Key Points
IFN-γ reduces functional HSCs and augments Fas expression and Fas-mediated apoptosis in hematopoietic stem and progenitor cells.
Disruption of the IFN-γ/IFN-γ-receptor 1 signaling axis by targeted gene deletion significantly attenuates immune-mediated BM failure.
Abstract
Interferon gamma (IFN-γ) has been reported to have both negative and positive activity on hematopoietic cells, adding complexity to the interpretation of its pleiotropic functions. We examined the effects of IFN-γ on murine hematopoietic stem cells (HSCs) and progenitors in vitro and in vivo by using mouse models. IFN-γ treatment expanded bone marrow (BM) c-Kit+Sca1+Lin– (KSL) cell number but reduced BM KLCD150+ and KLCD150+CD48– cells. IFN-γ-expanded KSL cells engrafted poorly when tested by competitive repopulation in vivo. KSL, KLCD150+, and KLCD150+CD48– cells from IFN-γ-treated animals all showed significant upregulation in Fas expression. When cocultured with activated T cells in vitro, KSL and KLCD150+ cells from IFN-γ-treated donors showed increased apoptosis relative to those from untreated animals, and infusion of activated CD8 T cells into IFN-γ-injected animals in vivo led to partial elimination of KSL cells. Exposure of BM cells or KSL cells to IFN-γ increased expression of Fas, caspases, and related proapoptotic genes and decreased expression of Ets-1 and other hematopoietic genes. In mouse models of BM failure, mice genetically deficient in IFN-γ receptor expression showed attenuation of immune-mediated marrow destruction, whereas effector lymphocytes from IFN-γ-deficient donors were much less potent in initiating BM damage. We conclude that the activity of IFN-γ on murine hematopoiesis is context dependent. IFN-γ-augmented apoptotic gene expression facilitates destruction of HSCs and progenitors in the presence of activated cytotoxic T cells, as occurs in human BM failure.
Introduction
Interferon gamma (IFN-γ) is a critical cytokine of the immune system. Its absence in genetically engineered mice and in humans with constitutional genetic defects profoundly influences susceptibility to microbial agents, especially chronic mycobacterial infection.1-4 When immunity is triggered inappropriately, as in autoimmune diseases or immune-mediated syndromes such as graft-versus-host disease,5-7 IFN-γ appears to mediate inflammation and target cell destruction, negative effects associated with type 1 T cells and Th1 cell response.8,9 However, the precise roll of IFN-γ in animal models, and particularly in human diseases, has not always been easy to define because of conflicting data among experiments and sometimes strikingly poor correlation between murine experiments and the clinic.10-12
Effects of IFN-γ on hematopoiesis, mainly assessed by progenitor assays in vitro, have been reported as both stimulatory13-17 and suppressive18-23 under various circumstances. IFN-γ has been reported to stimulate myelopoiesis under specific infectious conditions.24,25 Zhao and colleagues26 reported that murine Th1 supernatants led to expansion of c-Kit+Sca-1+Lin– (KSL) cells with high proliferative capacity skewed toward myelopoiesis, an effect attributed to IFN-γ signaling through STAT1. In a mouse model of mycobacterial infection, Baldridge et al27 reported increased proportions of long-term repopulating hematopoietic stem cells (HSCs) in infected animals, an effect dependent on IFN-γ, suggesting a positive role of IFN-γ in hematopoietic homeostasis.
In contrast, the role of IFN-γ in human hematopoietic disease has appeared to be mainly negative, as in chronic neutropenia, anemia of chronic diseases, and immune-mediated aplastic anemia (AA).28,29 Marrow failure in AA is reversible in most patients by immunosuppressive therapies, and blood counts in a large proportion of responding patients are dependent on continued administration of the calcineurin inhibitor cyclosporine.29,30 We and others have reported inhibition of hematopoiesis by IFN-γ in assays of human progenitor cells in vitro,20-22,31 overexpression of its gene in bone marrow (BM) cells and T cells,32,33 upregulation of genes downstream of IFN-γ signaling, and alterations of the T-bet regulator of IFN-γ in BM failure.12,34
In our murine models of immune-mediated marrow destruction, infusion of H2 or minor histocompatibility antigen mismatched lymph node (LN) cells rapidly induces AA with elevated circulating IFN-γ.35 Development of marrow failure can be ameliorated by both broadly acting immunosuppressive agents and monoclonal antibody specific to IFN-γ.36,37 Inhibitory effects of IFN-γ on human hematopoietic cells have been localized molecularly to an essential role for Mnk kinases and sprouty proteins.38,39 IFN-γ appears to suppress HSC self-renewal and multilineage differentiation, thus impairing normal hematopoiesis.11,40-43
With contrasting results in the literature, we have re-examined the role of IFN-γ in murine hematopoiesis, focusing on destruction of HSCs and hematopoietic progenitor cells, in particular in rodent models of BM failure that mimic human AA.
Methods
Mice and IFN-γ treatment
Inbred C57BL/6 (B6) and FVB/N (FVB), congenic C.B10-H2b/LilMcd (C.B10) and B6.SJL-PtprcaPep3b/Boy (CD45.1), and induced mutant B6.129S7-Ifngtm1Ts (IFN-γ−/−) and B6.129S7- IFNgR1tm1Agt(IFN-γR1−/−) mice were all from The Jackson Laboratory (Bar Harbor, ME) and were bred and maintained in National Institutes of Health animal facilities under standard care and nutrition. All animal studies were approved by the Animal Care and Use Committee at the National Heart, Lung, and Blood Institute.
Recombinant murine IFN-γ was obtained from BioLegend (San Diego, CA) or PeproTech (Rock Hill, NJ), diluted in Iscove modified Dulbecco medium (Life Technologies, Grand Island, NY), filtered through 0.22-μM Millex-GS filters (Millipore Ireland Ltd., Tullagreen, Ireland), and injected into 6- to 10-week-old B6, FVB, or CD45.1 mice at 10 μg per mouse in 200 μL volume via intraperitoneal injection. Mice were bled and euthanized 20 to 48 hours later to collect BM cells for analyses.
Blood counts, cell staining, and flow cytometry
Blood was collected from the retro-orbital sinus into Eppendorf tubes with EDTA added. Complete blood count was performed by using a HemaVet 950 analyzer (Drew Scientific Inc., Waterbury, CT). After mice were euthanized by using CO2, BM cells were extracted from tibiae and femurs, filtered through 95-μM nylon mesh, counted by using a Vi-Cell counter (Beckman Coulter, Miami, FL), stained with antibody mixtures on ice for 30 minutes in RPMI 1640 (Life Technologies) supplemented with fetal bovine serum, and acquired by using LSR II or FACSCanto II flow cytometers operated by using FACSDiva software (Becton Dickinson, San Diego, CA). Stained BM cells were sorted on a FACSAria cell sorter (Becton Dickinson) to collect KSL and KLCD150+ cells. BM cells from mice previously induced to develop BM failure were sorted to obtain activated CD4 and CD8 T cells.
Monoclonal antibodies for murine CD3 (clone 145-2C11), CD4 (clone GK 1.5), CD8 (clone 53-6.72), CD11b (clone M1/70), CD95 (Fas, clone Jo2), CD178 (FasL, clone MFL3), erythroid cells (clone Ter119), and granulocytes (Gr1/Ly6-G, clone RB6-8C5) were from BD Biosciences (San Diego, CA). Annexin V apoptosis kits were also purchased from BD Biosciences. Anti-mouse CD45R (clone RA3-6B2), CD45.1 (clone A20), CD45.2 (clone 104), CD48 (clone HM48-1), CD117 (c-Kit, clone 2B8), CD150 (SLAM, clone TC15-12F12.2), and stem cell antigen 1 (Sca1, clone E13-161) were from BioLegend. Antibodies were conjugated to fluorescein isothiocyanate, phycoerythrin (PE), PE-cyanin 5 (PE-Cy5), PE-cyanin 7 (PE-Cy7), allophycocyanin, or brilliant violet 421 (BV421).
BM cell culture
BM cells from B6 and FVB mice (1.0 to 1.5 × 106 cells per milliliter) or sorted KSL cells from B6 mice were cultured in RPMI 1640 medium supplemented with 100 U/mL penicillin, 100 U/mL streptomycin, 292 μg/mL l-glutamine, 10% fetal bovine serum (Life Technologies), 10 ng/mL interleukin-3 (IL-3), 10 ng/mL IL-6, 10 ng/mL stem cell factor, and 10 ng/mL Flt3-L (PeproTech) at 37°C with 5% CO2 overnight or for 6 days with or without 50 ng/mL IFN-γ. In some experiments, anti-Fas antibody (BD Biosciences) was added to BM culture at 1 μg/mL. Harvested cells were counted and saved for RNA extraction or flow cytometry analyses.
Assays of apoptosis
BM cells from B6 or FVB mice with or without injection of 10 μg IFN-γ per mouse 1 day earlier were stained and sorted for KSL cells or KLCD150+ cells, which were mixed with sorted CD4 and/or CD8 T cells from C.B10 or B6 mice induced to develop BM failure 12 to 14 days earlier. Cell mixtures were cultured in vitro at 37°C with 5% CO2 in RPMI 1640 media with added cytokine for 1 hour. Cells were harvested and stained with antibodies, annexin V, and 7-aminoactinomycin D to detect cell apoptosis by using annexin V apoptosis detection kits (BD Biosciences).
FVB mice injected with 10 μg IFN-γ per mouse at day 0 were infused at day 1 with nothing (IFN-γ) or 6 × 104 CD8 T cells from BM failure B6 mice (IFN-γ+CD8). FVB mice without IFN-γ treatment were also infused at day 1 with nothing (control) or 6 × 104 CD8 T cells (CD8). BM cells were extracted from animals euthanized at day 2, stained, and analyzed for KSL and Fas+KSL cell recovery to measure KSL cell elimination in vivo.
Colony assay and competitive repopulation
Sorted KSL cells from B6 mice with or without injection of 10 μg IFN-γ per mouse 1 day earlier were cultured in MethoCult GF medium (STEMCELL Technologies Inc., Vancouver, BC, Canada) at 37°C with 5% CO2 for 10 days. Colonies were counted under a Zeiss Axioskop2 Plus microscope. Sorted KSL cells from IFN-γ-injected and control B6 mice were also mixed with BM cells from a pool of CD45.1 competitors and engrafted into lethally irradiated (11 Gy from a 137Cesium gamma source [J.L. Shepherd & Associates, Glendale, CA]) CD45.1 recipients. Two experiments were performed using low or high KSL:competitor BM cell ratios. Recipients were bled once per month for 3 to 4 months, and engraftment of donor KSL cells was measured by flow cytometry analyses based on CD45.1/CD45.2 allelic difference.
PCR array
Total RNA was isolated from cultured BM cells, cultured KSL cells, or sorted KSL cells using the RNeasy Kit (QIAGEN, Valencia, CA), digested with RNase-free DNase I (QIAGEN), and assessed by using a Nanodrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). First-strand complementary DNA was synthesized by using RT2 First-Strand Kit (QIAGEN). Quantitative analysis of messenger RNA expression of hematopoiesis- and apoptosis-related genes was performed by using mouse polymerase chain reaction (PCR) arrays PAMM-054Z and PAMM-012Z (QIAGEN). Expression of genes in apoptosis and hematopoiesis pathways was compared between samples with and without IFN-γ treatment of the same cell type (BM cells or KSL cells) under the same treatment conditions (cell culture in vitro or injection in vivo). Two-way hierarchical cluster analysis was performed for genes that had more than twofold changes, based on PCR array data using Ward’s method of JMP statistical software (SAS Institute, Cary, NC).
Induction of BM failure
Inguinal, axillary, and lateral axillary LN cells from B6, IFN-γ−/−, and FVB mice were homogenized by using a mini tissue grinder (Daigger Scientific, Vernon Hills, IL) in Iscove modified Dulbecco medium. LN cells were filtered through 90-μM nylon mesh (Small Parts, Miami Lake, FL) and counted by a Vicell counter. LN cells from FVB donors were injected into pre-irradiated (6.5 Gy total body irradiation [TBI]) B6 or IFN-γR1−/− recipients, and LN cells from B6 and IFN-γ−/− donors were injected into pre-irradiated (5 Gy TBI) C.B10 recipients, all at 5 × 106 cells per recipient through the lateral tail vein. Recipient mice were bled and euthanized 12 or 14 days later, and BM cells were extracted from tibiae and femurs for cell counting and flow cytometry analyses. Sternae were fixed in 10% neutral buffered formalin, sectioned at 5µM thickness, stained with hematoxylin and eosin (GeneCopoeia Inc., Rockville, MD), and examined by using a Zeiss Axioskop2 Plus microscope equipped with a Zeiss AxioCam high-resolution, cooled camera (Carl Zeiss MicroImaging GmbH, Jena, Germany).
Statistics
JMP (SAS Institute) repeated measures analysis (mixed model with random effects) was used to analyze data from competitive repopulation assays with multiple measurements of the same recipients at different time points. Data from B6/IFN-γR1−/− BM failure mice were analyzed by using a linear square model, in which interaction of treatment and genotype was used to evaluate differences between B6 and IFN-γR1−/− mice in response to induction of BM failure. Data obtained from flow cytometry were analyzed by unpaired t test, variance analyses, and multiple comparisons using GraphPad Prism statistical software. Data are presented as means with standard errors. Statistical significance was declared at P < .05.
Results
IFN-γ-stimulated expansion of KSL cells but not of functional HSCs and progenitors
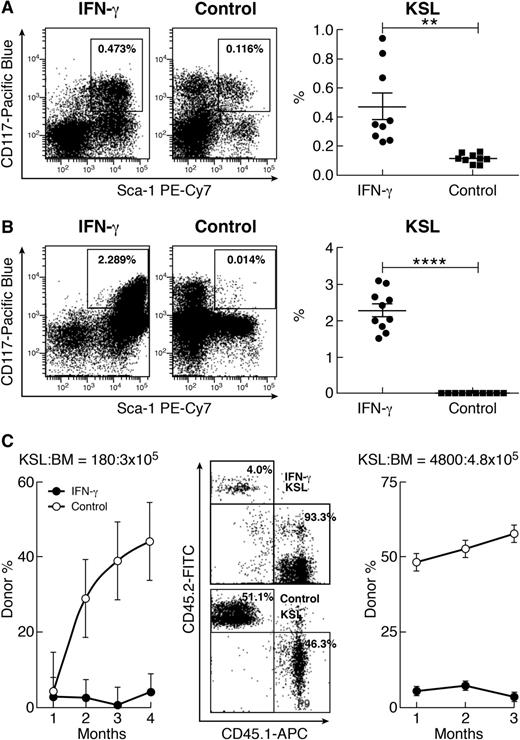
A single injection of IFN-γ at 10 µg per mouse caused a significant increase in the percentage of KSL cells in the BM of B6 mice measured 20 to 24 hours later (Figure 1A). Based on CD150 expression, the proportion of KSLCD150+ cells (SKSL) increased seven- to eightfold after treatment with IFN-γ (supplemental Figure 1A available on the Blood Web site). When BM cells from B6 mice were cultured in vitro for 6 days in hematopoietic cytokine-supplemented (Flt3-ligand, IL-3, IL-6, and stem cell factor) RPMI 1640 media, IFN-γ exposure stimulated a 163-fold increase in the proportion of KSL cells relative to cell culture without IFN-γ (Figure 1B).
Expansion of phenotypic KSL cells by IFN-γ without expansion of functional HSCs. (A) IFN-γ injection (10 µg per mouse; n = 9) caused a significant increase in the proportion of KSL cells relative to untreated controls (n = 9). (B) When B6 BM cells were cultured for 6 days with IFN-γ (50 ng/mL; n = 10), there was a significant increase in KSL cell proportion. (C) In a competitive repopulation assay with KSL:competitor BM cell ratio of 180:3 × 105, KSL cells from IFN-γ-treated donors (solid circles) contributed very little to recipient mature blood cells relative to KSL cells form normal donors (open circles). This was confirmed in a second competitive repopulation assay at KSL:competitor BM cell ratio of 4800:4.8 × 105 in which IFN-γ-expanded KSL cells (solid circles) had significantly lower donor contributions than normal KSL cells (open circles), shown as representative flow cytometry dot plots and means with standard errors. APC, allophycocyanin; FITC, fluorescein isothiocyanate; PE, phycoerythrin. **P < .01; ****P < .0001.
Expansion of phenotypic KSL cells by IFN-γ without expansion of functional HSCs. (A) IFN-γ injection (10 µg per mouse; n = 9) caused a significant increase in the proportion of KSL cells relative to untreated controls (n = 9). (B) When B6 BM cells were cultured for 6 days with IFN-γ (50 ng/mL; n = 10), there was a significant increase in KSL cell proportion. (C) In a competitive repopulation assay with KSL:competitor BM cell ratio of 180:3 × 105, KSL cells from IFN-γ-treated donors (solid circles) contributed very little to recipient mature blood cells relative to KSL cells form normal donors (open circles). This was confirmed in a second competitive repopulation assay at KSL:competitor BM cell ratio of 4800:4.8 × 105 in which IFN-γ-expanded KSL cells (solid circles) had significantly lower donor contributions than normal KSL cells (open circles), shown as representative flow cytometry dot plots and means with standard errors. APC, allophycocyanin; FITC, fluorescein isothiocyanate; PE, phycoerythrin. **P < .01; ****P < .0001.
Because no difference was observed in blood cell counts between B6 mice with or without IFN-γ injection (supplemental Table 1), we queried whether IFN-γ-augmented KSL cell expansion was accompanied by altered HSC and progenitor cell function. We first measured the frequency of colony-forming units in vitro: KSL cells from IFN-γ-injected mice formed hematopoietic colonies, and IFN-γ-expanded KSL cells contained more colony-forming units than KSL cells from untreated B6 mice (supplemental Figure 1B). Next, we mixed KSL cells from untreated or IFN-γ-treated B6 donors with BM cells from congenic competitors and transplanted cell mixtures into lethally irradiated recipients. In 2 experiments with low and high ratios of donor KSL to competitor BM cells, KSL cells from IFN-γ-treated donors had significantly lower donor contribution to recipient hematopoiesis than did KSL cells from untreated B6 donors (Figure 1C). Thus, IFN-γ-expanded KSL cells failed to reconstitute lethally irradiated recipients in vivo and therefore did not function normally as HSCs and progenitors.
IFN-γ-augmented KSL cell apoptosis and elimination
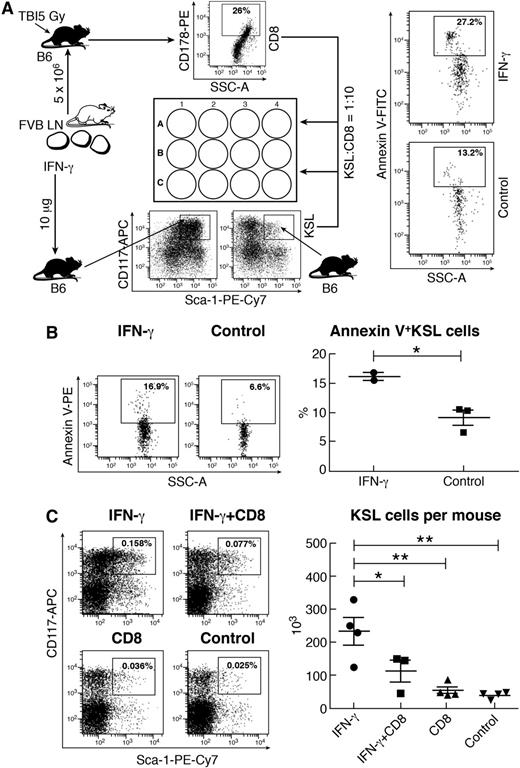
One function of IFN-γ is to sensitize target cells to killing. We hypothesized that IFN-γ-stimulated KSL expansion might facilitate IFN-γ-mediated cell destruction, especially under conditions of inflammatory stress and in the presence of activated T cells. We cultured KSL cells from IFN-γ-injected donor mice with activated CD8 T cells from mice induced for BM failure. There was a significantly higher proportion of KSL cells entering apoptosis relative to normal KSL cells not exposed to IFN-γ (Figure 2A). In another experiment, we cultured untreated or IFN-γ-expanded KSL cells with activated CD4 and CD8 T cells from BM failure mice, and again, IFN-γ-expanded KSL cells were more apoptotic than were KSL cells from normal mice (Figure 2B). Thus, IFN-γ-expanded KSL cells were more sensitive to CD8 T cell–mediated killing than were normal KSL cells.
T-cell-mediated apoptosis and elimination of IFN-γ-expanded KSL cells. (A) Activated CD8 T cells were sorted from the BM of B6 mice pretreated with sublethal TBI and FVB LN cell infusion (induced BM failure) and then mixed with KSL cells from IFN-γ-treated (10 μg per mouse) or control B6 mice at a CD8:KSL cell ratio of 10:1. Cells were cultured at 37°C for 60 minutes. IFN-γ-treated KSL cells (n = 4) had significantly higher proportion of annexin V+ cells compared with untreated KSL cells (n = 2). (B) In another experiment, KSL cells from IFN-γ-injected (n = 2) or untreated control (n = 3) B6 mice were mixed with activated CD4 and CD8 T cells at a KSL:CD4:CD8 ratio of 1:5:10 and cultured at 37°C for 60 minutes. Activated CD4 and CD8 T cells caused an increase in annexin V+ cells in IFN-γ-expanded KSL cells. (C) FVB mice were treated with IFN-γ (10 μg per mouse) on day 0 (IFN-γ; n = 4), IFN-γ (10 μg per mouse) on day 0 plus 6 × 104 activated CD8 T cells per mouse on day 1 (IFN-γ+CD8; n = 3), 6 × 104 activated CD8 T cells only per mouse on day 1 (CD8; n = 4), or nothing (control; n = 4) and were euthanized on day 2 to harvest BM cells for flow cytometry analyses. Total number of KSL cells recovered was significantly reduced in the IFN-γ+CD8 treatment group compared with the IFN-γ group. SSC-A, side scatter-area. *P < .05; **P < .01.
T-cell-mediated apoptosis and elimination of IFN-γ-expanded KSL cells. (A) Activated CD8 T cells were sorted from the BM of B6 mice pretreated with sublethal TBI and FVB LN cell infusion (induced BM failure) and then mixed with KSL cells from IFN-γ-treated (10 μg per mouse) or control B6 mice at a CD8:KSL cell ratio of 10:1. Cells were cultured at 37°C for 60 minutes. IFN-γ-treated KSL cells (n = 4) had significantly higher proportion of annexin V+ cells compared with untreated KSL cells (n = 2). (B) In another experiment, KSL cells from IFN-γ-injected (n = 2) or untreated control (n = 3) B6 mice were mixed with activated CD4 and CD8 T cells at a KSL:CD4:CD8 ratio of 1:5:10 and cultured at 37°C for 60 minutes. Activated CD4 and CD8 T cells caused an increase in annexin V+ cells in IFN-γ-expanded KSL cells. (C) FVB mice were treated with IFN-γ (10 μg per mouse) on day 0 (IFN-γ; n = 4), IFN-γ (10 μg per mouse) on day 0 plus 6 × 104 activated CD8 T cells per mouse on day 1 (IFN-γ+CD8; n = 3), 6 × 104 activated CD8 T cells only per mouse on day 1 (CD8; n = 4), or nothing (control; n = 4) and were euthanized on day 2 to harvest BM cells for flow cytometry analyses. Total number of KSL cells recovered was significantly reduced in the IFN-γ+CD8 treatment group compared with the IFN-γ group. SSC-A, side scatter-area. *P < .05; **P < .01.
We further tested the fate of IFN-γ-expanded KSL cells in vivo by sequential injection of IFN-γ on day 0 and of activated CD8 T cells on day 1. FVB mice injected with IFN-γ alone showed marked increase in the proportion and total number of BM KSL cells relative to untreated controls (Figure 2C). Infusion of activated CD8 T cells into IFN-γ-injected mice (IFN-γ+CD8) eliminated almost half the KSL cells gained by IFN-γ treatment, whereas infusion of CD8 T cells into normal mice produced no apparent change (Figure 2C). When SKSL cells were measured, IFN-γ+CD8 treatment also reduced the proportion of SKSL cells in BM (supplemental Figure 2A). Thus, activated CD8 T cells caused elimination of IFN-γ-expanded KSL and SKSL cells in vivo.
Upregulated Fas expression on IFN-γ-expanded KSL cells
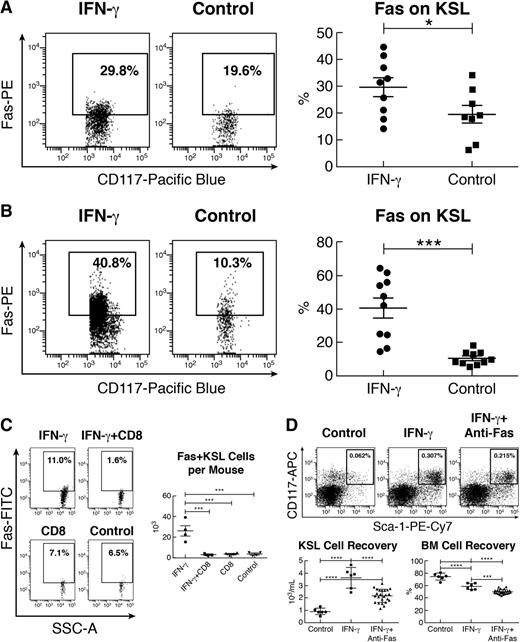
To investigate the mechanism of IFN-γ-mediated effects on KSL cells, we examined expression of Fas on KSL cells after historic observations that IFN-γ mediated Fas expression on human BM CD34+ cells.44-46 Indeed, KSL cells from IFN-γ-injected mice showed large proportions of cells expressing Fas (Figure 3A). In 6-day BM cell culture, as stated earlier, the proportion of Fas+KSL cells was about 40% on average in the IFN-γ-exposed BM culture, much higher than 10% Fas+KSL cells in culture without IFN-γ (Figure 3B). When mice were sequentially injected with IFN-γ and activated CD8 T cells, there was a significant decline in Fas+KSL cells relative to mice that received IFN-γ alone (Figure 3C). Thus, the decline in KSL cells (Figure 2C) likely represented partial elimination of Fas+KSL cells (Figure 3C). IFN-γ+CD8 treatment also reduced SKSL and Fas+SKSL cells in these same mice (supplemental Figure 2).
IFN-γ-upregulated KSL cell Fas expression and Fas-mediated destruction. (A) KSL cells from IFN-γ-injected mice (10 μg per mouse; n = 9) had a significantly higher proportion of cells expressing the apoptotic receptor Fas relative to KSL cells from untreated (n = 8) mice. (B) BM cells from B6 mice (n = 10) cultured with 50 ng/mL IFN-γ for 6 days in vitro also had significant upregulation in Fas expression on KSL cells compared with cultured BM cells without IFN-γ (n = 10). (C) From the same mice as described in Figure 2C, the proportion and total number of Fas+KSL cells were significantly reduced in those that received treatment with IFN-γ+CD8 T cells compared with those exposed to IFN-γ alone. (D) BM cells from FVB mice (n = 6) were cultured for 24 hours in RPMI 1640 media supplemented with 25 ng/mL IFN-γ (IFN-γ), 25 ng/mL IFN-γ plus 0.5 to 2.0 μg/mL anti-Fas antibody (IFN-γ+anti-Fas), or media only (control). The proportion of KSL cells was reduced in the IFN-γ+anti-Fas treatment group relative to the IFN-γ group. Recovery of total KSL cells was reduced (P < .01) with IFN-γ+anti-Fas treatment relative to treatment with IFN-γ alone. IFN-γ treatment also reduced total BM cell recovery relative to control, whereas IFN-γ+anti-Fas treatment further reduced BM cell recovery relative to treatment with IFN-γ alone. *P < .05; ***P < .001; ****P < .0001.
IFN-γ-upregulated KSL cell Fas expression and Fas-mediated destruction. (A) KSL cells from IFN-γ-injected mice (10 μg per mouse; n = 9) had a significantly higher proportion of cells expressing the apoptotic receptor Fas relative to KSL cells from untreated (n = 8) mice. (B) BM cells from B6 mice (n = 10) cultured with 50 ng/mL IFN-γ for 6 days in vitro also had significant upregulation in Fas expression on KSL cells compared with cultured BM cells without IFN-γ (n = 10). (C) From the same mice as described in Figure 2C, the proportion and total number of Fas+KSL cells were significantly reduced in those that received treatment with IFN-γ+CD8 T cells compared with those exposed to IFN-γ alone. (D) BM cells from FVB mice (n = 6) were cultured for 24 hours in RPMI 1640 media supplemented with 25 ng/mL IFN-γ (IFN-γ), 25 ng/mL IFN-γ plus 0.5 to 2.0 μg/mL anti-Fas antibody (IFN-γ+anti-Fas), or media only (control). The proportion of KSL cells was reduced in the IFN-γ+anti-Fas treatment group relative to the IFN-γ group. Recovery of total KSL cells was reduced (P < .01) with IFN-γ+anti-Fas treatment relative to treatment with IFN-γ alone. IFN-γ treatment also reduced total BM cell recovery relative to control, whereas IFN-γ+anti-Fas treatment further reduced BM cell recovery relative to treatment with IFN-γ alone. *P < .05; ***P < .001; ****P < .0001.
We further explored the role of Fas in IFN-γ-mediated KSL cell apoptosis/destruction by culturing BM cells with or without anti-Fas antibody. Addition of anti-Fas antibody significantly reduced the KSL cell percentage, total KSL cell recovery, and total BM cell recovery after IFN-γ treatment (Figure 3D), indicating that anti-Fas antibody mediated apoptosis and elimination of IFN-γ-treated BM cells and IFN-γ-expanded KSL cells.
Effects of IFN-γ on apoptotic and hematopoietic gene expression
In cultures of BM cells, IFN-γ treatment significantly upregulated the expression of Fas and caspases 1, 7, and 12 (Figure 4A). IFN-γ treatment stimulated expression of Fas, caspases 1, 4, and 7, Bcl2l11, Tnf, Traf1, and CD40 relative to BM cells absent IFN-γ (Figure 4B). Levels of apoptotic gene upregulation were highest at 3 hours and then gradually decreased at 6 and 20 hours in IFN-γ-treated and IFN-γ+anti-Fas-treated relative to untreated BM cells. At 20 hours of culture, expression of apoptotic genes Fas, Caspase 1, Caspase 4, and Bax were lower in BM cells exposed to IFN-γ+anti-Fas antibody relative to BM cells exposed only to IFN-γ, suggesting that Fas engagement accelerated elimination of apoptotic cells (Figure 4B). Consistent with findings from BM cell culture, KSL cells exposed to IFN-γ in vitro also upregulated expression of caspases 1, 2, 3, 4, 7, and 12 and other proapoptotic genes (Figure 4C), whereas KSL cells from IFN-γ-injected mice in vivo showed elevated expression of Caspase 3 (Figure 4D). These differentially expressed genes are all components of a signaling network that is triggered by engagement of apoptotic receptors like Fas (supplemental Figure 3).
IFN-γ-augmented apoptotic gene expression. (A) Heat map of apoptotic gene expression in normal B6 BM cells cultured for 20 hours with 50 ng/mL IFN-γ (IFN-γ) or media only (control). (B) Differential expression of apoptotic genes in B6 BM cells after 20 hours of culture with 50 ng/mL IFN-γ (IFN-γ), 50 ng/mL IFN-γ + 1 μg/mL anti-Fas antibody (anti-Fas), or media control (control). (C) Apoptotic gene expression heat map for KSL cells from B6 donors after 20 hours of culture with or without 50 ng/mL IFN-γ. (D) Apoptotic gene expression heat map for KSL cells from B6 mice with or without IFN-γ injection (10 µg per mouse) 20 hours earlier.
IFN-γ-augmented apoptotic gene expression. (A) Heat map of apoptotic gene expression in normal B6 BM cells cultured for 20 hours with 50 ng/mL IFN-γ (IFN-γ) or media only (control). (B) Differential expression of apoptotic genes in B6 BM cells after 20 hours of culture with 50 ng/mL IFN-γ (IFN-γ), 50 ng/mL IFN-γ + 1 μg/mL anti-Fas antibody (anti-Fas), or media control (control). (C) Apoptotic gene expression heat map for KSL cells from B6 donors after 20 hours of culture with or without 50 ng/mL IFN-γ. (D) Apoptotic gene expression heat map for KSL cells from B6 mice with or without IFN-γ injection (10 µg per mouse) 20 hours earlier.
We also analyzed the effect of IFN-γ on hematopoietic gene expression. Normal KSL cells from B6 mice cultured with IFN-γ in vitro for 20 hours downregulated Ets-1 and other transcription factors and signaling molecules such Pax5, Runx1, and Stat 3 (supplemental Figure 4A). In B6 mice injected with IFN-γ, KSL cells downregulated Ets-1 and Gata2 and upregulated genes that affect Notch function (supplemental Figure 4B). Thus, IFN-γ exposure in vitro and in vivo reduced Ets-1 expression in KSL cells, consistent with a negative role of IFN-γ in hematopoiesis.
IFN-γ-augmented Fas expression and apoptosis in KLCD150+ cells
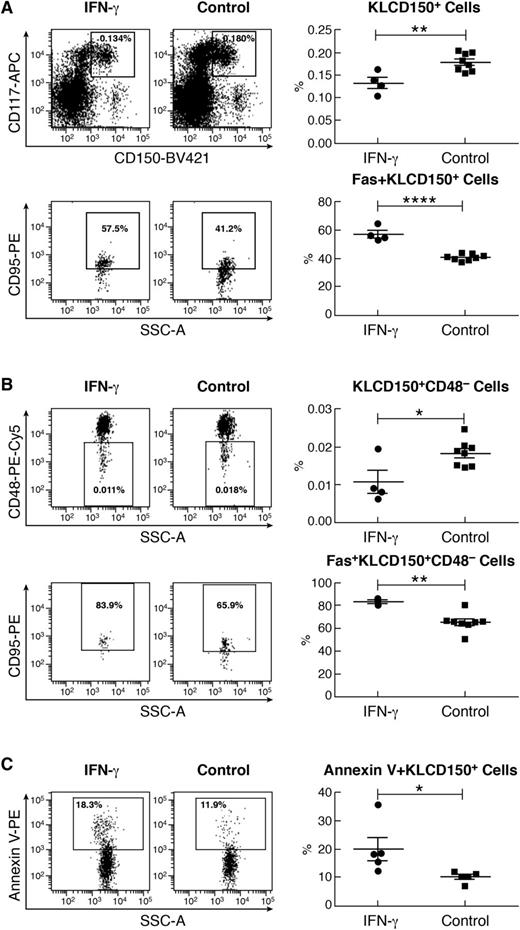
Because KSL cell engraftment was severely reduced after IFN-γ exposure, we reasoned that despite KSL cell expansion, IFN-γ might not stimulate hematopoiesis when other markers were used to define HSCs and progenitor cells. Toward this end, we measured Kit+Lin– (KL), KLCD150+, and KLCD150+CD48– cells along with KSL cells in a new set of mice with and without IFN-γ injection. Despite a marked increase in the proportion of KSL cells (supplemental Figure 5A), IFN-γ-treated mice had significantly decreased proportions of KL (supplemental Figure 5B), KLCD150+ (Figure 5A), and KLCD150+CD48– (Figure 5B) cells relative to untreated control mice. We determined that Fas expression was significantly upregulated in all hematopoietic cell fractions in response to IFN-γ treatment, including KSL (supplemental Figure 5A), KL (supplemental Figure 5B), KLCD150+ (Figure 5A), and KLCD150+CD48– (Figure 5B) populations. IFN-γ-mediated Fas upregulation was also apparent when measured by median fluorescent intensity (supplemental Table 2). Thus, IFN-γ augmented Fas expression on HSCs and progenitors regardless of cell surface markers used to detect them.
IFN-γ-upregulated Fas expression and Fas-mediated apoptosis in KLCD150+ cells. (A) IFN-γ-injected B6 mice (10 µg per mouse, n = 4) had a significantly lower proportion of KLCD150+ cells but a significantly higher proportion of Fas+ cells within the KLCD150+ population relative to untreated controls (n = 8). (B) Similarly, the proportion of KLCD150+CD48– cells was significantly lower but the proportion of Fas+ cells was significantly higher in the KLCD150+CD48– cell population in IFN-γ-injected mice than in controls. (C) Sorted KLCD150+ BM cells from IFN-γ-injected mice (n = 5) or untreated controls (n = 5) were mixed with activated CD4 and CD8 T cells (from BM failure mice) at a KLCD150+:CD4:CD8 ratio of 1:5:10 and cultured at 37°C with 5% CO2 for 60 minutes. The proportion of annexin V+ apoptotic KLCD150+ cells was significantly higher in IFN-γ-treated than in untreated control mice. *P < .05; **P < .01; ****P < .0001.
IFN-γ-upregulated Fas expression and Fas-mediated apoptosis in KLCD150+ cells. (A) IFN-γ-injected B6 mice (10 µg per mouse, n = 4) had a significantly lower proportion of KLCD150+ cells but a significantly higher proportion of Fas+ cells within the KLCD150+ population relative to untreated controls (n = 8). (B) Similarly, the proportion of KLCD150+CD48– cells was significantly lower but the proportion of Fas+ cells was significantly higher in the KLCD150+CD48– cell population in IFN-γ-injected mice than in controls. (C) Sorted KLCD150+ BM cells from IFN-γ-injected mice (n = 5) or untreated controls (n = 5) were mixed with activated CD4 and CD8 T cells (from BM failure mice) at a KLCD150+:CD4:CD8 ratio of 1:5:10 and cultured at 37°C with 5% CO2 for 60 minutes. The proportion of annexin V+ apoptotic KLCD150+ cells was significantly higher in IFN-γ-treated than in untreated control mice. *P < .05; **P < .01; ****P < .0001.
We further measured T-cell-mediated cell apoptosis in KLCD150+ cells. Upon interacting with activated CD4 and CD8 T cells for 60 minutes, a significantly larger proportion of KLCD150+ cells from IFN-γ-treated mice entered apoptosis relative to KLCD150+ cells from untreated control mice (Figure 5C). Thus, IFN-γ augmented apoptosis in both KSL (Figure 3) and KLCD150+ (Figure 5C) cells in the presence of activated T cells.
Attenuation of immune-mediated BM failure with IFN-γ/IFN-γR1 axis disruption
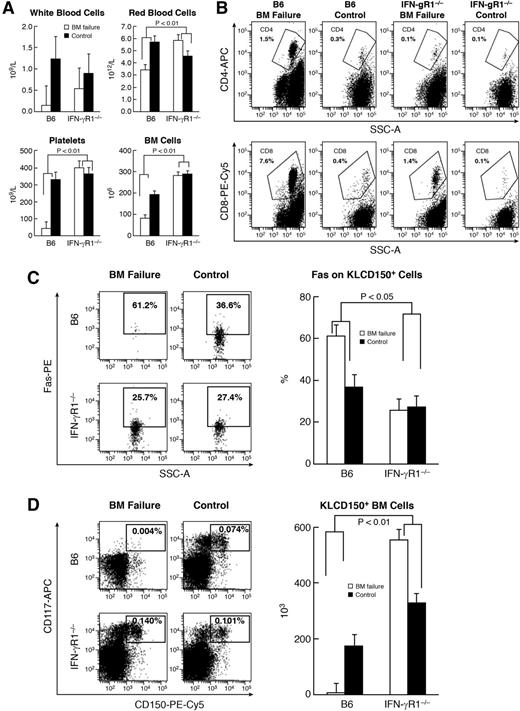
In animal models of immune-mediated BM failure, plasma IFN-γ levels were elevated, and administration of anti-IFN-γ antibody improved animal survival.34-36 In this study, we further tested the role of IFN-γ in BM failure. First, we used IFN-γR1−/− B6 mice as recipients to receive major histocompatibility complex–mismatched LN cells from FVB donors. B6 mice that received TBI plus FVB LN cells showed marked declines in white blood cells, red blood cells, platelets, and total BM cells relative to B6 mice that received TBI only (Figure 6A), as expected.47 In comparison, IFN-γR1−/− mice infused with FVB LN cells showed only slight declines in white blood cells without apparent change in red blood cells, platelets, or BM cells (Figure 6A). Attenuation in BM cell destruction in IFN-γR1−/− mice was accompanied by suppression of CD4 and CD8 T-cell expansion in the BM of IFN-γR1−/− mice infused with FVB LN cells (Figure 6B). In B6 mice, infusion of FVB LN cells caused a significant increase in Fas+ residual BM cells (CD4–CD8–) relative to TBI-only controls (Figure 6C). In IFN-γR1−/− mice, infusion of FVB LN cells did not cause Fas upregulation on residual BM cells relative to IFN-γR1−/− mice that had received TBI only (Figure 6C). Attenuated T-cell expansion and altered Fas upregulation on residual BM cells led to preservation of KLCD150+ (Figure 6D) and KSL cells (supplemental Figure 6) in IFN-γR1−/− recipients relative to B6 recipients after infusion of the same LN cells from FVB donors.
Attenuation of immune-mediated BM destruction with IFN-γR1 gene disruption. B6 mice and IFN-γR1−/− mice were treated with 6.5 Gy TBI (B6 control; n = 7; IFN-γR1−/− control; n = 10) or TBI plus infusion of 5 × 106 FVB LN cells (B6 BM failure; n = 10; IFN-γR1−/− BM failure; n = 8). Mice were bled and euthanized at day 12 to measure complete blood count (CBC) and cellular change. (A) B6 mice treated with FVB LN cells had severe declines in white blood cells (WBCs; 88%), RBCs (40%), platelets (40%), and total BM cells (57%) relative to TBI-only controls, whereas IFN-γR1−/− mice infused with FVB LN cells had only a 40% decline in WBCs relative to TBI-only controls without decreases in RBCs, platelets, or total BM cells, leading to significant differences (P < .01) between B6 and IFN-γR1−/− mice in response to the same number of infusions with FVB LN cells. (B) IFN-γR1−/− mice infused with FVB LN cells had lower proportions of CD4 and CD8 T cells (P < .05) in BM relative to B6 mice infused with FVB LN cells. (C) B6 mice infused with FVB LN cells (n = 6) had a significantly higher (P < .05) proportion of Fas+ cells in CD4–CD8– residual BM cells relative to their TBI-only controls (n = 4). IFN-γR1−/− mice (n = 5) infused with FVB LN cells showed no Fas upregulation on residual BM cells relative to their TBI-only controls (n = 6). (D) B6 mice, but not IFN-γR1−/− mice, infused with FVB LN cells had a significantly reduced proportion and total number of KLCD150+ hematopoietic stem and progenitor cells relative to their respective TBI-only controls, leading to significant treatment and strain interactions (P < .01).
Attenuation of immune-mediated BM destruction with IFN-γR1 gene disruption. B6 mice and IFN-γR1−/− mice were treated with 6.5 Gy TBI (B6 control; n = 7; IFN-γR1−/− control; n = 10) or TBI plus infusion of 5 × 106 FVB LN cells (B6 BM failure; n = 10; IFN-γR1−/− BM failure; n = 8). Mice were bled and euthanized at day 12 to measure complete blood count (CBC) and cellular change. (A) B6 mice treated with FVB LN cells had severe declines in white blood cells (WBCs; 88%), RBCs (40%), platelets (40%), and total BM cells (57%) relative to TBI-only controls, whereas IFN-γR1−/− mice infused with FVB LN cells had only a 40% decline in WBCs relative to TBI-only controls without decreases in RBCs, platelets, or total BM cells, leading to significant differences (P < .01) between B6 and IFN-γR1−/− mice in response to the same number of infusions with FVB LN cells. (B) IFN-γR1−/− mice infused with FVB LN cells had lower proportions of CD4 and CD8 T cells (P < .05) in BM relative to B6 mice infused with FVB LN cells. (C) B6 mice infused with FVB LN cells (n = 6) had a significantly higher (P < .05) proportion of Fas+ cells in CD4–CD8– residual BM cells relative to their TBI-only controls (n = 4). IFN-γR1−/− mice (n = 5) infused with FVB LN cells showed no Fas upregulation on residual BM cells relative to their TBI-only controls (n = 6). (D) B6 mice, but not IFN-γR1−/− mice, infused with FVB LN cells had a significantly reduced proportion and total number of KLCD150+ hematopoietic stem and progenitor cells relative to their respective TBI-only controls, leading to significant treatment and strain interactions (P < .01).
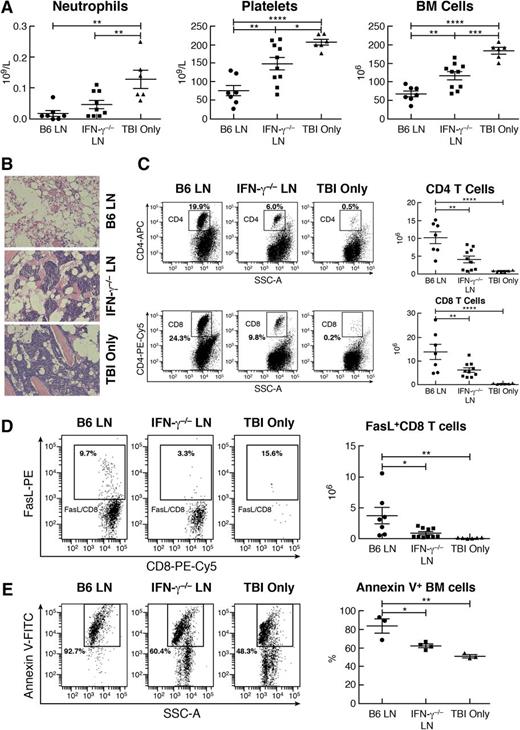
Next, we infused LN cells from B6 or IFN-γ−/− donors into C.B10 mice pre-irradiated with 5 Gy TBI. Relative to TBI controls, B6 LN cell treatment induced severe neutropenia, thrombocytopenia, and BM hypoplasia, similar to our previous report,37 whereas neutropenia, thrombocytopenia, and BM hypoplasia were far less severe in C.B10 recipients infused with IFN-γ−/− LN cells (Figure 7A). Sternal cavities appeared to be empty in recipients of B6 LN cells but remained cellular with clusters of hematopoietic cells in recipients of IFN-γ−/− LN cells (Figure 7B). CD4 and CD8 T-cell expansion was prominent in the BM of mice infused with B6 LN cells but was far less pronounced in the BM of mice infused with IFN-γ−/− LN cells (Figure 7C). A striking difference was a fivefold decrease in total BM FasL+CD8+ T cells in recipients of IFN-γ−/− LN cells relative to recipients of B6 LN cells (Figure 7D), which might account for the decline in residual BM cell apoptosis detected in recipients of IFN-γ−/− LN cells relative to recipients of B6 LN cells (Figure 7E).
Reduced efficacy of IFN-γ−/− LN cells in the induction of BM failure. C.B10 mice were treated with 5 Gy TBI (TBI only; n = 6), TBI plus 5 × 106 B6 LN cells (B6 LN; n = 7), or TBI plus 5 × 106 LN cells from IFN-γ−/− donors (IFN-γ−/−LN; n = 10) and were bled and euthanized on day 14 to measure CBC and cellular changes. (A) Recipients of IFN-γ−/− LN cells had more neutrophils, platelets, and total BM cells than did recipients of B6 LN cells. (B) Marrow cavities appeared to be empty in mice infused with B6 LN cells, had reduced cellularity but contained clusters of hematopoietic cells in mice treated with IFN-γ−/− LN cells, and had nearly normal cellularity in TBI-only control mice. (C) Proportion and total number of CD4+ T cells as well as proportion and total number of CD8+ T cells were significantly reduced in the BM of recipients infused with IFN-γ−/− LN cells than in the BM of recipients infused with B6 LN cells. (D) Total number of FasL+CD8 T cells was significantly lower in the BM of recipients infused with IFN-γ−/− LN cells than in the BM of recipients infused with B6 LN cells. (E) Residual (CD4–CD8–) BM cells from mice (n = 4) infused with IFN-γ−/− LN cells had a lower percentage of annexin V+ apoptotic cells than those from mice (n = 3) infused with B6 LN cells. *P < .05; **P < .01; ***P < .001; ****P < .0001.
Reduced efficacy of IFN-γ−/− LN cells in the induction of BM failure. C.B10 mice were treated with 5 Gy TBI (TBI only; n = 6), TBI plus 5 × 106 B6 LN cells (B6 LN; n = 7), or TBI plus 5 × 106 LN cells from IFN-γ−/− donors (IFN-γ−/−LN; n = 10) and were bled and euthanized on day 14 to measure CBC and cellular changes. (A) Recipients of IFN-γ−/− LN cells had more neutrophils, platelets, and total BM cells than did recipients of B6 LN cells. (B) Marrow cavities appeared to be empty in mice infused with B6 LN cells, had reduced cellularity but contained clusters of hematopoietic cells in mice treated with IFN-γ−/− LN cells, and had nearly normal cellularity in TBI-only control mice. (C) Proportion and total number of CD4+ T cells as well as proportion and total number of CD8+ T cells were significantly reduced in the BM of recipients infused with IFN-γ−/− LN cells than in the BM of recipients infused with B6 LN cells. (D) Total number of FasL+CD8 T cells was significantly lower in the BM of recipients infused with IFN-γ−/− LN cells than in the BM of recipients infused with B6 LN cells. (E) Residual (CD4–CD8–) BM cells from mice (n = 4) infused with IFN-γ−/− LN cells had a lower percentage of annexin V+ apoptotic cells than those from mice (n = 3) infused with B6 LN cells. *P < .05; **P < .01; ***P < .001; ****P < .0001.
Discussion
In this study, we explored the role of IFN-γ as a mediator of hematopoietic cell apoptosis and destruction. First, we observed that IFN-γ reduced numbers of KLCD150+ and KLCD150+CD48– cells and of functional HSCs, despite apparent expansion of KSL and SKSL cells. Second, apoptotic stimuli, such as anti-Fas antibody and FasL-bearing activated CD4 and CD8 T cells promoted apoptosis and elimination of IFN-γ-expanded KSL cells both in vitro and in vivo; activated T cells also promoted apoptosis of KLCD150+ cells from IFN-γ-treated mice. Third, expression of Fas and other proapoptotic and pro–cell death genes were upregulated in KSL cells and BM cells after IFN-γ exposure both in vitro and in vivo. Fourth, disruption of IFN-γ/IFN-γR1 signaling considerably attenuated immune-mediated BM failure in respective mouse models. These observations support IFN-γ as a stimulator of Fas-mediated hematopoietic cell apoptosis.
Although treatment with a single dose of IFN-γ caused great expansions of phenotypic KSL and SKSL cells, similar to previous reports,26,27 functional HSCs were markedly reduced by IFN-γ. This result is in good agreement with previous findings, in which whole BM cells from INF-γ-treated mice showed reduced engraftment27 and ex vivo IFN-γ-expanded KSL cells failed to engraft.40 Although KSL and SKSL cells are enriched for functional HSCs in normal mice,48-50 there were precedents in the literature for marked phenotype/function discordance.51-53 IFN-γ stimulates Sca-1 expression,26,54 and HSCs and progenitor cells can be defined by other cell surface markers.50,55 Thus, IFN-γ in our experiments reduced HSCs and hematopoietic progenitor cells as defined by the KLCD150+ and KLCD150+CD48– markers, as reported previously.40
Loss of normal HSC function after IFN-γ treatment suggests that IFN-γ plays a negative role in the regulation of hematopoiesis. This view is well supported by historic observations.18-23 In one recent study, IFN-γ appeared to impair HSC proliferation through downregulation of the Socs-Tpo-Stat5 signaling pathway.40 In another report, constitutive IFN-γ expression, achieved by deletion of an IFN-γ-adenylate-uridylate-rich element, resulted in AA with disruption of multilineage differentiation.43 Gene expression analyses revealed downregulation of Gata2, Pax5, Stat3, and Ets-1 in IFN-γ-expanded KSL cells; Ets-1 was downregulated in KSL cells treated with IFN-γ both in vitro and in vivo. As a key member of the Ets family of transcription factors, Ets-1 is a positive regulator of hematopoietic differentiation and lineage specification.56-61 Therefore, the effect of IFN-γ-mediated Ets-1 downregulation is consistent with IFN-γ as a negative regulator, acting directly on murine HSCs.
The putative role of IFN-γ in human AA has been modeled in animals,34,35 including mitigation of pancytopenia by treatment with anti-IFN-γ antibody.36 IFN-γ sensitizes target cells to killing by diverse mechanisms, including modulation of HLA expression,62,63 production of nitric oxide,3 activation of caspase death pathways,64-66 and induction of Fas expression.44-46,67 We previously reported that Fas/FasL is the major cell death pathway for marrow destruction in mouse models of immune-mediated BM failure,68 including as innocent bystanders.35 Results from this study are in agreement with our previous reports and those of others concerning IFN-γ in the development of BM failure.
In a recent publication, acute IFN-α exposure appeared to drive HSC proliferation by transient relaxation of HSC quiescence and rapid return of HSCs to quiescence to protect the HSC pool from exhaustion.69 In addition to literature concerning Fas-mediated cell apoptosis, there are reports of Fas-mediated cell proliferation under special circumstances.70-72 Thus, the fate of cells with Fas upregulation may be contextual to the environment. In some circumstances, particularly in response to infection, IFN-γ-expanded hematopoietic cells might usefully contribute to production of differentiated myeloid cells.25,27,73,74 However, under conditions of immune-mediated BM failure, in which activated FasL-bearing cytotoxic T cells expand in the local BM environment, IFN-γ would be deleterious because it drives Fas upregulation on hematopoietic cell components in the BM and serves as a critical component of an aberrant immune response that prepares hematopoietic cells for destruction mediated by Fas/FasL and other signals.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the National Heart, Lung, and Blood Institute Intramural Research Program, National Institutes of Health.
Authorship
Contribution: J.C. designed research, performed experiments, analyzed data, and wrote the paper; X.F. performed experiments and analyzed data; M.J.D. performed experiments; K.K. performed cell sorting; and N.S.Y. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jichun Chen, Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, NIH Bldg 10, Clinical Research Center, Room 3E-5272, 10 Center Dr, Bethesda, MD 20892-1202; e-mail: chenji@nhlbi.nih.gov.