Key Points
Fn14 activation is involved in intestinal apoptosis after allo-HCT and contributes to gastrointestinal GVHD.
Fn14 blockade with an ADCC-defective human immunoglobulin G1 antibody reduces GVHD severity without modulating GVL responses.
Abstract
Inhibition of the tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK)/fibroblast growth factor-inducible 14 (Fn14) system reduces intestinal cell death and disease development in several models of colitis. In view of the crucial role of TNF and intestinal cell death in graft-versus-host disease (GVHD) and the ability of TWEAK to enhance TNF-induced cell death, we tested here the therapeutic potential of Fn14 blockade on allogeneic hematopoietic cell transplantation (allo-HCT)–induced intestinal GVHD. An Fn14-specific blocking human immunoglobulin G1 antibody variant with compromised antibody-dependent cellular cytotoxicity (ADCC) activity strongly inhibited the severity of murine allo-HCT–induced GVHD. Treatment of the allo-HCT recipients with this monoclonal antibody reduced cell death of gastrointestinal cells but neither affected organ infiltration by donor T cells nor cytokine production. Fn14 blockade also inhibited intestinal cell death in mice challenged with TNF. This suggests that the protective effect of Fn14 blockade in allo-HCT is based on the protection of intestinal cells from TNF-induced apoptosis and not due to immune suppression. Importantly, Fn14 blockade showed no negative effect on graft-versus-leukemia/lymphoma (GVL) activity. Thus, ADCC-defective Fn14-blocking antibodies are not only possible novel GVL effect-sparing therapeutics for the treatment of GVHD but might also be useful for the treatment of other inflammatory bowel diseases where TNF-induced cell death is of relevance.
Introduction
Tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK) (TNFSF12) is a typical member of the TNF ligand family and its receptor fibroblast growth factor-inducible 14 (Fn14) (TNFRSF12a) belongs to the TNF receptor associated factor-interacting subgroup of the TNF receptor family.1,2 Like most other ligands of the TNF family, TWEAK is a single-spanning transmembrane protein with an extracellular carboxyl-terminal TNF homology domain followed by a stalk region connecting the TNF homology domain with the transmembrane domain and the cytoplasmic amino-terminal part of the molecule. Not unusual for a TNF ligand, the stalk region of TWEAK is subject to proteolytic processing and thus allows the generation of a soluble form of TWEAK. At the messenger RNA level, TWEAK expression has been documented for a variety of cell lines and in many tissues. Cell-surface exposed membrane bound TWEAK, however, has so far only been reported for monocytes, macrophages, dendritic cells, natural killer cells, and a few cancer cell lines. Fn14 is strongly expressed in all tissues during development but shows a differentiated expression pattern in the adult organism, reaching from high expression in heart and ovary over weak expression in brain and skeletal muscle to lack of detectable expression in the spleen.3 Particularly, in accordance with its identification as a fibroblast growth factor-inducible protein, Fn14 was found to be strongly induced by various growth factors and cytokines,4-8 as seen in situations of tissue damage.9,10 The TWEAK/Fn14 system triggers a diverse range of cellular effects including the stimulation of angiogenesis, proliferation, cell differentiation, and cell migration, as well as the activation of proinflammatory gene transcription programs and in rare cases, apoptosis. The range of activities of the TWEAK/Fn14 system and the tissue damage/injury-associated expression pattern of Fn14 argue for a role of TWEAK and Fn14 in wound healing, tissue repair, regeneration, and maintenance of tissue homeostasis.11 In line with this, it has been found that TWEAK and Fn14 are required for the regenerative responses occurring after muscle injury, partial hepatectomy, and partial pancreatectomy.12-14 In the case of exaggerated or chronic activation, however, the TWEAK/Fn14 system may also contribute to tissue injury.15,16 Indeed, in most disease models investigated so far, genetic or pharmacologic inactivation of the TWEAK/Fn14 system showed a beneficial effect.
Allogeneic hematopoietic cell transplantation (allo-HCT) is often the only curative treatment option for a number of malignant and nonmalignant diseases of the hematopoietic system.17,18 With respect to the treatment of leukemia by allo-HCT, a crucial issue is the so called graft-versus-leukemia/lymphoma (GVL) effect, a donor T cell and natural killer cell-mediated immune response against residual malignant cells in the recipient who has survived previous treatments with chemotherapy and/or radiotherapy. However, the GVL activity is closely linked to immune reactions of donor cells against normal nontransformed host cells leading to graft-versus-host disease (GVHD), one of the main reasons of mortality after allo-HCT. Acute GVHD mainly affects the gastrointestinal (GI) tract, liver, and skin.
Inhibition of TWEAK/Fn14 signaling showed a protective effect in 2,4,6-trinitrobenzene sulfonic acid-induced, interleukin (IL)-10 deficiency-induced, and γ-irradiation–induced colitis,19-21 Thus, we evaluated whether blockade of Fn14 would interfere with intestinal GVHD following allo-HCT. We found that a recombinant Fn14-specific blocking human immunoglobulin (Ig) G1 antibody strongly reduced the severity of allo-HCT–induced GVHD in mice without interfering with GVL activity. Whereas an antibody variant with compromised Fcγ-receptor (FcγR) binding was effective, an antibody variant with enhanced antibody-dependent cellular cytotoxicity (ADCC) activity failed to show any protective effect. This suggests that the therapeutic effect is indeed due to inhibition of Fn14 signaling and not related to ADCC-mediated depletion of Fn14-expressing cells or activation of Fn14 by FcγR-bound antibody.
Methods
Antibodies and animals
The anti-Fn14 hIgG1 variants 18D1-dead and 18D1-enhanced have been described in detail elsewhere.22 Rituximab, a therapeutic human IgG1 antibody recognizing human but not murine CD20, was purchased from Hoffmann La-Roche (Basel, Switzerland). Balb/c and C57Bl/6 (B6) mice were purchased from Charles River (Sulzfeld, Germany). Firefly luciferase-transgenic B6.L2G85.CD90.1 mice were described in detail elsewhere.23,24 For all experiments, female mice between 8 and 12 weeks of age were used. Mice were bred within the specified pathogen-free animal facility of the Center for Experimental Molecular Medicine of the University Hospital Würzburg, and received rodent chow and autoclaved drinking water ad libitum. All animal experiments were approved by local authorities (Regierung von Unterfranken, reference number 55.2-2531.01-103/11) and complied with German animal protection law.
Cells and cell culture
Bone marrow (BM) cells were isolated from B6 mice by flushing femur and tibia bones with phosphate buffered saline (PBS) and filtration of the obtained cell suspension through a 70-μm cell strainer (BD, Heidelberg, Germany). For preparation of T cells, spleens were directly filtered through a 70-μm cell strainer into erythrocyte lysis buffer (168 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) and incubated for 2 minutes. Two volumes of PBS were added to the single cell suspension and cells were spun down. Cells were resuspended in PBS, filtered through a new 70-μm cell strainer and spun down again. Cells were resuspended in PBS and T cells were enriched using the Dynal Mouse T-cell Negative Isolation Kit (Invitrogen, Darmstadt, Germany) according to the manufacturer’s instructions. IM380 plasmablastic lymphoma (H-2d) cells stably expressing firefly luciferase and eGFP (IM380 egfp/luc),25 and A20 yfp/luc mouse B-cell lymphoma (H-2d) cells stably expressing firefly luciferase and YFP26 were maintained in RPMI medium supplemented with 10% fetal calf serum (FCS) and 1% antibiotics (penicillin/streptomycin) and passaged twice weekly. Both cell lines are syngeneic to Balb/c mice.
allo-HCT and GVHD/GVL model
Female Balb/c mice (H-2d), 8 to 12 weeks of age, were lethally irradiated with a dose of 8 Gy using a Faxitron CP-160 X-ray Irradiation System (Faxitron X-ray; Lincolnshire, IL). To induce GVHD, conditioned mice were then injected with 5 × 106 allogeneic BM cells isolated from B6 donor mice (H-2b) and 1 × 106 enriched T cells from B6 or firefly luciferase-transgenic B6.L2G85.CD90.1 mice into the retro-orbital plexus. For GVL experiments with A20 yfp/luc cells, 1 × 105 cells in 100 μL PBS were injected into the lateral tail vein of host mice following irradiation, and transfer of BM cells and T cells. For GVL experiments with IM380 egfp/luc cells, 1 × 105 cells were injected into the lateral tail vein of host mice 6 days prior to allo-HCT. Drinking water of mice receiving allo-HCT was supplemented with the antibiotic Baytril (Bayer, Leverkusen, Germany) for 1 week to prevent infections following myeloablative irradiation. To target Fn14, mice were treated daily for a week with 100 μg of 18D1-enhanced or 18D1-dead (100 μL PBS IP) starting 1 day after allo-HCT. Rituximab (anti-human CD20 IgG1) served as a negative control. Mice were assessed daily for weight loss and clinical scoring of GVHD symptoms26 for the first 10 days after allo-HCT and then every other day until the end of the experiment. To avoid overestimation of late effects (ie, when only a few mice are still alive and have comparably low scores), the clinical scoring data were arranged in a way that the mice that died of GVHD or that were euthanized due to ethical reasons (score >8, weight loss >20%) were included in the statistical evaluation with their last score until the last mouse of the group had died. Thus, the fact that a graph ends earlier than the whole experiment indicates that all mice were severely affected, therefore they had to be euthanized.
Bioluminescence imaging
For in vivo bioluminescence imaging, mice were IP injected with 80 mg/kg esketamine hydrochloride (Pfizer, Berlin, Germany) and 16 mg/kg xylazine (CP-Pharma, Burgdorf, Germany) for anesthetization, together with 300 mg/kg of the luciferase substrate d-luciferin (Biosynth, Staad, Switzerland). Bioluminescence signals were recorded 10 minutes later from ventral and lateral views with a maximum exposure time of 5 minutes per picture using an IVIS Spectrum Imaging System (Caliper Life Sciences, Mainz, Germany). Pictures were evaluated using Living Image 4.0 software (Caliper Life Sciences). For ex vivo imaging, mice were injected with 300 mg/kg d-luciferin and euthanized 10 minutes later to prepare internal organs, which were immediately subjected to ex vivo bioluminescence imaging.
Immunohistochemistry and analysis of cytokine expression
Tissue samples were embedded in Tissue-Tek OCT (Sakura Finetek, Staufen, Germany) or stored in 4% paraformaldehyde for standard histopathologic analysis. Tissues were scored (0-4 depending on severity) for the following end points: small bowel (apoptosis of crypt cells and inflammation); large bowel (apoptosis of crypt cells and inflammation); and liver (bile duct injury, vascular injury, hepatocellular damage, and portal inflammation).27 For the evaluation of cytokine expression, serum samples were analyzed with the help of the BD Cytometric Bead Array Kit (BD Biosciences Pharmingen, Heidelberg, Germany) according to the manufacturer’s protocol. Data were analyzed with the FCAP Array version 2.0 software.
TNF-induced in vivo apoptosis of intestinal cells
Twenty-week-old male C57Bl/6 (n = 24) or Balb/c″ (n = 18) mice were randomized into 4 groups and injected IP with 18D1-dead (200 µg in PBS), recombinant murine TNF (10 µg in PBS), a mixture of both, or with saline. After 6 hours, mice were euthanized and samples of the small intestinal tissue were formalin-fixed and paraffin-embedded at the time of collection. After deparaffinization, rehydration, antigen-retrieval, and peroxidase blocking using standard protocols, apoptotic cell death in tissue sections was detected using an anti-cleaved caspase-3 antibody (Asp175; Cell Signaling Technology, Danvers, MA) and an anti-cleaved lamin A antibody (small subunit; Cell Signaling Technology) as described elsewhere.28 Briefly, after incubation with the primary antibodies, sections were washed with TBS and then incubated with Biotin-SP–conjugated AffiniPure, goat anti-rabbit IgG (1:200; Jackson ImmunoResearch, Suffolk, United Kingdom) for 45 minutes. Unbound antibodies were removed by two washes with TBS, and bound immunocomplexes were visualized using the Vectastain ABC Kit (Vector Laboratories, Peterborough, United Kingdom) and the diaminobenzidine-based ImmPACT DAB SK-4105 Staining Kit (Vector Laboratories). Sections were counterstained with hematoxylin, and evaluated and photographed with the PALM MicroBeam microscope (Carl Zeiss, Göttingen, Germany). The number of apoptotic cells per 100 crypts was determined based on at least 600 crypts.
RNA isolation and quantitative polymerase chain reaction (qPCR) analysis
Both small and large bowels were isolated from euthanized mice and snap frozen in liquid nitrogen for isolation of total RNA with the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the protocol of the supplier. Total RNA (1 µg) was subjected to reverse transcription using the QuantiTect Reverse Transcription Kit (Qiagen) to synthesize complementary DNA (cDNA). Two percent of the reverse transcription reactions were subsequently used as a template for quantitative real-time PCR with the Bio-Rad C1000 Thermal Cycler equipped with the CFX96 Real-Time System (Bio-Rad, Munich, Germany) and the QuantiTect SYBR Green PCR Kit (Qiagen). Reactions were run using the following program: (1) 95°C, hot start; and (2) 40 cycles of 15 seconds at 95°C, followed by 30 seconds at 52°C and 30 seconds at 72°C. In all experiments, a reaction without cDNA was included as a negative control. For amplification of murine TNF and β-actin cDNA, primer pairs from Qiagen (TNF: QT00104006; ActB: QT00095242) were used. The relative expression of the TNF messenger RNA was calculated using the threshold cycle (CT)-values of the TNF (CT[TNF]) and β-actin (CT[βact]) PCR reactions, and the following formula: relative expression = 2(CT[TNF]–CT[βact]).
Cleaved poly ADP-ribose polymerase 1 (PARP1) immunofluorescence microscopy
Sections of 3 µm thickness were cut from cryo-embedded tissues on a Leica CM1900 Cryostat (Leica Microsystems, Wetzlar, Germany). Slides were air-dried, fixed with acetone (room temperature, 7 minutes), and washed and blocked with 2% FCS in PBS for 15 minutes. Slides were incubated with the rabbit monoclonal anti-cleaved PARP antibody E51 (ab32064; Abcam, Cambridge, United Kingdom) for 1 hour at room temperature and unbound antibodies were removed by 3 washes with PBS. Bound antibodies were then detected with Alexa Fluor 647 goat anti-rabbit IgG (Invitrogen). After an additional 3 washes with PBS, the slides were counterstained with 4,6 diamidino-2-phenylindole and mounted with mounting medium (Vector Laboratories). Images were obtained with a Zeiss Imager.Z1m fluorescence microscope and evaluated using the Zeiss AxioVision software (Carl Zeiss).
Immunohistochemistry
Five formalin-fixed and paraffin-embedded human colonic biopsies showing moderate to strong alterations associated with GVHD were selected from the files of the Institute of Pathology, University of Würzburg. Five normal colonic biopsies served as controls. Ethics approval was obtained from the Ethics Committee of the Medical Faculty of the University of Würzburg. After antigen retrieval with target retrieval solution, pH6.1 (Dako, Hamburg, Germany), the slides were stained using an anti-Fn14 antibody (sc-56250; Santa Cruz, Heidelberg, Germany) at a dilution of 1:100. Representative images were taken using a Nikon Eclipse E600 microscope equipped with a Nikon DS-Fi1 camera.
Statistics
All data are shown as mean ± standard error of mean (SEM) and represent combined data from at least 2 independent experiments unless noted otherwise. Figures were prepared using GraphPad Prism 5 software (La Jolla, CA) and Adobe Photoshop 7 (San Jose, CA), or CorelDRAW X4 (Corel Corporation, Menlo Park, CA). Data were tested for normality using the Kolmogorov–Smirnov test or the Shapiro–Wilk test, where appropriate. Normally distributed groups (Figures 1C, 2, 3, and 4B) were compared by two-tailed Student t test and not normally distributed groups (Figures 1D and 4A) were compared by Mann–Whitney test. Survival data were analyzed with the log-rank (Mantel–Cox) test. Data reaching statistical significance are indicated as: *P ≤ .05; **P ≤ .01.
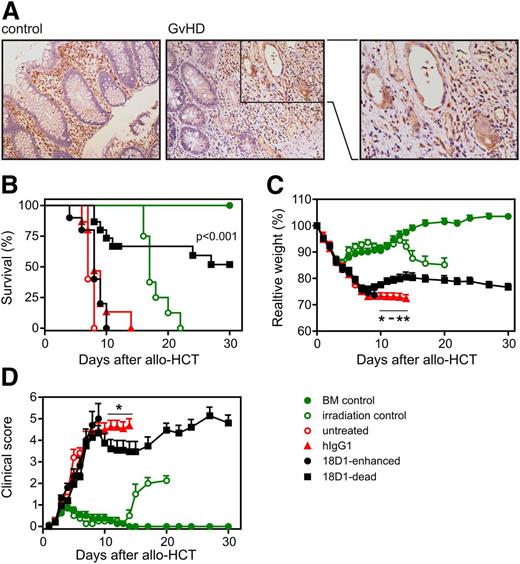
An ADCC-defective variant of the anti-Fn14 monoclonal antibody (mAb) 18D reduces acute GVHD and prolongs survival after allo-HCT. (A) Increased expression of Fn14 in intestinal epithelial cells of GVHD patients. Sections of normal mucosa (left) and mucosa with GVHD associated changes (middle and right) were stained with an anti-Fn14 antibody (ITEM-4) (shown in brown). Representative results are shown. Original magnification ×200. Fn14-positive immune cells are present in the lamina propria of both sample types, whereas Fn14-positive epithelial cells are only observed in GVHD samples. (B-D) Balb/c (H-2d) mice were myeloablatively irradiated and transplanted with 5 × 106 B6 (H-2b) BM cells and 1 × 106 enriched B6.L2G85.CD90.1 (H-2b) T cells. Starting on day 1 posttransplantation, mice were treated daily with 100 µg of 18D1-dead, 18D1-enhanced, or an irrelevant hIgG1 control antibody. Shown are combined data from 3 independent experiments (18D1-dead: n = 20; 18D1-enhanced: n = 10; and hIgG1: n = 20). To control the efficacy of the myeloablative conditioning (irradiation only) and BM engraftment (BM control), mice were irradiated without transplantation or were irradiated and only transplanted with B6 (H-2b) BM cells. (B) Survival of allo-HCT recipients. (C) Mice were weighed at the indicated times after allo-HCT. Weight measurements are shown in percent of initial weight. (D) Mice were assessed for clinical signs of GVHD at the indicated time points. Mean ± SEM. *P ≤ .05; **P ≤ .01 (hIgG1 vs 18D1-dead).
An ADCC-defective variant of the anti-Fn14 monoclonal antibody (mAb) 18D reduces acute GVHD and prolongs survival after allo-HCT. (A) Increased expression of Fn14 in intestinal epithelial cells of GVHD patients. Sections of normal mucosa (left) and mucosa with GVHD associated changes (middle and right) were stained with an anti-Fn14 antibody (ITEM-4) (shown in brown). Representative results are shown. Original magnification ×200. Fn14-positive immune cells are present in the lamina propria of both sample types, whereas Fn14-positive epithelial cells are only observed in GVHD samples. (B-D) Balb/c (H-2d) mice were myeloablatively irradiated and transplanted with 5 × 106 B6 (H-2b) BM cells and 1 × 106 enriched B6.L2G85.CD90.1 (H-2b) T cells. Starting on day 1 posttransplantation, mice were treated daily with 100 µg of 18D1-dead, 18D1-enhanced, or an irrelevant hIgG1 control antibody. Shown are combined data from 3 independent experiments (18D1-dead: n = 20; 18D1-enhanced: n = 10; and hIgG1: n = 20). To control the efficacy of the myeloablative conditioning (irradiation only) and BM engraftment (BM control), mice were irradiated without transplantation or were irradiated and only transplanted with B6 (H-2b) BM cells. (B) Survival of allo-HCT recipients. (C) Mice were weighed at the indicated times after allo-HCT. Weight measurements are shown in percent of initial weight. (D) Mice were assessed for clinical signs of GVHD at the indicated time points. Mean ± SEM. *P ≤ .05; **P ≤ .01 (hIgG1 vs 18D1-dead).
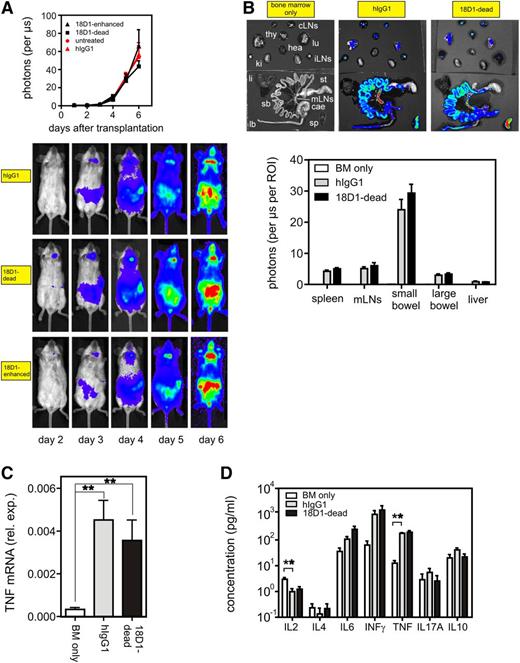
Treatment of allo-HCT recipients with the ADCC-defective anti-Fn14 mAb variant 18D1-dead does not affect donor T-cell target organ infiltration and cytokine production. Lethally irradiated Balb/c (H-2d) mice were transplanted with 5 × 106 B6 (H-2b) BM cells alone or together with 1 × 106 enriched B6.L2G85.CD90.1 (H-2b) T cells. Mice of the latter group were treated daily with 100 µg of 18D1-dead or an irrelevant hIgG1 control antibody for 6 days. All mice (n = 5 per group) were then euthanized for ex vivo assessment of T-cell expansion, cytokine production, and cell death induction in the GI tract. (A) In vivo bioluminescence imaging of B6.L2G85.CD90.1 (H-2b) cells in transplanted antibody-treated mice. Bioluminescence was imaged at indicated time points and light emission of donor T cells quantified. The upper panel shows the average light emission from ventral view and the lower panels show images from 1 representative mouse of each group. (B) Internal organs were analyzed ex vivo for donor T-cell–derived bioluminescence activity. Bioluminescence images of the organs of 1 representative mouse of each group are shown in the upper panel. The organ-derived averaged emissions are shown in the lower panel. (C) Large bowel biopsies of 18D1-dead and control hIgG1-treated GVHD mice and of untreated control mice were analyzed by qPCR for TNF expression. (D) Concentrations of various cytokines in serum were determined with the help of a cytometric bead array. Mean ± SEM. **P ≤ .01. cae, cecum; cLN, cervical lymph nodes; hea, heart; iLN, inguinal lymph nodes; ki, kidney; lb, large bowel; li, liver; lu, lung; mLN, mesenteric lymph nodes; sb, small bowel; sp, spleen; st, stomach; thy, thymus.
Treatment of allo-HCT recipients with the ADCC-defective anti-Fn14 mAb variant 18D1-dead does not affect donor T-cell target organ infiltration and cytokine production. Lethally irradiated Balb/c (H-2d) mice were transplanted with 5 × 106 B6 (H-2b) BM cells alone or together with 1 × 106 enriched B6.L2G85.CD90.1 (H-2b) T cells. Mice of the latter group were treated daily with 100 µg of 18D1-dead or an irrelevant hIgG1 control antibody for 6 days. All mice (n = 5 per group) were then euthanized for ex vivo assessment of T-cell expansion, cytokine production, and cell death induction in the GI tract. (A) In vivo bioluminescence imaging of B6.L2G85.CD90.1 (H-2b) cells in transplanted antibody-treated mice. Bioluminescence was imaged at indicated time points and light emission of donor T cells quantified. The upper panel shows the average light emission from ventral view and the lower panels show images from 1 representative mouse of each group. (B) Internal organs were analyzed ex vivo for donor T-cell–derived bioluminescence activity. Bioluminescence images of the organs of 1 representative mouse of each group are shown in the upper panel. The organ-derived averaged emissions are shown in the lower panel. (C) Large bowel biopsies of 18D1-dead and control hIgG1-treated GVHD mice and of untreated control mice were analyzed by qPCR for TNF expression. (D) Concentrations of various cytokines in serum were determined with the help of a cytometric bead array. Mean ± SEM. **P ≤ .01. cae, cecum; cLN, cervical lymph nodes; hea, heart; iLN, inguinal lymph nodes; ki, kidney; lb, large bowel; li, liver; lu, lung; mLN, mesenteric lymph nodes; sb, small bowel; sp, spleen; st, stomach; thy, thymus.
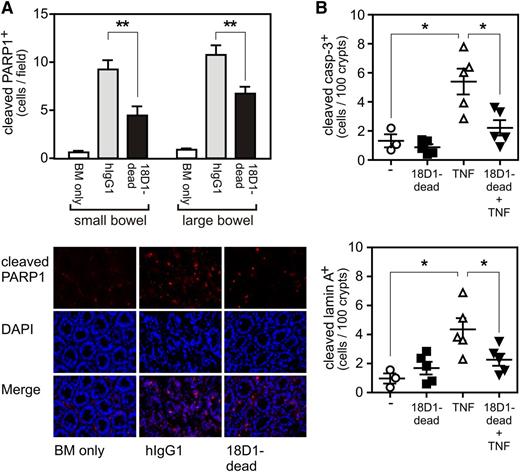
The ADCC-defective anti-Fn14 mAb variant 18D1-dead reduces allo-HCT–triggered and TNF-induced cell death of GI cells. (A) 3 µm intestinal tissue sections from mice shown in Figure 2 were analyzed by immunohistochemistry with a cleaved PARP1-specific antibody. Upper panel: graphic evaluation of apoptotic cells per field. Lower panel: representative photomicrographs. (B) Balb/c (H-2d) were injected with 18D1-dead (200 µg in PBS), murine TNF (10 µg in PBS), a mixture of both, or with saline. After 6 hours, the mice were euthanized and small intestinal tissues were analyzed by immunohistochemistry for the presence of apoptotic cells with anticleaved caspase-3 (upper panel) and anticleaved lamin A-specific antibodies (lower panel). Mean ± SEM. *P ≤ .05; **P ≤ .01.
The ADCC-defective anti-Fn14 mAb variant 18D1-dead reduces allo-HCT–triggered and TNF-induced cell death of GI cells. (A) 3 µm intestinal tissue sections from mice shown in Figure 2 were analyzed by immunohistochemistry with a cleaved PARP1-specific antibody. Upper panel: graphic evaluation of apoptotic cells per field. Lower panel: representative photomicrographs. (B) Balb/c (H-2d) were injected with 18D1-dead (200 µg in PBS), murine TNF (10 µg in PBS), a mixture of both, or with saline. After 6 hours, the mice were euthanized and small intestinal tissues were analyzed by immunohistochemistry for the presence of apoptotic cells with anticleaved caspase-3 (upper panel) and anticleaved lamin A-specific antibodies (lower panel). Mean ± SEM. *P ≤ .05; **P ≤ .01.
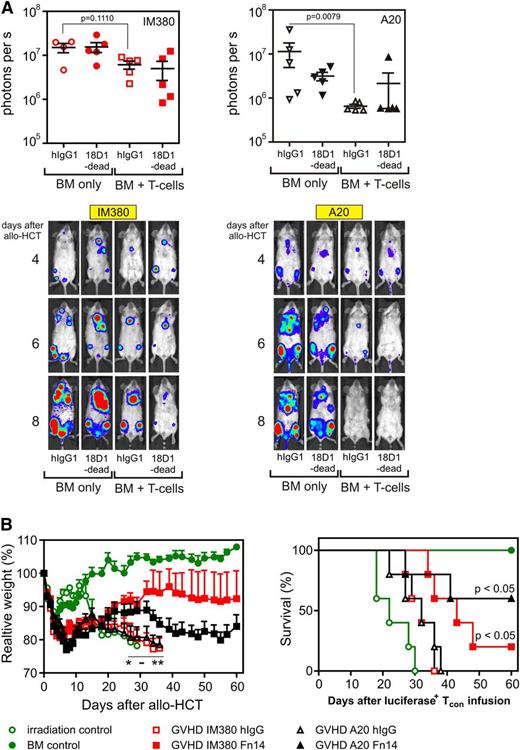
Treatment of allo-HCT recipients with the ADCC-defective anti-Fn14 mAb variant 18D1-dead delays the onset of acute GVHD while maintaining GVL effects. The effect of 18D1-dead on GVL activity was investigated in IM380 and A20 B-cell lymphoma models. Balb/c (H-2d) mice were either injected with 105 syngenic, luciferase-transgenic IM380 B-cell lymphoma cells iv 6 days before allo-HCT or with 105 syngenic, luciferase-transgenic A20 cells along with allogeneic 5 × 106 B6 (H-2b) BM cells alone or together with 1 × 106 enriched B6 (H-2b) T cells. Starting on day 1 post–allo-HCT, mice were treated daily for a week with 100 µg of 18D1-dead or with 100 µg of an irrelevant hIgG1 mAb (n = 5 for each group). (A) In vivo bioluminescence imaging of luciferase+ lymphoma cells of a representative mouse of the indicated groups. Upper panels represent data at 8 days after transplantation; lower panels show images of a representative mouse of each group throughout the first 8 days of the experiment. (B) Survival analysis of allo-HCT recipients (right panel). Data from both GVL models are displayed, along with data from mice of the myeloablative conditioning control (irradiation only) and the BM engraftment control (BM control). Weight change after allo-HCT in percent of the initial weight (left panel). Statistics of hIgG1 vs 18D1-dead are indicated: mean ± SEM. *P ≤ .05; **P ≤ .01.
Treatment of allo-HCT recipients with the ADCC-defective anti-Fn14 mAb variant 18D1-dead delays the onset of acute GVHD while maintaining GVL effects. The effect of 18D1-dead on GVL activity was investigated in IM380 and A20 B-cell lymphoma models. Balb/c (H-2d) mice were either injected with 105 syngenic, luciferase-transgenic IM380 B-cell lymphoma cells iv 6 days before allo-HCT or with 105 syngenic, luciferase-transgenic A20 cells along with allogeneic 5 × 106 B6 (H-2b) BM cells alone or together with 1 × 106 enriched B6 (H-2b) T cells. Starting on day 1 post–allo-HCT, mice were treated daily for a week with 100 µg of 18D1-dead or with 100 µg of an irrelevant hIgG1 mAb (n = 5 for each group). (A) In vivo bioluminescence imaging of luciferase+ lymphoma cells of a representative mouse of the indicated groups. Upper panels represent data at 8 days after transplantation; lower panels show images of a representative mouse of each group throughout the first 8 days of the experiment. (B) Survival analysis of allo-HCT recipients (right panel). Data from both GVL models are displayed, along with data from mice of the myeloablative conditioning control (irradiation only) and the BM engraftment control (BM control). Weight change after allo-HCT in percent of the initial weight (left panel). Statistics of hIgG1 vs 18D1-dead are indicated: mean ± SEM. *P ≤ .05; **P ≤ .01.
Results
Fn14 blockade reduces GVHD severity and prolongs survival after allo-HCT
The histopathology of spontaneous colitis of IL-10 knockout mice and acute 2,4,6-trinitrobenzene sulfonic acid-induced colitis, two disease models where the inhibition of the TWEAK/Fn14-system has a protective effect,19,21 resembles the clinical manifestations and histologic changes observed in intestinal GVHD following allo-HCT. Therefore, we evaluated normal colonic mucosa biopsies and mucosa biopsies derived of patients showing moderate-to-severe GVHD-associated changes for Fn14 expression. In the lamina propria of all biopsies, we observed numerous Fn14-positive immune cells (Figure 1A). However, the situation was different in intestinal epithelial cells. Whereas epithelial cells remained Fn14-negative in the normal mucosa, pre-apoptotic and apoptotic cryptic glands in the GVHD biopsies stained distinctly positive for Fn14 (Figure 1A and see supplemental Figure 1 on the Blood Web site). Together, this opened the possibility that Fn14 blockade could be protective in inflammatory scenarios with cell death of intestinal epithelial cells. Therefore, we evaluated the effect of the blocking human/murine Fn14-specific antibody 18D1 on GVHD in a major histocompatibility complex mismatch model in which lethally irradiated Balb/c recipient mice were reconstituted with B6 BM cells and firefly luciferase-transgenic B6 T cells. Starting on day 1 posttransplantation, mice were treated with an ADCC-defective (18D1-dead) and an ADCC-enhanced (18D1-enhanced) variant of 18D1, as well as with an irrelevant hIgG1 control antibody.22 Treatment of mice with the 18D1-dead antibody resulted in significantly prolonged survival compared with transplanted mice treated with the control hIgG1, whereas 18D1-enhanced had no effect (median survival: hIgG, 8 days; 18D1-enhanced, 8 days; 18D1-dead, >30 days) (Figure 1B). Mice receiving 18D1-dead after allo-HCT initially lost as much weight as did control hIgG1-treated mice, but recovered from allo-HCT by day 9 after transplantation (Figure 1C). Prolonged survival of mice treated with 18D-dead came along with a stabilization of the clinical score (Figure 1D).
Fn14 blockade reduces GI organ damage in GVHD mice
In vivo bioluminescence imaging showed no difference in global donor T-cell expansion after allo-HCT between mice treated with 18D1-dead and hIgG1 (Figure 2A). To assess donor T-cell proliferation and expansion patterns in more detail, we performed ex vivo imaging on day 6 after allo-HCT. Again, there was no evidence that 18D1-dead modified organ infiltration by alloreactive luciferase-transgenic T cells (Figure 2B). qPCR analysis revealed a strong increase in the expression of TNF in bowel biopsies of mice with GVHD compared with healthy mice, and this increase in TNF expression was not affected by treatment with 18D1-dead (Figure 2C). To evaluate further whether treatment with 18D1-dead affects systemic cytokine levels, we performed cytometric bead array analysis of serum samples. Serum levels of IL-6, IFN-γ, and TNF were markedly increased after allo-HCT, whereas the level of IL-2 was reduced; but there were no significant differences between mice treated with 18D1-dead and hIgG1 (Figure 2D). Collectively, these data suggest that the protective effect of 18D1-dead in GVHD is not related to the inhibition of potential proinflammatory Fn14 activities.
Fn14 blockade inhibits intestinal apoptosis after allo-HCT
Apoptosis induction in intestinal epithelial cells by γ-irradiation is reduced in TWEAK- and Fn14-knockout mice.19 It has been furthermore observed in an intestinal explant system that apoptosis induction by exogenous TNF is diminished in primary cultures derived from Fn14-knockout mice.21 Indeed, TNF is considered to be important in GVHD not only due to its stimulatory effects on T cells but also as an effector molecule that contributes to apoptosis induction in GVHD target tissues.29,30 Moreover, TNF is generally a prominent inducer of cell death in the intestinal epithelium.31-35 Against the background of the increased expression of TNF in the gut after allo-HCT (Figure 2C) and the established strong ability of the TWEAK/Fn14 system to enhance TNFR1-induced cell death,36-38 we addressed the possibility that 18D1-dead affects intestinal apoptosis after allo-HCT. Immunohistochemical evaluation of the GI tract of control hIgG1 and 18D1-dead treated mice revealed a significant reduction in the number of cleaved PARP1-positive cells within the small bowel (Figure 3A). This suggests that the protective effect of 18D1-dead on GVHD after allo-HCT is based on the inhibition of the apoptotic crosstalk of TWEAK and TNF in intestinal epithelial cells. In further accordance with this idea, 18D1-dead also showed a protective effect on intestinal cell apoptosis after exogenous administration of TNF in Balb/c (Figure 3B) as well as in B6 mice (supplemental Figure 2).
Fn14 blockade inhibits GVHD after allo-HCT while maintaining GVL effects
To assess whether blocking of Fn14 with 18D1-dead affects the capacity of the transplanted allogeneic T cells to control tumor cells, we performed GVL experiments with two different B-cell lymphoma cell lines, A20 and IM380. In the case of the A20 model, tumor cells were co-transplanted together with the BM graft and the allogeneic T cells, whereas in the genetically induced IM380 model, mice with pre-established tumors were subjected to allo-HCT. In both models, the tumors initially (4-5 days) developed similarly after allo-HCT irrespective of whether the mice were transplanted with BM cells alone or with BM cells supplemented with allogeneic T cells (Figure 4A). Mice transplanted with allogeneic T-cell supplemented BM cells subsequently rejected the tumors, whereas the BM cell-transplanted mice did not (Figure 4A). Treatment with 18D1-dead showed no effect on tumor rejection (Figure 4A), indicating that Fn14-blockade does not interfere with GVL activity. Moreover, 18D1-dead treatment again significantly prolonged survival, and prevented deadly weight loss of mice that showed GVL and developed GVHD (Figure 4A-B).
Discussion
By activation of its receptor Fn14, TWEAK stimulates the production of proinflammatory cytokines and chemokines in a variety of cell types.39-42 TWEAK also acts as a sensitizer for TNF-induced cell death.36-38 Noteworthy, in murine models of colitis, TWEAK crucially contributes to disease progression by inducing colon epithelial cells to secrete chemokines causing immune cell infiltration, as well as TNF-dependent cell death of intestinal epithelial cells.19-21 As inflammation and intestinal cell death induction propagate intestinal GVHD pathophysiology, we asked whether the TWEAK/Fn14 system may serve as a useful therapeutic target in this disease. To address this, we treated Balb/c mice that had been transplanted with T cells and BM cells derived from allogeneic B6 mice with the Fn14-specific antibody 18D1. This llama-derived antibody efficiently blocks TWEAK binding to human and murine Fn14.22 When expressed as a human IgG1, 18D1 elicits a strong inhibitory effect on soluble and membrane TWEAK-induced Fn14 signaling. However, like other Fn14-specific hIgG1 antibodies, 18D1 acquires a high agonistic potential upon oligomerization and FcγR binding and has then similar signaling activities as membrane TWEAK.22,43 To distinguish whether a potential effect of 18D1 depends on FcγR binding or not, we evaluated two variants of 18D1 with mutated Fc domains. One conferred an enhanced ability to bind to FcγRs and to trigger ADCC. The other displayed a reduced FcγR binding ability, and thus with impaired ADCC activity.22 The ADCC-enhanced variant of 18D1 showed no protective effect on intestinal GVHD after allo-HCT, whereas its ADCC-defective counterpart 18D1-dead strongly diminished GI GVHD (Figure 1). This observation suggests that antibody immune effector functions of 18D1 may limit protective effects related to Fn14 blockade, and we therefore focused in the following experiments on the evaluation of the 18D1-dead variant. The disease promoting effect of Fn14 in murine colitis models has been attributed to an enhancement of TNF-dependent apoptosis in intestinal epithelial cells and disturbance of the barrier function of the gut epithelium. The observation that the inhibition of intestinal tissue damage by 18D1-dead is accompanied by reduced numbers of apoptotic cells and reduced caspase processing in the intestine suggests that the therapeutic effect of 18D1-dead relies on the inhibition of TNF/TWEAK-induced cell death, too. Indeed, we found no evidence for an immune-suppressive effect of 18D1-dead in intestinal GVHD, indicating that early events after allo-HCT are not influenced by Fn14 blockade (Figure 2). The observation that 18D1-dead showed no major effect on the inflammatory aspects of GVHD may be due to the fact that (1) TWEAK mainly acts on parenchymal cells rather than adaptive immune cells, that do not express Fn14; and that (2) in the acute GVHD model used here, the main cytokine-producing cells are alloreactive T cells.10,44,45 Thus, blocking of Fn14 appears to act later in GVHD pathophysiology by inhibiting apoptotic tissue damage induced by infiltrating donor T cells. This corresponds to the fact that the protective effect of 18D1-dead becomes not apparent in the first week after allo-HCT (Figure 1). The finding that Fn14 blockade does not compromise the activity of donor-derived T cells also explains the unaffected GVL activity observed in 18D1-dead treated mice (Figure 4). This is of particular relevance because immune-suppressive treatment regimes typically not only ameliorate GVHD but also interfere with the therapeutically desired GVL effect. It is worth mentioning that TNF and its receptors are, at least in two different ways, of crucial relevance for donor T-cell activity and thus for GVHD and GVL. First, donor T-cell expressed TNF acts as an effector molecule in GVHD and GVL. Furthermore, both TNFR1 and TNFR2 are required by donor T cells in GVHD models for in vivo generation of a proper allo-specific cytotoxic T-cell response.46-48 Thus, in contrast to TNF blockers, which potentially interfere with GVHD and GVL and are used in clinical practice as an experimental mean to treat GVHD49 , inhibitors of the TWEAK/Fn14 system might elicit a more selective effect on GVHD only.
The fact that the ADCC-enhanced variant of 18D1 lacks therapeutic efficacy in GVHD after allo-HCT suggests that the ability to inhibit stimulation of Fn14 by endogenous TWEAK is indeed the main mode of action of 18D1 in this model. Although treatment with 18D1-enhanced would also prevent Fn14 stimulation by endogenous TWEAK, one has to consider that this protective effect could be counteracted by FcγR binding. This could result in a change from TNF/TWEAK-mediated killing of intestinal epithelial cells to ADCC-mediated cell lysis. In the case of insensitivity of intestinal epithelial cells to ADCC, the FcγR binding of 18D1-enhanced should unleash the latently present agonistic activity of the 18D1 antibody. Thus, the stimulation of Fn14 by endogenous TWEAK would only be substituted by stimulation with FcγR-bound 18D1, with no major effect on the TNF/TWEAK crosstalk. In line with this idea, it has been recently shown that human IgG1 antibodies targeting CD40 or the TRAIL death receptors, elicit strong FcγR-dependent agonistic activity in mice.50-53
Noteworthy, the blockade of Fn14 might not only reduce intestinal manifestations of acute GVHD as shown in this study, but it might also reduce disease severity in other organs. So, it has been reported that Fn14 knockout mice and mice treated with a TWEAK-neutralizing antibody are protected in a model of chronic graft-versus-host induced lupus erythematosus.54 In this model, activated donor CD4 T cells deliver T-cell help to recipient B cells and so trigger the production of autoantibodies and lupus disease symptoms.
TNF-induced cell death of intestinal epithelial cells has been identified as a pivotal step in the pathogenesis of various inflammatory diseases of the bowel. Typically, the TNF-dependent cell death enhancing TWEAK/Fn14 system is highly active in stressed/injured tissue. Therefore, it is tempting to speculate that TWEAK and Fn14 also contribute to other intestinal diseases where TNF-induced cell death is of relevance. Thus, ADCC-defective Fn14-blocking antibodies are not only potential novel GVL effect-sparing therapeutics for the treatment and prevention of GVHD, but might also be useful for the treatment of other inflammatory bowel diseases. Data and materials availability: the 18D1-dead and 18D1-enhanced antibodies must be obtained through a Materials Transfer Agreement.
There is an Inside Blood Commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Karen Silence from Argen-X BVBA for the Fn14-specific antibodies.
This work was supported by grants from the Deutsche Krebshilfe (109922) (H.W.), Deutsche Forschungsgemeinschaft (Wa1025/24-1) (H.W.), and Deutsche José Carreras Leukämie-Stiftung e.V. (R/06/17) (H.W.) and (F11/05) (C.A.B.), as well as by the Interdisziplinäre Zentrum für Klinische Forschung of the University of Würzburg (IZKF B-233) (A. Beilhack).
Authorship
Contribution: H.W., A. Beilhack, M.C., and T.B. designed the experiments; H.W., A. Beilhack, M.C., T.B., H.E., and A.R. wrote and edited the manuscript; M.C., A.Beilhack, A.Brandl, M.B., S.K., C.A.B., M.R., K.M., and S.S. performed and evaluated the GVHD-related animal experiments; T.G. and T.B. conducted the TNF-related animal experiment; V.S. produced and purified the Fn14-specific antibody variants and the recombinant TNF; D.S. and A.S. performed the qPCR analysis; and A.M. and A.R. executed the immunohistochemistry evaluation of the GVHD biopsies.
Conflict-of-interest disclosure: H.W. is a consultant of Argen-X BVBA, the developer of the Fn14-specific antibodies used in this study. The remaining authors declare no competing financial interests.
The current affiliation for M.C. is Department of Molecular Medicine and Pathology, Faculty of Medical and Health Sciences, The University of Auckland, Auckland, New Zealand.
The current affiliation for A.M. is Department of Pathology and Laboratory Medicine, University of British Columbia and Centre for Lymphoid Cancer, BC Cancer Agency, Vancouver, Canada.
Correspondence: Andreas Beilhack, IZKF Research Group for Experimental Stem Cell Transplantation, Department of Internal Medicine II, Center for Experimental Molecular Medicine, University Hospital Würzburg, Zinklesweg 10, D-97078 Würzburg, Germany; e-mail: beilhack_a@ukw.de; and Harald Wajant, Division for Molecular Internal Medicine, Department of Internal Medicine II, University Hospital Würzburg, Röntgenring 11, D-97070 Würzburg, Germany; e-mail: harald.wajant@mail.uni-wuerzburg.de.
References
Author notes
A. Beilhack and H.W. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal