Key Points
The mTOR pathway is constitutively activated in the TCL cells and is responsible for TCL proliferation.
This is first trial to demonstrate that mTORC1 inhibitors (everolimus) have substantial antitumor activity (44% overall response rate) in patients with relapsed TCL.
Abstract
Everolimus is an oral agent that targets the mammalian target of rapamycin (mTOR) pathway. This study investigated mTOR pathway activation in T-cell lymphoma (TCL) cell lines and assessed antitumor activity in patients with relapsed/refractory TCL in a phase 2 trial. The mTOR pathway was activated in all 6 TCL cell lines tested and everolimus strongly inhibited malignant T-cell proliferation with minimal cytotoxic effects. Everolimus completely inhibited phosphorylation of ribosomal S6, a raptor/mTOR complex 1 (mTORC1) target, without a compensatory activation of the rictor/mTORC2 target Akt (S475). In the clinical trial, 16 patients with relapsed TCL were enrolled and received everolimus 10 mg by mouth daily. Seven patients (44%) had cutaneous (all mycosis fungoides); 4 (25%) had peripheral T cell not otherwise specified; 2 (13%) had anaplastic large cell; and 1 each had extranodal natural killer/T cell, angioimmunoblastic, and precursor T-lymphoblastic leukemia/lymphoma types. The overall response rate was 44% (7/16; 95% confidence interval [CI]: 20% to 70%). The median progression-free survival was 4.1 months (95% CI, 1.5-6.5) and the median overall survival was 10.2 months (95% CI, 2.6-44.3). The median duration of response for the 7 responders was 8.5 months (95% CI, 1.0 to not reached). These studies indicate that everolimus has antitumor activity and provide proof-of-concept that targeting the mTORC1 pathway in TCL is clinically relevant. This trial was registered at www.clinicaltrials.gov as #NCT00436618.
Introduction
The lymphomas are the sixth most common neoplasms in the United States, with nearly 80 000 new cases of non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL) each year.1 Substantial progress has been made in the treatment of B-cell NHL and chemoimmunotherapy is now the standard of care. Novel agents that target the B-cell receptor signal pathway and immunomodulatory drugs are now approved for chronic lymphocytic leukemia and mantle cell NHL.2-4 Progress has also been made in T-cell lymphoma (TCL) with the approval of the antifolate pralatrexate,5 and the histone deacetylase inhibitors romidepsin6,7 and belinostat.8 These drugs are now being moved upfront in combination with chemotherapy. Despite these advances, the overall prognosis of TCL remains inferior to B-cell NHL with only 30% of patients cured with frontline therapy.9
The PI3K/Akt/mammalian target of rapamycin (mTOR) pathway has become an important focus for cancer therapeutic interventions with the approval of the PI3Kδ inhibitor idelalisib for chronic lymphocytic leukemia and indolent B-cell NHL,10,11 and the mTORC1 inhibitors temsirolimus and everolimus for solid tumors. We have reported that the PI3K/mTOR pathway is activated in NHL cells12 and that mTORC1 inhibitors have activity in relapsed B-cell NHL.13-21 TCLs account for approximately 10% of all NHL in the United States.22 Just as in B-cell NHL, there are a number of clinicopathological types that vary in presentation and outcome. Cell lines of TCL are only available for the anaplastic large cell lymphoma (ALCL) and cutaneous T-cell lymphoma (CTCL) types and the effect of mTORC1 inhibitors on TCL have not been reported. Patients with TCL often have B symptoms suggesting that cytokines are likely elevated. Many of these cytokines are pro-inflammatory and signal through the PI3K/mTOR pathway. For these reasons, we studied the effects of everolimus on TCL in vitro, and then in vivo, in a pilot phase 2 trial in patients with relapsed TCL.
Material and methods
Reagents
Novartis Pharmaceuticals provided the mTOR inhibitor, everolimus (RAD001) for in vitro use. Phospho-specific antibodies for mTOR, AKT, S6, 4EBP1, and eIF4E were purchased from Cell Signaling Technologies. Antibodies for mTOR, AKT, S6, 4EBP1, cyclin D1, D2, D3, c-Myc P27, and eIF4E were also purchased from Cell Signaling Technologies.
Human TCL cell lines.
Six human peripheral TCL (PTCL) cell lines were used for these studies. The ALCL PTCL cell lines used were SUDHL1 (DSMZ, Germany), SR786 (DSMZ), and Karpas 299 (American Type Culture Collection, Manassas, VA). The CTCL cell lines were SeAx (Sézary Syndrome) and MyLa (mycosis fungoides), and were generous gifts from Dr Robert Gniadecki (University of Copenhagen) and HuT 78 (Sézary Syndrome; American Type Culture Collection). All of these cell lines were grown in RPMI 1640 supplemented with 10% fetal bovine serum. CD3 cells were sorted from peripheral blood from normal controls.12
Thymidine incorporation assay.
Annexin V/propidium iodide flow cytometry assay.
Survival inhibition was assessed by Annexin V/propidium iodide staining by flow cytometry as described before.12
Western blotting.
Western blotting was performed as described before.24
Plasma cytokine analysis in paired patient samples.
Plasma was collected and cryopreserved from patients before starting everolimus treatment and after 2 cycles of therapy, for analysis of changes in plasma cytokine levels. These were analyzed as previously reported.25 We focused on changes in 7 cytokines: epidermal growth factor, interleukin (IL)-6, IL-12, IP-10, soluble IL-2Rα, monokine induced by interferon-γ, and IL-1RΑ. These were selected because elevations of these 7 cytokines have been shown to predict event-free survival in patients with new, untreated TCL (unpublished data).
Patient selection
MC048G was a phase 2 study for patients with relapsed lymphoma. The study allowed the accrual of uncommon lymphomas on an exploratory basis. The results of the trial for other major lymphoma subtypes (diffuse large B-cell lymphoma [DLBCL], mantle cell lymphoma [MCL], and Waldenström macroglobulinemia) have been reported13-17 ; however, the results for the TCL patients have never been reported. This study was conducted through the Mayo Clinic Cancer Center and was approved by the Mayo Clinic Institutional Review Board. Patients were eligible for this trial if they had previously received therapy, and had relapsed or were refractory to their last treatment. There was no limit on the number of prior therapies. Patients were required to be ≥18 years old, have measurable disease by computed tomography or magnetic resonance imaging with at least one lesion with a single diameter of >2 cm or tumor cells in the peripheral blood >5 × 109/L. Skin lesions (plaques, patches, or tumors) could be used if the area was >2 cm in at least one diameter and photographed with a ruler. Patients were to have a life expectancy of >3 months; Eastern Cooperative Oncology Group performance status of 0, 1, or 2; absolute neutrophil count >1000; platelets >75 000; hemoglobin >8 g/dL; serum creatinine <2× the upper limit of normal (UNL); serum bilirubin <2 UNL (if total bilirubin >2 then a direct bilirubin of <1.5 UNL was acceptable); aspartate aminotransferase ≤3 × ULN (≤5 × ULN if liver involvement is present). Patients could not have known HIV infection.
Treatment
Patients were treated with a flat dose of 10 mg of everolimus by mouth in the fasting state. Treatment was daily and 4 weeks was considered 1 cycle. A complete blood count was performed each week during the first cycle and with each subsequent cycle. If the platelet count was >40 000 and the absolute neutrophil count was >1000, and there were no grade 3 or 4 nonhematologic toxicities (National Cancer Institute Common Toxicity Criteria version 3.0), the full dose of everolimus was prescribed for the next cycle. Patients who did not meet the retreatment criteria had the dose held until recovery and followed by a stepwise dose modification to 5 mg daily, 5 mg every other day, and 5 mg every third day. Patients were not to receive prophylactic white blood cell growth factors to maintain dosing but could receive them at the physician’s discretion if neutropenia developed. Erythropoietin treatment of anemia was permitted at the physician’s discretion.
Patients were restaged for tumor response after 2 and 6 cycles and every 3 cycles thereafter. Responses for NHL were categorized using the International Workshop Criteria.26 Patients who progressed at any time went off study. Patients who had a complete remission (CR) at 6 months were to receive 2 cycles past CR, and then could discontinue everolimus and be observed or they could continue on treatment. Those patients with stable disease (SD) after 6 cycles could continue treatment at the physician’s discretion. Patients with SD or partial remission (PR) continued until progression or toxicity.
Translational research
Plasma was collected in consenting patients pretreatment and after 2 cycles of everolimus for cytokine analysis. Plasma cytokine levels in various subtypes of TCL patients and controls were measured using a custom-designed standard 30-plex ELISA kit (Invitrogen, Camarillo, CA) as previously described.25 All samples were analyzed on the same plate.
Statistical design
Due to the expected modest accrual rate for the uncommon lymphoma group in this study, accrual was open to this group while the aggressive and indolent groups were accruing; however, a formal statistical design was not used due to uncertainty in the sample size. The primary end point in this group was overall response rate (ORR), where a 20% ORR would be of interest. The proportion of responses was calculated and the 95% exact binomial confidence interval (CI) for the true ORR was calculated (with all eligible patients accrued), assuming that the number of responses was binomially distributed.
Duration of response (DR) was defined as the time from the date of first documented response to the date of progression. Progression-free survival was defined as the time from registration to progression or death due to any cause. Overall survival was defined as the time from registration to death resulting from any cause. The distributions of these time-to-event end points were each estimated using the Kaplan–Meier method (SAS version 9.3).27 Toxicity was defined as an adverse event felt to be possibly, probably, or definitely related to everolimus.
Results
mTORC1 and mTORC2 pathways are constitutively expressed in TCL
To provide a rationale for studying everolimus in patients with TCL, mTOR activity in six PTCL cell lines was compared with normal T cells (CD3) sorted from the blood of healthy controls. The cell lines used were Karpas 299, SUDHL1, SR786 (all derived from anaplastic large cell NHL), and HuT 78, SeAX, and MyLa (all derived from cutaneous TCL). The phosphorylation profile of mTOR at the serine 2481 autophosphorylation site and the activation of mTORC1 downstream targets 4E-BP1, and mTORC2 targets AKT using specific antibodies. As shown in Figure 1, mTOR at the autophosphorylation site was overexpressed in all six TCL lines compared with CD3 control. The mTORC1 target 4EBP1 and was not phosphorylated in normal CD3 cells but was significantly activated in all TCL lines (Figure 1). The mTORC2 target AKT (serine 475) and was phosphorylated in all cell lines, as well as in the CD3 control (Figure 1).
Activation of mTOR signaling in TCL cell lines (n = 6) and normal T cells (n = 3). Western blotting was performed on 6 TCL cell lines of ALCL (Karpas 299, SUDHL1, and SR786) and CTCL (HuT 78, SeAx, and MyLa) subtypes, along with CD3+ T cells using phosphorylation site-specific antibodies for mTOR, AKT, and 4EBP1.
Activation of mTOR signaling in TCL cell lines (n = 6) and normal T cells (n = 3). Western blotting was performed on 6 TCL cell lines of ALCL (Karpas 299, SUDHL1, and SR786) and CTCL (HuT 78, SeAx, and MyLa) subtypes, along with CD3+ T cells using phosphorylation site-specific antibodies for mTOR, AKT, and 4EBP1.
In vitro effects of everolimus treatment on TCL proliferation and survival
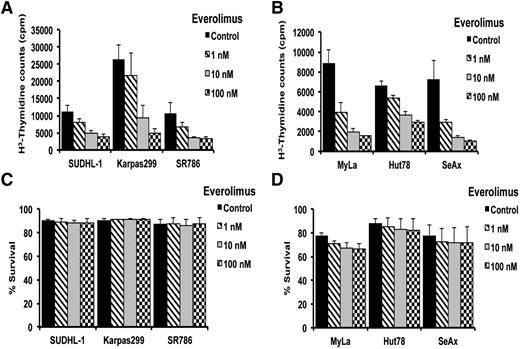
The finding that the mTOR signaling pathway is constitutively activated in human TCL cell lines suggested that mTOR inhibition might inhibit growth of these cells. To assess the potential effects of mTOR inhibition on cell proliferation and survival, malignant TCL lines (n = 6) were treated with 1, 10, and 100 nM of everolimus, and then examined using 3H-thymidine incorporation and flow cytometry, respectively. The thymidine incorporation assay revealed a dose-dependent inhibition of DNA synthesis in both ALCL and CTCL cell lines examined (Figure 2A-B), with 50% inhibition at a concentration of 10 nM and >90% inhibition at 100 nM. Flow cytometry data showed that everolimus had no inhibitory effect on the survival of most of the T-cell lines examined, except MyLa, which showed 10% inhibition at 10 and 100 nM (Figure 2C-D). These data suggest that everolimus is an antiproliferative drug in TCL.
Effect of everolimus on TCL proliferation and survival. (A-D) After ALCL (n = 3) and CTCL (n = 3) cell lines were treated with everolimus as indicated, thymidine incorporation assay (A-B) and flow cytometry (C-D) assay was performed to a assess cell proliferation and survival inhibition.
Effect of everolimus on TCL proliferation and survival. (A-D) After ALCL (n = 3) and CTCL (n = 3) cell lines were treated with everolimus as indicated, thymidine incorporation assay (A-B) and flow cytometry (C-D) assay was performed to a assess cell proliferation and survival inhibition.
Clinical outcome of everolimus-treated TCL patients in a phase 2 trial
Sixteen patients with relapsed TCL were treated between October 2005 and May 2010, and followed through September 2014 (Table 1). The median age was 60 years and the patients had received a median of 3 prior therapies (range, 1 to 11) with 31% having had a stem cell transplant. There were a variety of histologic subtypes with the most common being CTCL and PTCL not otherwise specified (NOS). All 7 CTCL patients were mycosis fungoides (MF)-type with 6 patients being stage IIB and 1 stage III by International Society for Cutaneous Lymphomas/European Organization of Research and Treatment of Cancer criteria.28 Patients received a median of 3.1 months of treatment (range, 0.2 to 47.3 months) or a median of 3.5 cycles (range, 1 to 25). The ORR in all patients was 44% (7/16; 95% CI, 20-70) with 1 patient achieving a CR (PTCL-NOS) and 6 patients a PR (3 MF, 2 PTCL-NOS, and 1 ALCL). The response rates within the different types of TCL were: 43% (3/7) in patients with CTCL; 75% (3/4) in patients with PTCL-NOS; and 20% (1/5) in other patients with TCL. The patient with precursor T-cell lymphoblastic leukemia/lymphoma was a nonresponder.
Patient characteristics of TCL (N = 16)
| Characteristic . | Total (N = 16) Number (%) . |
|---|---|
| Age (median, range) | 60 y (35-81) |
| Gender, male | 13 (81%) |
| Years from diagnosis to registration | |
| Median, range | 3.5 (1.0-20.4) |
| Ann Arbor stage for PTCL patients | |
| 2 | 1 (11%) |
| 4 | 8 (89%) |
| ISCL/EORTC stage for CTCL patients | |
| IIB | 6 (86%) |
| III | 1 (14%) |
| Performance score | |
| 0 | 4 (25%) |
| 1 | 11 (69%) |
| 2 | 1 (6%) |
| No. previous treatments (median, range) | 3 (1-11) |
| Prior stem cell transplant | 5 (31%) |
| Type of TCL | |
| CTCL (mycosis fungoides) | 7 (44%) |
| Peripheral TCL, unspecified | 4 (25%) |
| Anaplastic large cell lymphoma (systemic type) | 2 (13%) |
| Angioimmunoblastic TCL | 1 (6%) |
| Extranodal natural killer/TCL, nasal-type | 1 (6%) |
| Precursor T-lymphoblastic leukemia/lymphoma | 1 (6%) |
| Characteristic . | Total (N = 16) Number (%) . |
|---|---|
| Age (median, range) | 60 y (35-81) |
| Gender, male | 13 (81%) |
| Years from diagnosis to registration | |
| Median, range | 3.5 (1.0-20.4) |
| Ann Arbor stage for PTCL patients | |
| 2 | 1 (11%) |
| 4 | 8 (89%) |
| ISCL/EORTC stage for CTCL patients | |
| IIB | 6 (86%) |
| III | 1 (14%) |
| Performance score | |
| 0 | 4 (25%) |
| 1 | 11 (69%) |
| 2 | 1 (6%) |
| No. previous treatments (median, range) | 3 (1-11) |
| Prior stem cell transplant | 5 (31%) |
| Type of TCL | |
| CTCL (mycosis fungoides) | 7 (44%) |
| Peripheral TCL, unspecified | 4 (25%) |
| Anaplastic large cell lymphoma (systemic type) | 2 (13%) |
| Angioimmunoblastic TCL | 1 (6%) |
| Extranodal natural killer/TCL, nasal-type | 1 (6%) |
| Precursor T-lymphoblastic leukemia/lymphoma | 1 (6%) |
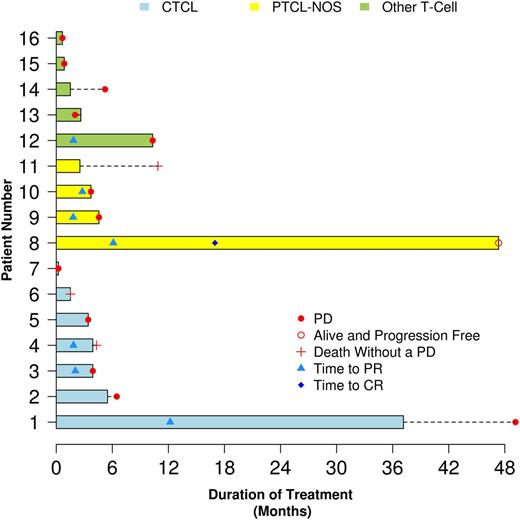
The survival outcomes of each of the 16 patients are contained in Figure 3. The median progression-free survival for all patients was 4.1 months (95% CI, 1.5-6.5) and the median overall survival was 10.2 months (95% CI, 2.3-44.3). The median DR of the 7 responders was 8.5 months (95% CI, 1.0 to not reached). Five of the 7 responding patients eventually progressed (3 have died and 2 remain alive), 1 died without progression of unrelated congestive heart failure, and 1 remains alive without progression (Figure 3). The median follow-up for patients still alive is 63 months (range, 47 to 97 months). Twelve patients have progressed and 12 have died (including 3 patients without TCL progression). The causes of death include disease progression in 9 patients, adverse event unrelated to everolimus in 1 patient (myocardial infarction), heart failure in 1 patient, and cause of death unknown in 1 patient. Fifteen patients have gone off study with 9 progressing on treatment, 2 patients with SD went to other therapy, 1 patient died in remission from an unrelated illness (heart failure), 1 patient developed a new primary cancer (small cell carcinoma), 1 patient went off study in a PR due to side effects (dysguesia), and 1 patient died of a myocardial infarction while on study (thought to be unrelated to everolimus).
Bar diagram of the outcome of each of the 16 patients with relapsed TCL treated with everolimus on this protocol.
Bar diagram of the outcome of each of the 16 patients with relapsed TCL treated with everolimus on this protocol.
Everolimus was well tolerated in this heavily pretreated study population. Seven patients did experience a grade 3 or higher hematologic toxicity and 6 patients had grade 3 nonhematologic toxicity (see supplemental Table 1A-B, available on the Blood Web site). All 16 patients were initially treated with everolimus 10 mg daily; however, 38% (6/16) required dose reductions to 5 mg daily. The reasons for dose reduction were due to 1 patient each having paroxysmal supraventricular tachycardia, worsening preexisting congestive heart failure, diarrhea, and no stated reason. Two additional patients experienced a skin rash that was considered everolimus-related. Three patients were able to return back to the standard dose of 10 mg daily at a later cycle. One additional patient experienced a dose delay due to diarrhea.
Mechanisms of action of everolimus in TCL in vitro
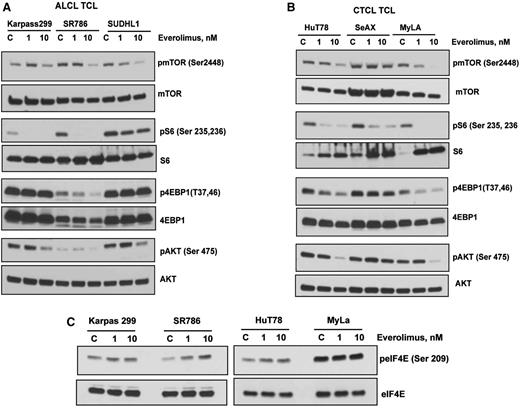
This clinical trial of everolimus for relapsed TCL patients demonstrated a 44% ORR (Figure 3), which is somewhat higher than the 30% and 32% found for relapsed DLBCLs and MCLs, respectively.16 To elucidate the mechanisms of sensitivity of everolimus in the PTCL, 3 ALCL and 3 CTCL cell lines were treated with 1 and 10 nM of everolimus overnight, and the effects on mTOR phosphorylation and its downstream targets were examined by western blotting. Everolimus at a dose of 10 nM inhibited the phosphorylation of mTOR at autophosphorylation sites in both of the ALCL and CTCL cell lines examined (Figure 4A-B). Moreover, everolimus at both 1 and 10 nM markedly inhibited the phosphorylation of the mTORC1 target ribosomal S6 in all TCL lines tested except for SUDHL1, where the inhibition was only modest. With respect to the other mTORC1 target 4EBP1, everolimus inhibited phosphorylation in the CTCL cell lines HuT and MyLa with less inhibition of SeAX (Figure 4A-B). There was inhibition of the phosphorylation of 4EBP1 in the ALCL cell line SR786 but not Karpas 299 or SUDHL1.
Effects of everolimus on mTOR signaling in TCL cell lines. (A-B) ALCL and CTCL cell lines were treated with 1 and 10 nM of everolimus overnight. Whole cell lysate were then subjected to western blotting with phospho-specific antibodies to mTOR, S6, 4EBP1, and AKT as indicated. (C) Karpas 299, SR786, HuT 78, and MyLa TCL cell lines were treated with everolimus overnight and western blotting was performed using phosho-eIF4E antibody.
Effects of everolimus on mTOR signaling in TCL cell lines. (A-B) ALCL and CTCL cell lines were treated with 1 and 10 nM of everolimus overnight. Whole cell lysate were then subjected to western blotting with phospho-specific antibodies to mTOR, S6, 4EBP1, and AKT as indicated. (C) Karpas 299, SR786, HuT 78, and MyLa TCL cell lines were treated with everolimus overnight and western blotting was performed using phosho-eIF4E antibody.
We have previously demonstrated12 that rapamycin, another mTORC1 inhibitor, triggers a negative feedback mechanism by activating survival pathways involving Akt at serine 475 and eIF4E at serine 209 in DLBCL. We examined whether a similar Akt activation occurred with everolimus in TCL cells. Interestingly, rather than activating Akt, everolimus at 10 nM decreased phosphorylation of Akt at serine 475 in 5/6 ALCL and CTCL cell lines (Figure 4A-B). We then evaluated the effect of everolimus on eIF4E, another important survival pathway in cancer cells.29 Everolimus treatment increased the phosphorylation of eIF4E at serine 209 in 3 of 4 TCL cell lines tested, suggesting that this compensatory feedback mechanism plays a role in protecting the cell from apoptosis (Figure 4C).
Effect of everolimus on cell cycle regulatory proteins in TCL cells
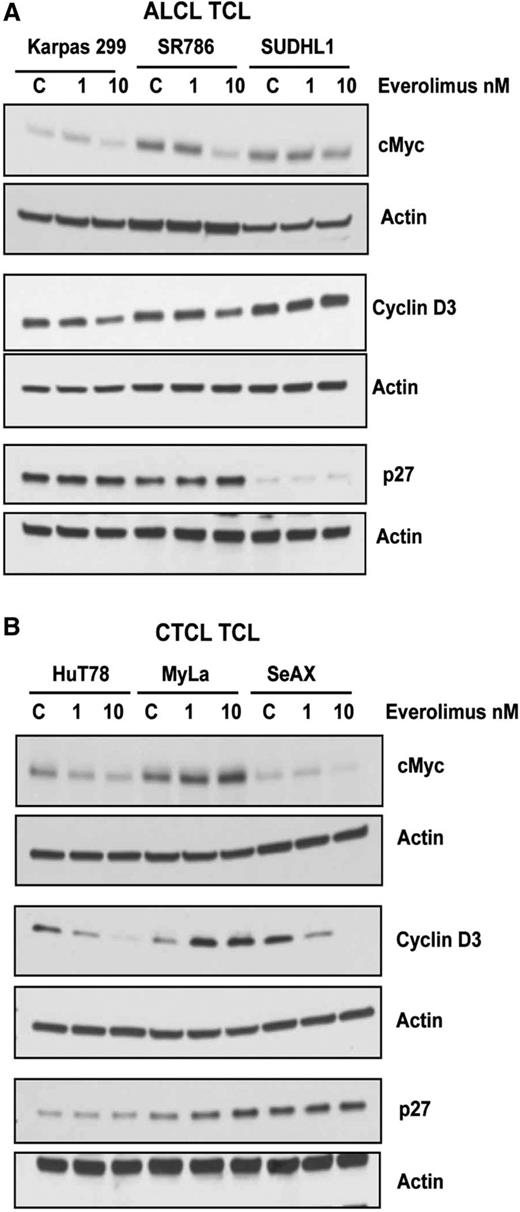
The potent antiproliferative effects of everolimus in TCL cells (Figure 2) led to studies examining whether critical cell cycle regulatory proteins are affected by everolimus. The 6 TCL lines were treated with 1 and 10 nM of everolimus and cyclins D1-3, c-Myc, and p27 protein expression measured by western blot (Figure 5). Everolimus reduced c-Myc levels in all TCL lines except the MyLa CTCL cell line (Figure 5A-B). Cyclin D3 expression was reduced by everolimus in Karpas 299, SR786, HuT78, and SeAX TCL cells at 10 nM (Figure 5A-B). Expression of p27 was unaffected by everolimus in all TCL lines (Figure 5A-B). In addition, there was no effect of everolimus on cyclin D1 and cyclin D2 expression (data not shown).
Effects of everolimus on cell cycle regulatory proteins in TCL. (A-B) ALCL (A) and CTCL (B) TCL cell lines were treated with everolimus for 24 hours and then subjected to western blot analysis for cell cycle regulatory proteins, such as c-Myc, cyclin D1, and P27.
Effects of everolimus on cell cycle regulatory proteins in TCL. (A-B) ALCL (A) and CTCL (B) TCL cell lines were treated with everolimus for 24 hours and then subjected to western blot analysis for cell cycle regulatory proteins, such as c-Myc, cyclin D1, and P27.
In vivo inhibition of plasma cytokines
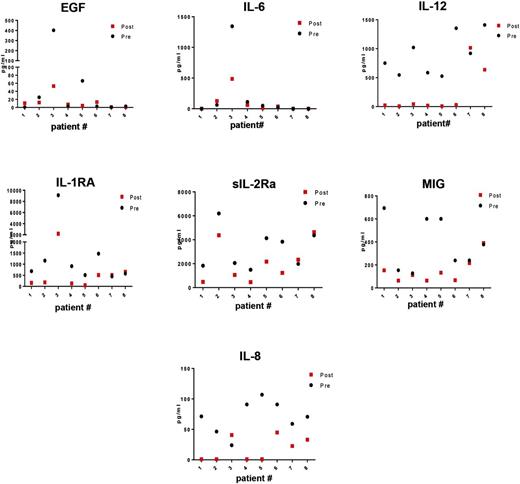
Eight patients had paired plasma samples available for analysis for changes in cytokine levels with everolimus treatment. The samples were batched and run on the same plate at the end of the study. Four patients were PTCL NOS, 3 had CTCL (MF), and 1 patient was T lymphoblastic. Treatment with single-agent everolimus for 2 cycles (56 days) led to a reduction in plasma levels of several cytokines but was most evident for IL-12, IL-1RA, sIL-2Rα, and IL-8 (Figure 6). Specifically, IL-12 and IL-8 plasma levels were inhibited in 7/8 patients, whereas IL-1RA and sIL-2Rα plasma levels were inhibited in 6/8 patients (Figure 6). The best response to everolimus for these 8 patients was CR in patient 4; PR in patients 1, 2, 6, and 7; and SD in patients 3, 5, and 8. Because this was a single-agent trial, it is clear that everolimus can inhibit secretion of biologically active cytokines. Due to the small number of patients, we were unable to draw any conclusions on reductions of the plasma cytokines with tumor response or DR.
Change in plasma cytokine levels in 8 patients after 2 cycles of everolimus therapy. The best response to everolimus for these 8 patients was CR in patient 4; PR in patients 1, 2, 6, and 7; and SD in patients 3, 5, and 8.
Change in plasma cytokine levels in 8 patients after 2 cycles of everolimus therapy. The best response to everolimus for these 8 patients was CR in patient 4; PR in patients 1, 2, 6, and 7; and SD in patients 3, 5, and 8.
Discussion
The treatment of TCL remains problematic.9,30 Despite the recent approval of the histone deacetylase inhibitors romidepsin and belinostat and the antifolate pralatrexate, additional new agents are needed to improve outcome for these patients. In this report, we present promising results with the single-agent mTORC1 inhibitor everolimus for relapsed TCL. This phase 2 trial demonstrated a 44% (95% CI, 20-70) ORR in patients with relapsed TCL, thus achieving the statistical goal of a >20% ORR in this heavily pretreated patient population. These responses were associated with an encouraging median DR of 8.5 months (95% CI, 1.0 to not reached). This ORR is similar to the experience with everolimus in relapsed HL15 ; higher than what we observed in relapsed DLBCL and MCL16 and lower than that found for relapsed Waldenström macroglobulinemia.13,14 Everolimus in this study and others31,32 has a reasonable toxicity profile as a single agent at the Food and Drug Administration-approved dose of 10 mg/day.31 In patients with solid tumors, the incidence of high-grade anemia, neutropenia, and thrombocytopenia has been found in a meta-analysis to be 8%, 2%, and 4%, respectively.32 The incidence of hematologic toxicity in patients with relapsed, heavily pretreated, blood disorders is higher. For example, in our previous large study of single-agent everolimus, we found the incidence of grade 3/4 hematologic toxicity to be 14%, 18%, and 38% for anemia, neutropenia, and thrombocytopenia, respectively.16,32 Everolimus can also cause a drug rash in approximately 29% of patients.31 Interstitial lung disease due to lymphocytic infiltration is rare and usually responds to corticosteroids or drug dose adjustments.33 Treatment of the above toxicities may require dose reductions to 5 mg daily, 5 mg every other day, or even cessation of the drug.
These results provide a rationale to combine mTORC1 inhibitors with other agents to improve the outcome for patients with TCL. Combinations of mTORC1 inhibitors with other chemotherapy agents have been found feasible and safe in early studies in B-cell NHL.19,20 In vitro studies on combinations12,34,35 and the single-agent trial results have led to a phase 1 trial of everolimus with standard RCHOP-21 for untreated DLBCL that was recently completed (#NCT01334502). In addition, trials of everolimus with the histone deacetylase inhibitor LBH589, the multikinase inhibitor sorafenib, and the IMiD lenalidomide are ongoing or recently completed for patients with relapsed NHL, including TCL.
The in vitro effects of everolimus in TCL are primarily antiproliferative as has been found for B-cell NHL.16 We did find one significant difference between the mTORC1 inhibitor effects on mTORC2 targets in TCL compared with B-cell NHL. In B-cell NHL, mTORC1 inhibitors block the phosphorylation of pS6 quite efficiently but trigger an activation of the mTORC2 target Akt.12 In the present study, when everolimus was added to TCL lines, the phosphorylation of Akt at serine 475 was inhibited (Figure 4). Everolimus was unable to block the phosphorylation of eIF4E but did downregulate c-Myc and cyclin D3 protein expression. The results of the plasma cytokine analysis demonstrate that inhibition of the mTORC1 pathway cannot only produce tumor responses but also lower levels of pro-inflammatory cytokines, potentially improving symptoms related to disease. We recently demonstrated, using the same multiplex enzyme-linked immunosorbent assay methodology, that untreated patients with TCL have significant elevations (compared with controls) of 7 cytokines: epidermal growth factor, IL-6, IL-12, IP-10, soluble IL-2Rα, monokine induced by interferon-γ, and IL-1RΑ (unpublished data). These cytokines can be derived from tumor cells (autocrine) or the microenvironment (paracrine). In our paired analysis (Figure 6), treatment with 2 cycles of single-agent everolimus was able to decrease circulating levels of these cytokines in most patients. These results could derive from a direct effect of everolimus on tumor cells or cells in the tumor microenvironment. Future studies with novel agents will likely demonstrate a differential effect on plasma cytokine secretion depending on the mechanism of action of the agent. This has the potential to tailor therapy to TCL patients based on the cytokine profile.
The strengths of this study are that is the first to report single-agent activity in TCL of an agent that specifically targets the mTORC1 kinase, a member of the PI3K/mTOR signal transduction pathway. Secondly, the in vitro work with everolimus in ALCL and CTCL cell lines supports the rationale for further studies with this class of agents. The lack of compensatory Akt activation and the downregulation of c-Myc and cyclin D3 in response to everolimus in TCL are particularly interesting given the importance of these signal components in the pathophysiology of NHL. The weaknesses of this study are the small number of patients with a variety of disease types within the category of TCL. Much larger studies (111 to 130 patients) were required to validate the 25% ORR of romidepsin,6 29% ORR for pralatrexate,5 and 26% for belinostat.8 However, to encourage large definitive trials, small pilots and in vitro data are required, especially when dealing with unusual types of lymphoma such as TCL.
In conclusion, this study provides the rationale to include mTORC1 inhibitors in future trials of novel combinations for TCL.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by grants from the National Institutes of Health National Cancer Institute (CA127433 and CA97274), and the Predolin Foundation.
Authorship
Contribution: T.E.W. designed and conducted the clinical trial study, and wrote the report; B.L. did the statistical analysis; W.M. did the pathology review; J.J.H. performed in vitro studies; M.S. performed cytokine correlative studies; C.R., H.W.T., S.M.A., T.M.H., D.J.I., I.N.M., P.B.J., L.F.P., J.P.C., S.M., and G.S.N. enrolled patients on to the trial and reviewed the manuscript; and M.G. designed the in vitro and correlative studies, interpreted and analyzed the data, finalized the figures, and wrote the paper.
Conflict-of-interest disclosure: Novartis provided everolimus for the trial and support to the Mayo Clinic to conduct the research. No investigator received funding for the trial. T.E.W. and P.B.J. have participated on Advisory Boards for Novartis but were personally uncompensated. The remaining authors declare no competing financial interests.
Correspondence: Mamta Gupta, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: gupta.mamta@mayo.edu; and Thomas E. Witzig, Division of Hematology, Mayo Clinic, Rochester, MN; e-mail: witzig.thomas@mayo.edu.