects on wall shear stresses. Viscosity increases disproportionally to the change in blood mass, reducing the HVR.28 However, mechanical interactions at the endothelial surface are also tempered by changes in RBC deformability through the reduction of HbSS concentration.29 HVR was initially developed to describe blood flow properties in the microcirculation and the potential for oxygen delivery to systemic and pulmonary tissues.15 Therefore, HVR may be an indicator for both oxygen delivery and the physical properties of whole blood, driving microcirculatory flow and affecting FMD upstream in the conduit arteries.
The negative impact of blood age on FMD response to transfusion is fascinating and warrants further investigation. Although other reports demonstrate an increase in plasma free Hb following transfusion, we did not see the same change across our sample; differences may depend on the amount of blood given, the initial level of plasma Hb, and transfusion storage conditions.30,31 Stored red cells undergo both biochemical and mechanical changes (so-called storage lesion) that could impair their endothelial interactions independently from circulating cell free Hb. Therefore, our data suggest that the pretransfusion FMD may be a more robust predictor of vascular dysfunction in patients with SCD.
Cell free Hb appears to link systemic and pulmonary vascular disease. Cross-sectional analysis demonstrated that higher FMD was associated with lower TR jet velocity (Figure 2), and cell free Hb was also the strongest predictor of TR jet velocity. However, the use of chronic transfusion therapy was also an independent, powerful, predictor of TR jet velocity, suggesting that cell free Hb is only 1 component of the pathophysiology. Although Lezcano and colleagues demonstrated that chronic transfusion therapy decreases circulating cell free Hb after being on transfusion therapy for 1 year,32 CT patients still had cell free Hb levels comparable to nontransfused patients. Chronic transfusion therapy is generally reserved for patients with severe vascular disease, and there was a protective effect demonstrated by a lower TR jet velocity in the transfusion group. These effects were retained after correction for sex, Hb, and cardiac output, suggesting that protection is not simply due to correction of anemia. Patients on chronic transfusion therapy have a lower risk of microvascular complications, including acute chest syndrome, vaso-occlusive crisis, and silent stroke. Thus reduction in pulmonary hypertension prevalence is not inherently surprising. Endogenesis erythropoiesis, pulmonary hypertension, and vascular function have been shown to have significant sex differences, consistent with our findings that females have higher FMD and lower TR jet velocity.33-35 Transfusion therapy also suppresses endogenous erythropoiesis and improves vascular function, decreasing angiogenic factors produced by erythroid precursors, such as placental growth factor, that have been implicated in pulmonary hypertension.36,37
TR jet velocity is a controversial surrogate for pulmonary hypertension in SCD.10 Despite this, there is a significantly higher incidence of all-cause pulmonary hypertension in adults with SCD (6% to 10%). Pulmonary hypertension confirmed by cardiac catheterization is increased compared with the general population (15 to 50/million).38 Mortality in these patients is exceptionally high. In fact, mortality is markedly elevated (15% over 2 years) even in patients with mild increases in TR jet velocity (250 to 300 cm/s).7 In patients, TR jet velocity likely represents a broad spectrum of vascular stress and loss of functional integrity ranging from reversible endothelial dysfunction to irreversible destruction of pulmonary vasculature, as seen in the Heath-Edward Classification of pulmonary vascular disease.39 Decreased FMD and increased TR jet velocity both signal vascular stress, and may reduce the patient’s ability to respond to acute stressors, including crisis events.
Mild increases in TR jet velocity secondary to impaired endothelial function are likely the result of high cardiac output necessary to preserve organ oxygen delivery.40,41 Under these conditions, a healthy vasculature would maintain pulmonary artery pressures within normal limits, similar to the normal response with exercise. More severe elevations in TR jet and impaired endothelial function are likely from a progression of histological changes associated with irreversible fibromuscular vascular changes. Nitric oxide is a well-known inhibitor of adverse fibromuscular remodeling in patients with primary pulmonary hypertension.42,43 Our study is likely exposing 2 overlapping, yet different mechanisms of vascular dysfunction in SCD, the acute effects of a single transfusion and the chronic effects of hemolysis.
There are a few limitations in our study. Although the present study suggests a key role for cell free Hb in vascular dysfunction, it does not indicate possible mechanisms of vascular toxicity. Furthermore, we cannot exclude contributions from other products of hemolysis. The cross-sectional nature of our study does not allow us to determine whether plasma free Hb is a direct mechanism of vascular dysregulation vs important unmeasured covariates including arginase, asymmetric dimethylarginine, and labile iron that could cause vascular dysfunction. The chronic effect of transfusion therapy on cell free Hb and vascular function could not be assessed in our study because of the cross-sectional nature and chronic nature of vascular disease development. However, the lower prevalence of elevated TR jet velocity in the CT group compared with the nontransfused group is compelling.
Our patient population consisted primarily of pediatric and young adult patients. Because vascular remodeling progresses over time, the relationship between plasma free Hb and markers of vascular function may be lost.39 Our NTN group had a higher percentage of patients on hydroxyurea therapy than the CT patients (reflecting standard clinical practice). Although there was no difference in FMD between patients on or off hydroxyurea, this was a post hoc analysis. Despite screening for recent transfusions, the NTN group also had 5 patients that had not fully cleared Hb A from their system. Use of echocardiography for the assessment of cardiac index and the use of TR jet velocity as a surrogate for pulmonary vascular disease are not gold standards. However, when used in a strict research protocol with preserved and strict methodology, these measures are accurate and reproducible with acceptable variance.44 Furthermore, by assessing the transfused group pre- and posttransfusion, we may study the physiologic effects of replacing HbS with HbA, controlling for important intersubject bias and variability.
Conclusions
In both transfused and nontransfused patients with SCD, endothelial dysfunction is associated with higher TR jet velocity. Plasma free Hb persists as an independent predictor of both endothelial dysfunction and pulmonary hypertension, linking systemic and pulmonary vascular disease in patients with SCD.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Hui Gao for her echocardiography support and Rosalinda B. Wenby for her rheology laboratory support, Dr Istvan Seri in the Division of Neonatal Medicine for technical support of our research, and the Division of Hematology at Children’s Hospital Los Angeles for their support, particularly Vasili Berdoukas and Thomas Hofstra.
This work was supported by grants from the National Institutes of Health National Heart, Lung, and Blood Institute (grants 1 U54 HL090511-01 and 1 U54 HL117718) (TC); Sickle Cell Scholar Award (grant 5 RC1 HL099412-01), K12 scholar award (grants 2 K12 HD 52954-6 A1 and 1 K23 HL 119627-01A1) (J.A.D.); and by the Children’s Hospital Los Angeles General Clinical Research Center (National Institutes of Health #RR00043-43). The authors acknowledge support from the Elias, Genevieve, Georgianna Atol Charitable Trust.
Authorship
Contributor: J.A.D. designed/performed the experiments, collected and analyzed data, performed statistical analysis, and wrote the paper; R.M.K. performed experiments and collected data; M.R. performed experiments and collected data; H.J.M. designed/performed experiments, collected and analyzed data, and wrote the paper; T.D.C. designed the experiments, analyzed data, and wrote the paper; J.CW. designed the experiments, analyzed data, performed statistical analysis, and wrote the paper; and all authors reviewed and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jon A. Detterich, Division of Cardiology, Children’s Hospital Los Angeles, 4650 Sunset Blvd Mailstop 34, Los Angeles, CA 90027; e-mail: jdetterich@chla.usc.edu.