In this issue of Blood, Witzig et al report on the promising in vitro and in vivo activity of everolimus in T-cell lymphoma (TCL) and pave the way for future combination studies.1
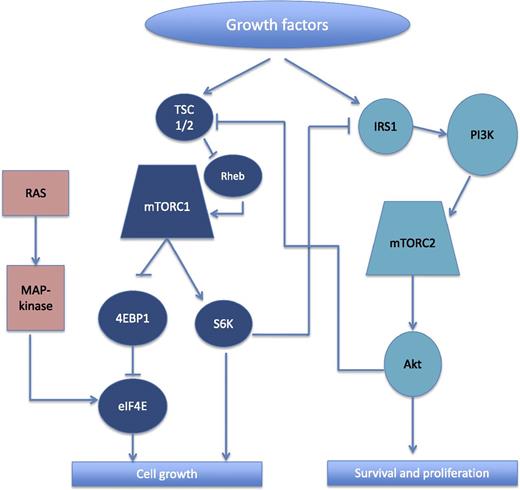
Simplified schema showing the interactions between mTORC1 and mTORC2, and associated pathways. The downstream effectors of mTORC1 include S6K and 4EBP1. When phosphorylated, 4EBP1 releases eIF4E, which then facilitates messenger RNA translation and contributes to cell growth. Activation of eIF4E also occurs via the ras/MAPK pathway. Among the key downstream effectors of mTORC2 is Akt, which suppresses apoptosis by inhibiting pro-apoptotic proteins and further promotes cell growth through activation of mTORC1 (via inhibition of TSC 1/2). The activation of mTORC1 also causes downregulation of mTORC2 by inhibition of IRS1 by S6K. 4EBP1, eukaryotic translation initiation factor 4E–binding protein 1; eIF4E, eukaryotic translation initiation factor 4E; IRS1, insulin receptor substrate 1; Rheb, ras homolog enriched in brain; S6K, ribosomal S6 kinase; TSC 1/2, tuberous sclerosis 1/2; PI3K, phosphatidylinositol 3-kinase.
Simplified schema showing the interactions between mTORC1 and mTORC2, and associated pathways. The downstream effectors of mTORC1 include S6K and 4EBP1. When phosphorylated, 4EBP1 releases eIF4E, which then facilitates messenger RNA translation and contributes to cell growth. Activation of eIF4E also occurs via the ras/MAPK pathway. Among the key downstream effectors of mTORC2 is Akt, which suppresses apoptosis by inhibiting pro-apoptotic proteins and further promotes cell growth through activation of mTORC1 (via inhibition of TSC 1/2). The activation of mTORC1 also causes downregulation of mTORC2 by inhibition of IRS1 by S6K. 4EBP1, eukaryotic translation initiation factor 4E–binding protein 1; eIF4E, eukaryotic translation initiation factor 4E; IRS1, insulin receptor substrate 1; Rheb, ras homolog enriched in brain; S6K, ribosomal S6 kinase; TSC 1/2, tuberous sclerosis 1/2; PI3K, phosphatidylinositol 3-kinase.
The peripheral TCLs are heterogeneous diseases typically associated with unfavorable prognosis and limited treatment options. Standard frontline therapies, such as cyclophosphamide, hydroxydaunorubicin, Oncovin (vincristine), and prednisone (CHOP), produce low rates of cure. Outside of brentuximab vedotin for anaplastic large cell lymphoma, the approved drugs for relapsed disease, pralatrexate, romidepsin, and belinostat, are associated with response rates ranging from 25% to 29% and produce intermediate or long-term benefit for only a minority of patients.2-5 With an overall response rate of 44% (95% confidence interval [CI], 20-70) and median duration of response of 8.5 months in a small phase 2 study, it is likely that everolimus sits within range of the other approved and useful agents for TCL. It is also likely that, as a single agent, everolimus is not a game-changer or an otherwise breakthrough drug in TCL; however, the data presented by Witzig et al confirm the relevance of this pathway in TCL and pave the way for rationally designed combination studies.
Developing new drugs for TCLs is an ongoing challenge due to the rarity of these diseases and the marked diversity among them. We lack predictive biomarkers to aid in selecting individual treatments for individual patients. Even in the case of a targeted agent such as brentuximab vedotin, the degree of CD30 expression has been poorly correlated with the likelihood of response or resistance.6 The activity of our established agents was noted empirically and the combination studies that exist have often added active agents without a strong preclinical rationale or testable hypothesis for response or resistance. It is here that the study by Witzig et al gives a clue to a path forward.
The mammalian target of rapamycin (mTOR) pathway is commonly activated in cancer due to its role in promoting cell growth, proliferation, and survival.7 The mTOR protein is a member of at least 2 distinct protein complexes, mTOR complex 1 (mTORC1) and mTORC2, that activate separate, yet inter-related pathways (see figure). A negative feedback loop between these 2 pathways is potentially responsible for the variable sensitivity of cell lines and tumors to rapamycin and its analogs (or rapalogs).7 In fact, the inhibition of mTORC1 is often associated with upregulation of Akt through the release of its inhibitory effects on mTORC2 and Akt. Although rapalogs primarily inhibit mTORC1, in some cell types they have the potential to inhibit both mTORC1 and mTORC2 and therefore overcome this feedback loop. As Witzig et al demonstrate, this appears to be the case in TCL. In addition to the inhibition of the typical mTORC1 effectors, S6K and 4EBP1, decreased phosphorylation of Akt was seen also, indicating that everolimus may be inhibiting both mTORC1 and mTORC2 and thus avoiding compensatory mTORC2 activation. Interestingly, despite eIF4E being downstream from mTORC1, the authors observed increased phosphorylation of eIF4E following everolimus treatment in 3 of 4 cell lines tested; however, as the authors point out, this could represent another feedback mechanism, potentially mediated through the mitogen-activated protein kinase (MAPK) pathway, which is also known to activate eIF4E.
These findings provide a framework for further investigation of mTOR inhibitors in relapsed/refractory peripheral TCL. In particular, the preclinical data suggest potential markers of response or resistance to mTOR inhibitors and imply certain combinations that warrant further evaluation. Given the increased activation of eIF4E observed, an inhibitor of eIF4E is likely to enhance sensitivity to everolimus.8 In addition, increased phosphorylated Akt following treatment with everolimus is likely to be associated with resistance, which may be overcome with a PI3K inhibitor. This would be a particularly attractive approach since PI3K inhibitors have recently been shown to have activity in TCL.9
Most combination studies in TCL have been primarily empiric. CHOP has been the most commonly used regimen, and newer agents such as romidepsin, brentuximab vedotin, belinostat, and pralatrexate are being evaluated in combination with CHOP in an effort to improve efficacy. The rationale for these combinations is primarily to add active agents together and success will be determined by comparing overall results to CHOP alone. However, with agents that inhibit specific pathways, such as mTOR inhibitors or PI3K inhibitors, we have the ability to interrogate in vitro and in vivo pathway activity following treatment and ultimately combine drugs in a mechanistically logical way. Success would be measured by better responses, coupled with an understanding of where novel combinations work, and importantly, where and why they do not work. Identifying predictive biomarkers will help us better select therapies for individual patients and understanding mechanisms of resistance will inform successive combination studies. Whether or not everolimus becomes an important drug in our treatment of TCL remains to be seen and will no doubt be the subject of future studies. However, identifying new effective therapies in TCL with testable mechanisms of action and resistance, and designing rational combinations based on those results, may allow us to understand predictors of response or modes of resistance, will certainly elevate the quality of our clinical trials, and hopefully will move the field forward at an accelerated rate.
Conflict-of-interest disclosure: S.M.H. is supported by Spectrum, Seattle Genetics, Millennium, and Celgene for consultancy work; and A.J.M. is supported by Seattle Genetics and Merck for the same.