Abstract
Introduction: It has been previously reported that pegylated interferon alpha-2a can induce hematologic and molecular responses in patients with essential thrombocythemia "ET" and polycythemia vera "PV", but the follow up in these studies were relatively short.
Objective: We present longer-term efficacy and safety results of a prospective phase II study of pegylated interferon alpha-2a in patients with ET and PV after a median follow up of 82.5 months (range, 8-107).
Methods: Patients with a diagnosis of ET or PV, in a need of therapy, either newly diagnosed or previously treated, were eligible for this study. Median interferon starting dose of 180 mcg/week SQ (range, 450-90; 39% started on 90mcg/week) was modified in majority of the patients based on toxicity or lack of efficacy. Clinical and molecular responses were assessed every 3 to 6 months.
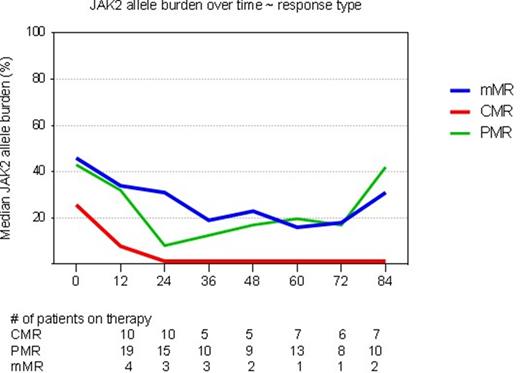
Results: Among 83 enrolled patients (43 PV, 40 ET), 32 patients (39%) are still on study (but in 8 therapy is on hold: 5 due to toxicity, and 3 for financial reasons). Median age was 53 years (range, 19-78). Overall 37% of patients did not receive prior cytoreductive treatment. The overall median exposure to therapy was 87 months (range, 58-107) and was no different for patients still enrolled on the study and those who stopped study participation. Nine (28%) patients still on study are currently on a dose equal or higher than 90 mcg/week and 15 (47%) are on dose equal or smaller than 45mcg/week. JAK2 status or allele burden had no impact on achievement of response (clinical or molecular), time to response or duration of therapy. 55 of 59 (71%) JAK2V617F positive patients were evaluable for molecular response (Figure); 8 patients carried CARL mutation, 3 carried MPL and in 13 were triple negative. Median duration of hematologic and molecular response was 66 and 53 months, respectively; and directly correlated with treatment length and type of response (CMR had the longest duration of response). Overall yearly discontinuation rate were gradually decreasing for first 5 years, from 17% to 5%, and slowly increasing afterward to 10%. Of the 51 patients not on the study anymore, 27 (35% of the total) discontinued therapy primarily due to treatment toxicity. New late (≥24 months from start of therapy) G3/4 toxicity occurred in 17% of patients. Among patients in complete hematologic response treatment failure due to vascular adverse event or disease transformation was seen in 5 patients each. Three patients died on study (not related to therapy or disease), and 8 after stopping participation. Mean changes in allele burden over time in JAK2 positive patients are depicted in figure.
Conclusions: Although pegylated interferon alpha-2a can induce significant hematologic and molecular responses; toxicity still limits its use over longer period of time and loss of response or transformation is encountered.
| Response . | Characteristics . | First response . | Last response . |
|---|---|---|---|
| Hem Resp, N. of patients (No), (%) | CHR | 62 (76) | 25 (40)a |
| PHR | 4 (5) | 1 (25) | |
| ORR | 66 (79) | 26 (39)a | |
| Mol Resp, No, (%) | CMR | 10 (18) | 9 (90) |
| PMR | 20 (36) | 5 (25)* | |
| mMR | 5 (9) | 2 (40) | |
| ORR | 35 (74) | 16 (46) | |
| Safety | Any grade | Grade≥3 | |
| Overall Adverse Events (AE), No, (%) | any AE | 83 (100) | 57 (67) |
| recurrent AE | 74 (89) | 13 (16) | |
| AE subtypes, No, (%) | musculoskeletal | 73 (88) | 6 (8) |
| neurological | 53 (64) | 2 (4) | |
| psychiatric | 38 (46) | 4 (11) | |
| gastrointestinal | 54 (65) | 11 (20) | |
| LFT elevation | 27 (33) | 5 (18) | |
| skin | 18 (22) | 2 (11) | |
| infection/fever | 26 (31) | 3 (12) | |
| respiratory | 23 (28) | 2 (9) | |
| cardiovascular | 13 (16) | 3 (23) | |
| metabolic | 16 (19) | 2 (13) | |
| neutropenia | 37 (45) | 21 (57) | |
| thrombocytopenia | 18 (22)a | 1 (6) | |
| anemia | 36 (43) | 1 (3) | |
| Autoimmune toxicity, No, (%) | hepatitis | 1 (2.5) | |
| CNS vasculitis | 1 (2.5) | ||
| lupus nephritis | 1 (2.3) | ||
| Sjogren sy & dermatitis | 1 (2.5) | ||
| Vascular AE (TEE/bleeding), | Unprovoked | 6 (7) | 5 (83) |
| No, (%) | Provoked | 4 (5) | 3 (75) |
| Disease transformation, No, (%) | Myelofibrosis | 6 (7) | |
| AML | 1 (1) | ||
| Safety over ≥24 months** | Any grade | Grade≥3 | |
| New AE, No (%) | 3th year | 10 (17) | 4 (40) |
| 4th year | 6 (11) | 4 (67) | |
| 5th year | 5 (10) | 1 (20) | |
| ≥ 6th year | 10 (24) | 1 (10) |
| Response . | Characteristics . | First response . | Last response . |
|---|---|---|---|
| Hem Resp, N. of patients (No), (%) | CHR | 62 (76) | 25 (40)a |
| PHR | 4 (5) | 1 (25) | |
| ORR | 66 (79) | 26 (39)a | |
| Mol Resp, No, (%) | CMR | 10 (18) | 9 (90) |
| PMR | 20 (36) | 5 (25)* | |
| mMR | 5 (9) | 2 (40) | |
| ORR | 35 (74) | 16 (46) | |
| Safety | Any grade | Grade≥3 | |
| Overall Adverse Events (AE), No, (%) | any AE | 83 (100) | 57 (67) |
| recurrent AE | 74 (89) | 13 (16) | |
| AE subtypes, No, (%) | musculoskeletal | 73 (88) | 6 (8) |
| neurological | 53 (64) | 2 (4) | |
| psychiatric | 38 (46) | 4 (11) | |
| gastrointestinal | 54 (65) | 11 (20) | |
| LFT elevation | 27 (33) | 5 (18) | |
| skin | 18 (22) | 2 (11) | |
| infection/fever | 26 (31) | 3 (12) | |
| respiratory | 23 (28) | 2 (9) | |
| cardiovascular | 13 (16) | 3 (23) | |
| metabolic | 16 (19) | 2 (13) | |
| neutropenia | 37 (45) | 21 (57) | |
| thrombocytopenia | 18 (22)a | 1 (6) | |
| anemia | 36 (43) | 1 (3) | |
| Autoimmune toxicity, No, (%) | hepatitis | 1 (2.5) | |
| CNS vasculitis | 1 (2.5) | ||
| lupus nephritis | 1 (2.3) | ||
| Sjogren sy & dermatitis | 1 (2.5) | ||
| Vascular AE (TEE/bleeding), | Unprovoked | 6 (7) | 5 (83) |
| No, (%) | Provoked | 4 (5) | 3 (75) |
| Disease transformation, No, (%) | Myelofibrosis | 6 (7) | |
| AML | 1 (1) | ||
| Safety over ≥24 months** | Any grade | Grade≥3 | |
| New AE, No (%) | 3th year | 10 (17) | 4 (40) |
| 4th year | 6 (11) | 4 (67) | |
| 5th year | 5 (10) | 1 (20) | |
| ≥ 6th year | 10 (24) | 1 (10) |
**Effective sample size for patients on therapy/year: Initial number of patients at risk at the beginning of period minus half of patients censored during that period
*% calculated from 19 patients
astatistically significant differences by Fisher's exact test
Abbr. CMR= complete molecular remission (undetectable JAK2 allele burden), PMR= partial molecular remission (>50% decrease in allele burden), mMR= minor molecular remission (20-49% decrease in allele burden)
Off Label Use: Pegylated Interferon alfa-2a used for patients with essential thrombocythemia and polycythemia vera. Cortes:Novartis: Consultancy, Research Funding; BerGenBio AS: Research Funding; Teva: Research Funding; BMS: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Ariad: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Ambit: Consultancy, Research Funding; Arog: Research Funding; Celator: Research Funding; Jenssen: Consultancy. Konopleva:Novartis: Research Funding; AbbVie: Research Funding; Stemline: Research Funding; Calithera: Research Funding; Threshold: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal