Key Points
An improvement of 4-year OS for acute and lymphoma types of ATL was observed in comparison with that of the 1991 report.
The prognosis of the smoldering type ATL was worse than expected from the 1991 report.
Abstract
Adult T-cell leukemia/lymphoma (ATL) is a malignancy of mature T lymphocytes caused by human T-lymphotropic virus type I. Intensive combination chemotherapy and allogeneic hematopoietic stem cell transplantation have been introduced since the previous Japanese nationwide survey was performed in the late 1980s. In this study, we delineated the current features and management of ATL in Japan. The clinical data were collected retrospectively from the medical records of patients diagnosed with ATL between 2000 and 2009, and a total of 1665 patients’ records were submitted to the central office from 84 institutions in Japan. Seventy-one patients were excluded; 895, 355, 187, and 157 patients with acute, lymphoma, chronic, and smoldering types, respectively, remained. The median survival times were 8.3, 10.6, 31.5, and 55.0 months, and 4-year overall survival (OS) rates were 11%, 16%, 36%, and 52%, respectively, for acute, lymphoma, chronic, and smoldering types. The number of patients with allogeneic hematopoietic stem cell transplantation was 227, and their median survival time and OS at 4 years after allogeneic hematopoietic stem cell transplantation was 5.9 months and 26%, respectively. This study revealed that the prognoses of the patients with acute and lymphoma types were still unsatisfactory, despite the recent progress in treatment modalities, but an improvement of 4-year OS was observed in comparison with the previous survey. Of note, one-quarter of patients who could undergo transplantation experienced long survival. It is also noted that the prognosis of the smoldering type was worse than expected.
Introduction
Adult T-cell leukemia/lymphoma (ATL) is a peripheral T-cell malignancy caused by human T-cell lymphotropic virus type I (HTLV-1).1-3 Southwestern Japan is one of the most endemic areas for the malignancy, along with the Caribbean basin, Central and South America, and Western Africa. The Japan Clinical Oncology Group-Lymphoma Study Group proposed 4 clinical subtypes, namely, acute, lymphoma, chronic, and smoldering, based on nationwide surveys of patients with ATL who were newly diagnosed from 1983 to 1987 (1991 database).4 Moreover, patients with the chronic type are further divided into 2 categories by the presence of any unfavorable prognostic factors, defined by levels of blood urea nitrogen (BUN) or lactate dehydrogenase (LDH) at higher than the upper limit of normal or having albumin levels lower than the lower limit of normal. Patients with acute, lymphoma, and chronic type with unfavorable prognostic factors, and those with chronic type without unfavorable prognostic factors and with smoldering type, are categorized as having aggressive and indolent ATL, respectively.5 Many patients with indolent ATL have been treated by dermatologists as a result of the frequent presence of cutaneous lesions.
In terms of treatment, an international consensus meeting recommended first-line treatment with multiagent chemotherapies with or without subsequent allogeneic hematopoietic stem cell transplantation (allo-HSCT) for patents with aggressive ATL. In patients with indolent ATL, interferon α combined with zidovudine (IFN/AZT) or watchful waiting are recommended if patients are symptomatic, and watchful waiting alone is recommended if patients are asymptomatic.
In this study, we delineated the current clinical features and practical management of patients with ATL in Japan by conducting a nationwide survey of patients diagnosed from 2000 to 2009.
Patients and methods
Patients
We conducted the ATL–Prognostic Index (ATL-PI) Project, a nationwide retrospective survey of patients with ATL newly diagnosed between January 1, 2000, and May 31, 2009, by hematologists in participating hospitals in Japan for the purpose of establishing a PI for patients with ATL.6 The diagnostic criteria for ATL in this study are seropositivity of anti-HTLV-1 antibody and histologically or cytologically proven peripheral T-cell malignancy. We further collected clinical information on patients cared for by dermatologists with chronic and smoldering types who were newly diagnosed during the same period for the ATL-PI Project in 3 major hospitals located on Kyushu Island, on which HTLV-1 is highly endemic. Detailed information on the features of skin eruptions, using the definition from Sawada et al7 , was collected in patients treated by dermatologists. All clinical data as well as the validity of diagnosis of ATL were centrally reviewed by 2 expert hematologists.
Statistical analysis
Baseline characteristics were summarized by frequency distributions and descriptive statistics. The current data were compared with the 1991 database4 for age survival. Overall survival (OS) was calculated from the time of diagnosis to the date of death from any cause or to the date of last follow-up. Survival curves were calculated by the Kaplan-Meier method. Differences in Kaplan-Meier curves were assessed using the log-rank test. A 2-sided value of P < .05 was considered statistically significant. The Bonferroni correction was used for a multiple-comparison correction. Analyses were performed using JMP software, version 9 (SAS Institute, Cary, NC).
Approval of the study was obtained from the Ethics Committee and Institutional Review Board of the coordinating center (Fukuoka University) and at each participating center based on their institutional policies. Research was in accordance with the Declaration of Helsinki.
Results
Cases
A total of 1665 patients’ (1552 from hematologists and 113 from dermatologists) records were sent to the central office from 84 institutions in Japan. In total, 1270 and 395 patients were confirmed to have either acute or lymphoma and chronic or smoldering types of ATL, respectively, by central review. Seventy-one patients were excluded for the following reasons: 30 for loss of data, 35 for diagnostic error, and 6 for double registration. Finally, 895, 355, 187, and 157 patients with acute, lymphoma, chronic, and smoldering types remained, respectively, and they were analyzed in this study (Figure 1). Baseline characteristics are shown in Table 1. The rates of concordance in determining subtype between institutional diagnosis and central review were 92%, 70%, 85%, and 86% for acute, lymphoma, chronic, and smoldering types, respectively (supplemental Table 1).
Flowchart of patients. In total, 1552 and 113 patients were collected by hematologists and dermatologists, respectively. Overall, 1270 patients diagnosed with acute or lymphoma types ATL were registered. Of these, 20 patients were excluded. A total of 395 patients diagnosed with chronic or smoldering types were registered, and 51 patients were excluded. Finally, 895, 355, 187, and 157 patients were diagnosed with acute, lymphoma, chronic, and smoldering types, respectively.
Flowchart of patients. In total, 1552 and 113 patients were collected by hematologists and dermatologists, respectively. Overall, 1270 patients diagnosed with acute or lymphoma types ATL were registered. Of these, 20 patients were excluded. A total of 395 patients diagnosed with chronic or smoldering types were registered, and 51 patients were excluded. Finally, 895, 355, 187, and 157 patients were diagnosed with acute, lymphoma, chronic, and smoldering types, respectively.
Baseline characteristics of all patients enrolled in this study
| Characteristic . | Acute (n = 895) . | Lymphoma (n = 355) . | Chronic (n = 187) . | Smoldering (n = 157) . | n . |
|---|---|---|---|---|---|
| Age, median, years | 63 | 66 | 61 | 67 | 1594 |
| Sex, female, % | 48 | 41 | 49 | 45 | 1594 |
| White blood cell count, median, ×109/L | 11.2 | 6.2 | 14.8 | 6.6 | 1594 |
| Neutrophil count, median, ×109/L | 5.6 | 4.4 | 4.4 | 3.6 | 1594 |
| Hemoglobin level, median, g/dL | 13.3 | 13.1 | 13.5 | 13.7 | 1581 |
| Platelet count, median, × 109/L | 189 | 23.1 | 22.5 | 21.9 | 1594 |
| Serum total protein, median, g/dL | 6.4 | 6.8 | 7 | 7.2 | 1466 |
| Serum albumin, median, g/dL | 3.6 | 3.8 | 4.2 | 4.3 | 1441 |
| BUN, median, mg/dL | 15.7 | 15.1 | 12.9 | 14.4 | 1489 |
| LDH, median, IU/L | 665 | 459 | 291 | 234 | 1594 |
| LDH > 2 × ULN, % | 60 | 43 | 0 | 0 | 1594 |
| Soluble IL-2R, median, U/mL | 27 500 | 11 017 | 5190 | 1320 | 1455 |
| Hypercalcemia present | 41 | 17 | 0 | 0 | 1576 |
| Ann Arbor stage, I-II,% | 5 | 23 | 1 | 12 | 1594 |
| ECOG PS, 0-1, % | 50 | 65 | 92 | 96 | 1586 |
| Monoclonal integration of HTLV-1 proviral DNA present, % | 96 | 94 | 95 | 91 | 761 |
| B symptoms present, % | 32 | 30 | 15 | 3 | 1578 |
| Number of lymph node lesions, median | 2 | 3 | 0 | 0 | 1594 |
| Number of extranodal sites, median | 1 | 1 | 1 | 1 | 1594 |
| Skin lesion involvement present, % | 25 | 10 | 41 | 76 | 1594 |
| Pulmonary involvement present, % | 11 | 6 | 6 | 2 | 1594 |
| Liver involvement present, % | 16 | 6 | 2 | 0 | 1594 |
| Spleen involvement present, % | 22 | 10 | 9 | 0 | 1594 |
| Pleural effusion involvement present, % | 11 | 8 | 0 | 0 | 1594 |
| Ascites involvement present, % | 7 | 7 | 0 | 0 | 1594 |
| Bone involvement present, % | 5 | 5 | 0 | 0 | 1594 |
| Gastric involvement present, % | 10 | 9 | 0 | 0 | 1594 |
| Central nervous system involvement present, % | 1 | 0 | 0 | 0 | 1594 |
| Characteristic . | Acute (n = 895) . | Lymphoma (n = 355) . | Chronic (n = 187) . | Smoldering (n = 157) . | n . |
|---|---|---|---|---|---|
| Age, median, years | 63 | 66 | 61 | 67 | 1594 |
| Sex, female, % | 48 | 41 | 49 | 45 | 1594 |
| White blood cell count, median, ×109/L | 11.2 | 6.2 | 14.8 | 6.6 | 1594 |
| Neutrophil count, median, ×109/L | 5.6 | 4.4 | 4.4 | 3.6 | 1594 |
| Hemoglobin level, median, g/dL | 13.3 | 13.1 | 13.5 | 13.7 | 1581 |
| Platelet count, median, × 109/L | 189 | 23.1 | 22.5 | 21.9 | 1594 |
| Serum total protein, median, g/dL | 6.4 | 6.8 | 7 | 7.2 | 1466 |
| Serum albumin, median, g/dL | 3.6 | 3.8 | 4.2 | 4.3 | 1441 |
| BUN, median, mg/dL | 15.7 | 15.1 | 12.9 | 14.4 | 1489 |
| LDH, median, IU/L | 665 | 459 | 291 | 234 | 1594 |
| LDH > 2 × ULN, % | 60 | 43 | 0 | 0 | 1594 |
| Soluble IL-2R, median, U/mL | 27 500 | 11 017 | 5190 | 1320 | 1455 |
| Hypercalcemia present | 41 | 17 | 0 | 0 | 1576 |
| Ann Arbor stage, I-II,% | 5 | 23 | 1 | 12 | 1594 |
| ECOG PS, 0-1, % | 50 | 65 | 92 | 96 | 1586 |
| Monoclonal integration of HTLV-1 proviral DNA present, % | 96 | 94 | 95 | 91 | 761 |
| B symptoms present, % | 32 | 30 | 15 | 3 | 1578 |
| Number of lymph node lesions, median | 2 | 3 | 0 | 0 | 1594 |
| Number of extranodal sites, median | 1 | 1 | 1 | 1 | 1594 |
| Skin lesion involvement present, % | 25 | 10 | 41 | 76 | 1594 |
| Pulmonary involvement present, % | 11 | 6 | 6 | 2 | 1594 |
| Liver involvement present, % | 16 | 6 | 2 | 0 | 1594 |
| Spleen involvement present, % | 22 | 10 | 9 | 0 | 1594 |
| Pleural effusion involvement present, % | 11 | 8 | 0 | 0 | 1594 |
| Ascites involvement present, % | 7 | 7 | 0 | 0 | 1594 |
| Bone involvement present, % | 5 | 5 | 0 | 0 | 1594 |
| Gastric involvement present, % | 10 | 9 | 0 | 0 | 1594 |
| Central nervous system involvement present, % | 1 | 0 | 0 | 0 | 1594 |
ECOG PS, Eastern Cooperative Oncology Group performance status; IL-2R, interleukin-2 receptor; ULN, upper limit of normal.
Monoclonal integration of HTLV-1 proviral DNA was detected in 95% (722/761) among 48% (761/1594) of the patients who were examined. In detail, it was detected in 96% (379/393), 94% (144/153), 95% (90/95), and 91% (109/120) of patients with acute, lymphoma, chronic, and smoldering types, respectively, and in 88% (28/32), 95% (35/37), 91% (69/76), and 96% (590/616) of patients with Ann Arbor stages I, II, III, and IV, respectively.
Deaths were observed in 710 (79%), 254 (72%), 94 (50%), and 70 patients (45%), and median follow-up times were 7.2, 8.9, 19.8, and 27.4 months for acute, lymphoma, chronic, and smoldering types, respectively. The median survival times (MSTs) were 8.3 (95% confidence interval [CI], 7.5-8.9), 10.6 (95% CI, 9.3-11.9), 31.5 (95% CI, 25.9-41.1), and 55.0 (95% CI, 36.6-90.4) months, and the 4-year OS rates were 11%, 16%, 36%, and 52% for acute, lymphoma, chronic, and smoldering types, respectively (Figure 2). The most common cause of death was progressive disease, at rates of 77%, 79%, 67%, and 76%, whereas the rates of death from infection without disease progression were 14%, 12%, 15%, and 9% for acute, lymphoma, chronic, and smoldering types, respectively.
Management and outcome for acute and lymphoma types of ATL
A total of 95% (1186/1250) of patients with acute and lymphoma types received treatments. Among them, 98% (n = 1168) of patients received chemotherapy, and the remaining 2% (n = 18) received radiation therapy to their limited lesions. A combination of cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP), or a CHOP-like regimen was the most commonly used treatment (n = 579; 50%): 439 and 140 patients received tri-weekly CHOP (CHOP21) and bi-weekly CHOP (CHOP14), respectively, followed by VCAP-AMP-VECP (a sequential combination chemotherapy consisting of vincristine, cyclophosphamide, doxorubicin, and prednisolone; doxorubicin, ranimustine, and prednisolone; and vindesine, etoposide, carboplatin, and prednisolone; n = 365; 31%),8 ATL-G-CSF (vincristine, vindesine, doxorubicin, mitoxantrone, cyclophosphamide, etoposide, ranimustine, and prednisolone with prophylactic support by granulocyte-colony stimulating factor; n = 56; 5%),9 and modified EPOCH (mEPOCH; etoposide, prednisolone, vincristine, carboplatin, and doxorubicin; carboplatin was substituted for cyclophosphamide; n = 42, 4%). Single-agent chemotherapy, such as etoposide and sobuzoxane, was used in 61 patients (5%). The overall response rates, MST, and 4-year OS by the chemotherapeutics frequently used are shown in supplemental Table 2.
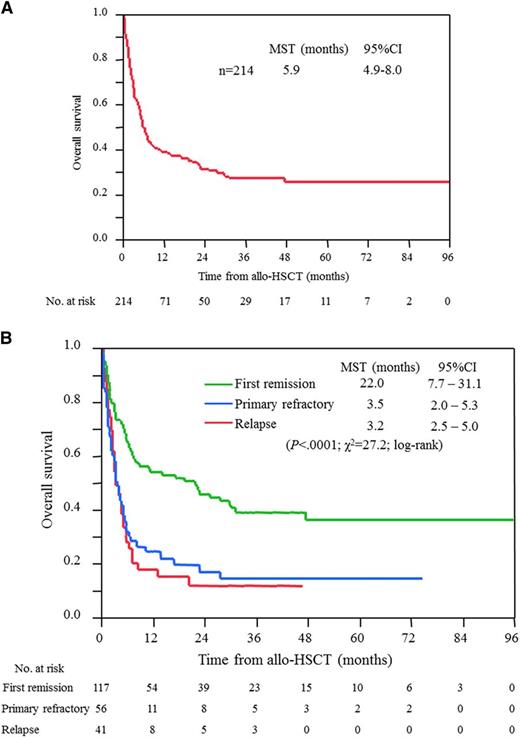
Two hundred twenty-seven patients with acute and lymphoma types received allo-HSCT; among them, 13 patients had inadequate data at transplantation. Allo-HSCT was performed in 33% of patients who were aged 65 years or younger, whereas it was performed in 1% of patients older than 65 years with acute and lymphoma types (median age, 52 years; range, 25-68 years; supplemental Table 3). MSTs from the date of diagnosis for ATL, and those from transplantation, were 14.0 (95% CI, 12.1-17.9) and 5.9 (95% CI, 4.9-8.0) months, respectively; however, patients who had undergone allo-HSCT experienced long survival, with 4-year OS of 26%, as shown in Figure 3A. The sources of HSC were related, unrelated, and cord blood in 101, 78, and 36 patients, respectively and the MSTs from transplantation were 6.8 (95% CI 5.3-19.2), 5.0 (95% CI 3.2-8.7), and 5.3 (95% CI, 1.9-21.5) months, respectively (supplemental Figure 1). In total, 117, 56, and 41 patients underwent allo-HSCT in the first remission, primary refractory, and relapse, respectively. Importantly, their MSTs from transplantation clearly differed by status: 22 months for patients with first remission at the time of transplantation and 3 months for the others (Figure 3B).
OS for patients with acute and lymphoma types of ATL who received allo-HSCT. (A) All patients, and (B) patients according to disease status at the time of allo-HSCT. Survival time is calculated from the date of allo-HSCT.
OS for patients with acute and lymphoma types of ATL who received allo-HSCT. (A) All patients, and (B) patients according to disease status at the time of allo-HSCT. Survival time is calculated from the date of allo-HSCT.
Management and outcome for chronic and smoldering types of ATL
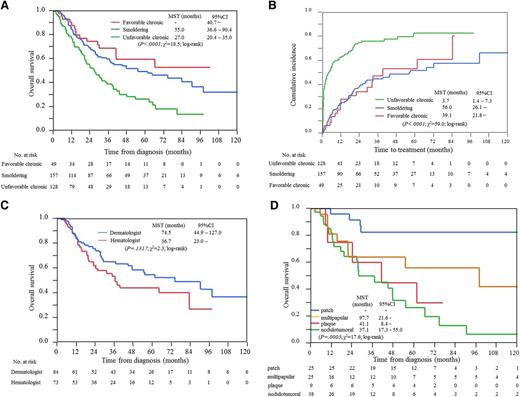
Of 187 patients with chronic type, data for determination of unfavorable factors were not available in 10 patients; 49 (28%) and 128 (72%) patients were finally diagnosed with the favorable and unfavorable chronic types, respectively, and 172 and 15 patients were treated by hematologists and dermatologists, respectively. The MSTs and 4-year OS for favorable and unfavorable chronic types and patients with smoldering type were not reached (95% CI, 40.7 to not estimable), 27.0 (95% CI, 20.4-35.0), and 55.0 (95% CI, 36.6-90.4) months, and 60%, 29%, and 52%, respectively (Figure 4A).
Analysis of chronic and smoldering types of ATL. (A) OS. Patients with unfavorable chronic type have at least one of the following factors: BUN or LDH higher than the upper limit of normal or albumin lower than the lower limit of normal. (B) Cumulative incidence of the introduction of systemic chemotherapies. (C) OS for patients with smoldering type treated by hematologists or dermatologists. (D) OS of patients with smoldering type treated by dermatologists according to eruption type. The data of purpura type are not shown because the number of patients was too small (n = 2). Hematologists, patients treated by hematologists; Dermatologists, patients treated by dermatologists.
Analysis of chronic and smoldering types of ATL. (A) OS. Patients with unfavorable chronic type have at least one of the following factors: BUN or LDH higher than the upper limit of normal or albumin lower than the lower limit of normal. (B) Cumulative incidence of the introduction of systemic chemotherapies. (C) OS for patients with smoldering type treated by hematologists or dermatologists. (D) OS of patients with smoldering type treated by dermatologists according to eruption type. The data of purpura type are not shown because the number of patients was too small (n = 2). Hematologists, patients treated by hematologists; Dermatologists, patients treated by dermatologists.
A total of 55% of patients with chronic and smoldering types received chemotherapy at a median follow-up time of 22.8 months. The median times to the first-line systemic chemotherapy from diagnosis, which are not exactly the same but generally correspond to the times for transformation to aggressive ATL, were 39.1 (95% CI, 21.8 to not estimable) and 56.0 (95% CI, 26.1 to not estimable) months in patients with favorable chronic and smoldering types, respectively (Figure 4B). On the other hand, that for unfavorable chronic types were 3.7 (95% CI, 1.4-7.3) months. The most commonly used chemotherapy was the CHOP regimen (n = 58), followed by mLSG15 (n = 55), single agent (n = 36), and ATL-G-CSF (supplemental Table 4). The MST and 4-year OS from the start of the first-line systemic chemotherapy were 26.4 (95% CI, 9.0 to not estimable), 13.1 (95% CI, 8.5-16.8), and 8.9 (95% CI, 7.0-11.3) months and 48%, 11%, and 7% in patients with favorable and unfavorable chronic types and smoldering type, respectively. Among chronic and smoldering types, 26 patients eventually received allo-HSCT, and their MST and 4-year OS from transplantation were 6.2 (95% CI, 2.9-19.6) months and 29.9%, respectively.
Among 157 patients with smoldering type, all patients (n = 84) treated by dermatologists and 35 of 73 patients treated by hematologists had skin lesions. The MSTs and 4-year OS in patients with smoldering type treated by hematologists and dermatologists were 36.7 (95% CI, 23.0 to not estimable) and 74.5 (95% CI, 44.9-127.0) months, and 44% and 58%, respectively (Figure 4C). Among 99 patients with chronic and smoldering types treated by dermatologists, nodulotumoral, multipapular, patch, plaque, and purpura types were observed in 38%, 25%, 25%, 9%, and 2%, respectively, whereas erythroderma was not observed in patients enrolled in this study. Survival curves according to skin eruptions are shown in Figure 4D, which showed a significant difference between patch and plaque or nodulotumoral, using a Bonferroni corrected α = .017 (P = .008 and P < .0001, respectively). The most commonly used skin-directed treatment was topical steroids, which were applied in 52 patients, followed by narrow-band ultraviolet B therapy in 17, psoralen photochemotherapy in 15, electron beam therapy in 11, radiation therapy in 6, and resection in 4 patients.
Comparison between 1991 database and current study
Because mean age, but not median age at diagnosis, was shown in the 1991 database,4 the comparison of mean age between the 1991 database and the current one revealed that patients are getting older, with shifts from 56 to 63, 59 to 66, 58 to 61, and 59 to 67 years in patients with acute, lymphoma, chronic, and smoldering types, respectively. It was reported in the 1991 database that the MSTs and 4-year OS were 6.2 months and 5.0% for acute type and 10.2 months and 5.7% for lymphoma type, respectively. In this study, the equivalent values were 8.3 months and 11.4% for acute and 10.6 months and 16.2% for lymphoma type, respectively. MST and 4-year OS for patients with smoldering type were not reached and were 62.3% in the 1991 database and 55 months and 51.9% in this study, respectively (Table 2).
A comparison of the prognosis between the 1991 database and the current study
| . | 1991 database4 (1983-1987) . | Current study (2000-2009) . | ||
|---|---|---|---|---|
| Total . | Without allo-HSCT . | With allo-HSCT . | ||
| Acute | ||||
| n | 465 | 895 | 717 | 178 |
| Mean age, years | 56.0 | 63 | 66 | 51 |
| MST, months | 6.2 | 8.3 | 6.7 | 14.0 |
| 4-year OS, % | 5.0 | 11.4 | 6.8 | 27.8 |
| Lymphoma | ||||
| n | 156 | 355 | 306 | 49 |
| Mean age, years | 59.2 | 66 | 69 | 53 |
| MST, months | 10.2 | 10.6 | 9.7 | 13.9 |
| 4-year OS, % | 5.7 | 16.2 | 13.7 | 32.3 |
| Chronic | ||||
| n | 152 | 187 | ||
| Mean age, years | 57.5 | 61 | ||
| MST, months | 24.3 | 31.5 | ||
| 4-year OS, % | 26.9 | 35.6 | ||
| Smoldering | ||||
| n | 45 | 157 | ||
| Mean age, years | 59.3 | 67 | ||
| MST, months | not reached | 55.0 | ||
| 4-year OS, % | 62.8 | 51.9 | ||
| . | 1991 database4 (1983-1987) . | Current study (2000-2009) . | ||
|---|---|---|---|---|
| Total . | Without allo-HSCT . | With allo-HSCT . | ||
| Acute | ||||
| n | 465 | 895 | 717 | 178 |
| Mean age, years | 56.0 | 63 | 66 | 51 |
| MST, months | 6.2 | 8.3 | 6.7 | 14.0 |
| 4-year OS, % | 5.0 | 11.4 | 6.8 | 27.8 |
| Lymphoma | ||||
| n | 156 | 355 | 306 | 49 |
| Mean age, years | 59.2 | 66 | 69 | 53 |
| MST, months | 10.2 | 10.6 | 9.7 | 13.9 |
| 4-year OS, % | 5.7 | 16.2 | 13.7 | 32.3 |
| Chronic | ||||
| n | 152 | 187 | ||
| Mean age, years | 57.5 | 61 | ||
| MST, months | 24.3 | 31.5 | ||
| 4-year OS, % | 26.9 | 35.6 | ||
| Smoldering | ||||
| n | 45 | 157 | ||
| Mean age, years | 59.3 | 67 | ||
| MST, months | not reached | 55.0 | ||
| 4-year OS, % | 62.8 | 51.9 | ||
Discussion
In this study, clinical features and care for patients with ATL were shown using retrospectively collected data of 1665 patients newly diagnosed from 2000 to 2009 in Japan. Importantly, this is the largest study elucidating practical management and its outcome for patients with ATL. This study includes 15% to 20% of entire ATL patients diagnosed during the period of this study estimated from the annual incidence of this disease.10 Because the monoclonal integration of HTLV-1 by Southern blotting has not always been evaluated for the diagnosis of ATL in practice, it might raise a concern about the possible migration of non-ATL peripheral T-cell lymphoma occurring in HTLV-1-infected individuals, especially in lymphoma type. However, 52% of all patients and 43% of lymphoma patients registered in this study were examined for the integration of HTLV-1 provirus DNA in tumor cells, and its monoclonal integration was detected in 95% and 94% of these patients, respectively. In consideration of the sensitivity of the test and the possibility of inadequate sampling of specimens, these facts confirmed the accuracy of diagnosis for ATL in the patients included in this study.
Among acute and lymphoma types, the most used multiagent chemotherapy was CHOP21, followed by CHOP14, VCAP-AMP-VECP, ATL-G-CSF, and mEPOCH. The overall response rate and MSTs of these treatments seem to be similar, but the 4-year OS was significantly better in patients receiving ATL-G-CSF or mEPOCH, probably because these regimens were applied to younger patients who more frequently underwent allo-HSCT than patients treated by other regimens. No patients with acute and lymphoma types received IFN/AZT because of the lack of approval of both agents for the treatment of ATL under the national health insurance system in Japan.
Allo-HSCT for patients with aggressive ATL has been reported to have the potential to cure some patients, as shown by their 3-year OS of approximately 35%, whereas allo-HCST caused high transplant-related mortality of up to or more than 40%.11-14 The Japanese guidelines indicate that allo-HSCT should be considered as postremission therapy in patients experiencing a response to induction therapy. In general, a myeloablative conditioning regimen is considered an option for patients aged 55 years and younger, whereas a reduced-intensity conditioning regimen was recommended for those aged 70 years and younger. In our study, MST and 4-year OS from transplantation were 5.9 months and 26%, respectively, with a significant difference according to disease status at the time of transplantation. This indicates that patients who can receive allo-HSCT at the first remission have a higher possibility of achieving prolonged survival. Of course, it should be taken into consideration that, as far as performing a retrospective comparison between patients with or without allo-HSCT, huge biases in both cohorts of patients are inevitable; however, these results suggest that allo-HSCT should be performed for eligible patients during the first remission outside a clinical trial.
Among chronic and smoldering types, standard care for indolent ATL in Japan is watchful waiting, with skin-directed therapy if needed, whereas that for unfavorable chronic type is the same as for acute and lymphoma types. Surprisingly, about 40% of patients with unfavorable chronic type remained free from systemic chemotherapy more than 1 year from diagnosis, suggesting diverse clinical courses especially for this cohort of patients. We are now attempting to elucidate the clinical features that are related to the withholding of systemic chemotherapy after the diagnosis of unfavorable chronic type, and factors that are related to the period from diagnosis to transformation to aggressive ATL for patients with indolent ATL in our ongoing analysis.
The type of skin eruption has been reported as an independent prognostic indicator by analyzing 4 clinical subtypes altogether: the erythrodermic type was the poorest, followed by the nodulotumoral and multipapular types. Although the patch and plaque types were reported to be associated with better prognosis than the others, all patients with the erythrodermic type had the acute type, and most of those with patch type had the smoldering type in the study.7 The current study showed similar findings by analyzing in patients limited to chronic and smoldering ATL: No patients with erythroderma were observed in this cohort of patients, and the prognosis of patients with patch type was better than for the others.
A poorer prognosis in the smoldering type with skin manifestations has been suggested.7,15-20 Our results showed that the prognosis of patients presenting with patch type was better than that of the other skin manifestations. However, no significant difference was recognized between the presence and absence of skin manifestations in both patients with chronic and smoldering types (data not shown). These results indicate that the type of skin lesions, rather than the presence, might predict prognosis in patients with smoldering type.
Of note, there are 3 significant findings upon comparison with the 1991 database. First, an increase of the age at diagnosis was confirmed. It has already been reported,21 and it is suggested that it can be explained by the high HTLV-1 prevalence in elderly people resulting from the decrease of HTLV-1 carriers among the young as a result of the birth cohort effects, as well as the continual development of ATL from this pool of HTLV-1-infected elderly individuals.22 Second, an improvement of 4-year OS, but not MST, was observed in patients with acute type who had allo-HSCT, and in patients with lymphoma type, even in the patients who did not have allo-HSCT. These data suggest that the outcome of a particular proportion of patients who are primarily resistant to chemotherapy or not suitable for appropriate treatment of various reasons, such as poor performance status, organ insufficiency, and infectious complications, has not improved. In contrast, those who could be managed successfully by the first-line treatment could survive for a longer time via the incorporation of allo-HSCT for patients with acute type and the establishment of more effective chemotherapy for patients with lymphoma type, along with the progress of supportive care, even though the age at diagnosis has been getting older. Third, a worse prognosis of smoldering type than in the 1991 database was observed. In fact, another Japanese study reported similar results to the current study.19 To explain this, we compared the characteristics of patients included in the 2 studies, but we could not identify the reason for the worse prognosis (data not shown). The outcome of smoldering type should thus be subjected to further evaluation, using a larger cohort of patients with longer follow-up.
Mogamulizumab, which is a CCR4 monoclonal antibody, has been recently approved in Japan for the treatment of ATL, and a phase 2 study of lenalidomide has been completed after a phase 1 study showing promising activity for ATL. A retrospective meta-analysis showed that OS for 5 years was 100% and 42% in patients with indolent ATL treated by IFN/AZT and chemotherapy, respectively.23 An ongoing phase 3 trial comparing IFN/AZT with watchful waiting for indolent ATL by the Japan Clinical Oncology Group will enable to elucidate the benefits by this strategy.
In this study, we showed the current features and management of patients with ATL, using data collected across Japan. It is disappointing that the improvement of survival of patients with acute and lymphoma types is limited to a particular proportion of patients who could be saved by induction chemotherapy, in comparison with the 1991 database. For patients with chronic and smoldering ATL, further studies are warranted to improve the outcome of patients, especially by establishing a risk-stratified approach.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms Yukimi Ito, Noriko Gushima, Kazuko Nakata, Yasuko Koga, Emi Ageishi, Etsuko Kumakawa, and Noriko Ikoma for supporting this study through data management and manuscript preparation.
This work was supported by Health and Labor Sciences Research Grants for Clinical Research (H23-rinkensui-ippan-011, H26-kakushintekigann-ippan-136) and for Cancer Research (H23-gan rinsho-ippan-020 and 022, H25-gan rinsho-ippan-011) from the Ministry of Health, Labour and Welfare of Japan (K.I.), by the Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development (AMED) (K.I.), and funds from the Clinical Hematology Oncology Treatment Study Group (Fukuoka, Japan) (H.K.).
Authorship
Contribution: H.K. and K.I. designed research, performed research, collected data, analyzed data, and wrote the manuscript; A.U., S.H., T.E., Y.M., Y.S., M.M., E.S., N.U., S.Y., K.Y., K.T., H.S., Y.O., H.M., T.J., M.A. and R.H. collected data; M.S. analyzed data; K.K. designed research and collected data; and J.S. and K.T. designed and performed research.
Conflict-of interest-disclosure: A.U. received honoraria from Kyowa Hakkou Kirin, Chugai Pharmaceutical, and Bristol-Myers Squibb. K.T. acted as a consultant for Takeda Pharmaceutical, Symbio Pharmaceuticals, and Huya Bioscience International and received research funding from Novartis, Pfizer, Chugai Pharmaceutical, Eisai, Celgene, Nippon Shinyaku, and Takeda Pharmaceutical. J.S. acts as a consultant for Kyowa Hakkou Kirin and Zenyaku Kogyo, received research funding from Chugai, and had a patent by Cellgentech. K.T. received honoraria from Asahi Kasei Pharm and Kyowa Hakkou Kirin and received research funding from Astellas Pharm, Eisai, Shionogi, and Sumitomo Dainippon Pharma. The remaining authors declare no competing financial interests.
A complete list of the participating investigators appears in the Appendix.
Correspondence: Kenji Ishitsuka, Division of Oncology, Hematology and Infectious Diseases, Department of Internal Medicine, Fukuoka University, 7-45-1 Nanakuma Jonan, Fukuoka 814-0180, Japan; e-mail: kenjiishitsuka@fukuoka-u.ac.jp or kenjiishitsuka2015@gmail.com.
Appendix: study group members
The following investigators participated in this study: S. Okamura (National Hospital Organization Kyushu Medical Centre); M. Ogata (Oita University Hospital); T. Murayama (Hyogo Cancer Center); M. Nakagawa (Nissay Hospital); S. Iida (Nagoya City University Graduate School of Medical Sciences); H. Nagoshi (Yokohama City Seibu Hospital, St. Marianna University School of Medicine); H. Teshima (Osaka City General Hospital); H. Take (Toyonaka Municipal Hospital); K. Nosaka (Kumamoto University Hospital); H. Tsuda (Kumamoto Municipal Hospital); N. Harada (Kyushu University Graduate School of Medical Science); N. Uoshima (Matsushita Memorial Hospital); J. Tsukada (University of Occupational and Environmetal Health); Y. Yakushijin (Ehime University Graduate School of Medicine); H. Mori (Showa University Fujigaoka Hospital); Y. Abe (Kyushu University Graduate School of Medical Science); T. Okamura (Kurume University School of Medicine); Y. Kubuki (Miyazaki University); Y. Yamano (Kyushu Kosei-nenkin Hospital); T. Kakimoto (Yokohama Municipal Citizen's Hospital); T. Kamimura (Harasanshin General Hospital); M. Koike (Juntendo University Shizuoka Hospital); Y. Adachi (Kobe Central Hospital of Insurance); K. Hodohara (Shiga University of Medical Science Hospital); M. Yamaguchi (Minoh City Hospital); T. Murase (Nishio Municipal Hospital); K. Ohbayashi (Toyota Memorial Hospital); F. Kawano (National Hospital Organization Kumamoto Medical Center); A. Miyata (Chugoku Chuo Hospital); T. Miyake (Asahikawa City Hospital); S. Tamaki (Yamada Red Cross Hospital); R. Tabata (Hyogo Prefectural Tsukaguchi Hospital); M. Iwahashi (Saiseikai-Hita Hospital); K. Izutsu (NTT Kanto Medical Center); M. Shinohara (Anan Kyoei Hospital); S. Motomura (Kyushu University Beppu Hospital); S. Okamoto (Keio University School of Medicine); Y. Maeda (Kinki University School of Medicine); S. Ito (Iwate Medical University School of Medicine); K. Kitamura (Ichinomiya Municipal Hosital); T. Okamoto (Takarazuka City Hospital); M. Morioka (Aiiku Hospital); T. Shimomura (National Hospital Organization Hiroshima-Nishi Medical Center); T. Hayashi (Tenri Hospital); H. Matsubara (Shinkokura Hospital); C. Hashimoto (Kanagawa Cancer Center); T. Takeichi (Health Insurance Naruto Hospital); A. Horikoshi (Nihon University School of Medicine, Nerima-Hikarigaoka Hospital); A. Wakita (East Medical Center Higashi Municipal Hospital); F. Urase (Sakai Hospital Kinki University Faculty of Medicine); N. Hirase (Kyushu Rosai Hospital); Y. Masaki (Kanazawa Medical University); N. Sakai (Iwate Prefectural Iwai Hospital); Y. Yamanaka (Akita University Graduate School of Medicine); T. Sakura (Saiseikai Maebashi Hospital); M. Tsukaguchi (Sakai Municipal Hospital); J. Tanabe (Fujieda Municipal General Hospital); T. Takahashi (Caress Alliance Tenshi Hospital); A. Itoh (JA Shizuola Kohseiren Enshu Hospital); H. Kaneko (Aiseikai Yamashina Hospital); M. Iino (Yamanashi Prefectural Central Hospital); H. Kimura (Northern Fukushima Medical Center); S. Matsuda (Ohta Nishinouchi Hospital); H. Ifuku (Amagasaki Chuou Hospital); K. Sato (Asahikawa Medical University).



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal