Abstract
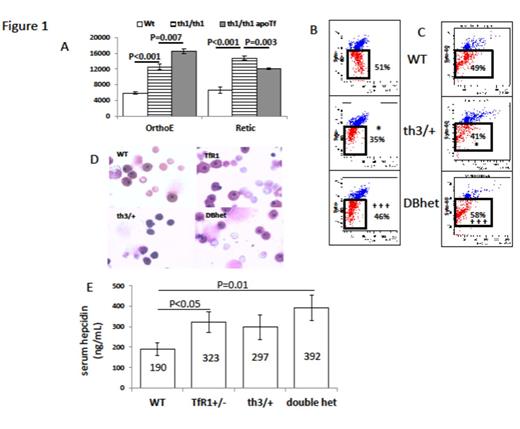
Transferrin-bound iron binding to transferrin receptor 1 (TfR1) is essential for erythropoiesis, and TfR1 is found in highest concentrations on erythroid precursors due to high iron requirement for hemoglobin (Hb) synthesis. Diseases of ineffective erythropoiesis such as β-thalassemia, are characterized by anemia, expanded and extramedullary erythropoiesis, and iron overload. Iron overload results from insufficient hepcidin, a peptide hormone secreted by hepatocytes in response to iron load. In β-thalassemia, hepcidin is relatively suppressed as a consequence of erythroid expansion. Erythroferrone (ERFE), a recently described erythroid-derived hepcidin suppressor, has been proposed as the mechanism and found in higher concentration in bone marrow of β-thalassemic mice. We previous demonstrate that exogenous transferrin (Tf) ameliorates anemia in β-thalassemic mice, reversing splenomegaly, hepcidin suppression, and iron overload and recently confirmed a decrease in Erfe expression in erythroid precursors from Tf-treated β-thalassemic mice. We observed that although Tf-treated β-thalassemic mice exhibit a further decrease in MCV and MCH, suggesting a relatively more iron restricted erythropoiesis, TfR1 expression is decreased. We hypothesize that TfR1 is central to Tf's effect on erythropoiesis in β-thalassemic mice. Last year, we presented our analysis of th3/+ TfR1+/- double heterozygote mice which exhibit reversal of all erythropoiesis- and iron-related pathology in th3/+ mice, confirming our observations in Tf-treated β-thalassemic mice and further supporting our hypothesis. To evaluate the mechanism involved, we observed that despite suppressed TfR1 concentration in reticulocyte (P=0.006) and sorted bone marrow erythroid precursors (P=0.0004) from Tf-treated th1/th1 mice, cell surface TfR1 expression decreased on reticulocytes (P=0.003) but was surprisingly increased on late stage erythroid precursors (P=0.007) (Figure 1A), suggesting that exogenous Tf influences erythroid precursor enucleation. Because we previously demonstrate decreased serum soluble TfR1 in Tf-treated th1/th1 mice [Liu J Blood 2013], we hypothesize that exogenous Tf alters TfR1 shedding from erythroid precursor membranes, promoting enucleation and improved terminal differentiation. We observed decreased enucleation using syto60 in flow cytometry of fetal liver cells (FLC) from th3/+ relative to wild type (WT) embryos (35 vs. 51%, P=0.03) which is normalized by exposure of th3/+ FLCs to Tf in vitro (58 vs. 41%, P=0.001) (Figure 1B). Tf-treated th3/+ FLCs shed more TfR1 to the nuclear fraction relative to reticulocyte during enucleation (P=0.0001) (Figure 1C). Furthermore, enucleation isdecreased in vivo in th3/+ relative to WT FLCs and peripheral blood at E14.5 and normalized in th3/+ TfR1+/- double heterozygote mice (45 vs. 35%, P=0.002) (Figure 1D). Interestingly, we analyzed iron status in TfR1+/- mice revealing that serum hepcidin is increased relative to WT (323 vs. 190 ng/ml, P=0.04) despite minimally decreased serum and liver iron concentrations (no statistically significant differences) and increased Erfe expression in erythroid precursors (5-fold, P=0.04). Relative to th3/+ mice, double heterozygote mice exhibit decreased serum iron (94 vs. 133 ug/dl), non-heme liver iron (0.31 vs. 0.74 mg iron/g dry weight, P=0.02), and Erfe expression (0.3-fold, P=0.04). Although no difference is observed between double heterozygote mice and th3/+, serum hepcidin is significantly increased in double heterozygote mice compare to WT (392 vs. 190 ng/ml, P=0.01), suggesting a more appropriate hepcidin response to iron overload (Figure 1E). Taken together, we postulate that decreased TfR1 expression plays a critical role in reversing ineffective erythropoiesis by increasing enucleation and influences hepcidin regulation in an ERFE independent manner.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal