Abstract
Introduction
Gene Expression Profiling studies have resulted in signatures capable of providing robust prognosis for Multiple Myeloma (MM) patients, such as the EMC92 [SKY92, Kuiper et al. Leukemia 2012]. Recently, data from 4720 MM patients from the HOVON-65/GMMG-HD4, UAMS-TT2, UAMS-TT3, MRC-IX, APEX and IFM trials were employed to assessed the majority of currently identified prognostic markers and their combinations (GEP, FISH, and biochemistry data, [Kuiper et al ASH 2014]). The "SKY92 + ISS" was identified and validated as the statistically most optimal (i.e. most significant and robust) prognostic marker combination for MM patients. The combination of the EMC92 (=SKY92) and ISS prognostic features proves to be a very powerful, unprecedented and straightforward prognostic system. It identifies four prognostic risk classes: low risk (ISS I-SKY92 standard risk (SR)), intermediate-low (ISS II-SKY92 SR), intermediate-high (ISS III-SKY92 SR) and high risk (ISS I-III, SKY92 high risk).
Previously, the SKY92 has been validated on the 91 MM cases in the MMGI cohort [Van Beers et al. ASH 2013]. Here we present an extension of that validation with the SKY92 + ISS combination on the 78 MM cases for whom both GEP and ISS is available, by both assessing as the "4 risk group" model defined above, but also a "3 risk group" model (the two intermediate groups combined) as this may be more relevant and useful for clinical application.
Materials and Methods
A public untreated MM dataset (Multiple Myeloma Genomics Initiative, MMGI) had n=78 cases for which OS, GEP, and ISS were available for analysis. The prognostic markers SKY92 and ISS were applied as proposed [Kuiper et al ASH 2014] to classify cases into the risk categories.
Results
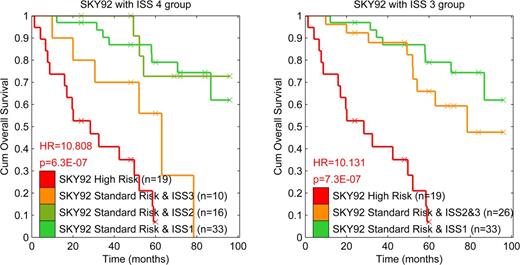
The risks for the 4 group classification model are shown in Table 1 and Figure 1, and the 3 risk group model is shown in Table 2 and Figure 1. In the 4 group model, the intermediate-low group (SKY92 standard + ISS II) was a small and not significantly different (p=0.79) from the low risk. The intermediate-high group (SKY92 standard + ISS III) was also a small group, with significantly worse outcome compared with the low risk group (p=0.012). Although stratification into four groups was statistically superior in the training and validation data [Kuiper et al ASH 2014] the interpretability benefits from aggregation of the middle two groups [Fig 1 right].
Classification results for the four risk groups (Fig 1 left)
| Risk group . | n . | % . | Median OS . | HR vs low risk . |
|---|---|---|---|---|
| SKY92 High Risk regardless of ISS | 19 | 24 | 28.4 m | 10.8 |
| SKY92 standard risk + ISS-III | 10 | 13 | 63.0 m | 4.1 |
| SKY92 standard risk + ISS-II | 16 | 21 | NR | (0.8) |
| SKY92 standard risk + ISS-I (low risk) | 33 | 42 | NR | NA |
| Risk group . | n . | % . | Median OS . | HR vs low risk . |
|---|---|---|---|---|
| SKY92 High Risk regardless of ISS | 19 | 24 | 28.4 m | 10.8 |
| SKY92 standard risk + ISS-III | 10 | 13 | 63.0 m | 4.1 |
| SKY92 standard risk + ISS-II | 16 | 21 | NR | (0.8) |
| SKY92 standard risk + ISS-I (low risk) | 33 | 42 | NR | NA |
NR= Not Reached at 96 months, HR= hazard ratio
Classification results for the three risk groups (Fig 1 right)
| Risk group . | n . | % . | Median OS . | HR vs low risk . |
|---|---|---|---|---|
| SKY92 High Risk regardless of ISS | 19 | 24 | 28.4 m | 10.1 |
| SKY92 standard risk + ISS-II/III | 26 | 34 | 78.5 m | (1.8) |
| SKY92 standard risk + ISS-I | 33 | 42 | NR | NA |
| Risk group . | n . | % . | Median OS . | HR vs low risk . |
|---|---|---|---|---|
| SKY92 High Risk regardless of ISS | 19 | 24 | 28.4 m | 10.1 |
| SKY92 standard risk + ISS-II/III | 26 | 34 | 78.5 m | (1.8) |
| SKY92 standard risk + ISS-I | 33 | 42 | NR | NA |
NR= Not Reached at 96 months, HR= hazard ratio, () not significant
Conclusions
By applying the SKY92 + ISS risk stratification model in an independent validation cohort of newly diagnosed Multiple Myeloma patients, besides a high risk group of 19 patients (24%), a group of 33 patients (42%) with superior prognosis could be predicted (SKY92 standard risk and ISS I) that translated into 62% OS at 96 months. The 19 high risk (SKY92 high risk) cases had very poor prognosis (median survival of 28 months). The combination of SKY92 Standard Risk and ISS II and III seems useful for definition of "intermediate risk" although sample size currently is insufficient for significance compared to low risk. The intention is to also perform this validation on the relapsed samples from the MMGI cohort, once OS data has been collected. This validated risk model could play a role in the design of future treatment strategies for high and low risk MM patients.
Kaplan Meier curves on the 78 MMGI cases, split into four (left) or three (right) risk groups based on the combination of SKY92 + ISS. Hazard Ratios (HR) are from a Cox proportional Hazards model comparing a particular group to the low risk group.
Kaplan Meier curves on the 78 MMGI cases, split into four (left) or three (right) risk groups based on the combination of SKY92 + ISS. Hazard Ratios (HR) are from a Cox proportional Hazards model comparing a particular group to the low risk group.
van Beers:SkylineDx: Employment. van Vliet:SkylineDx: Employment. de Best:SkylineDx: Employment. Chari:Novartis: Consultancy, Research Funding; Millennium/Takeda: Consultancy, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Biotest: Other: Institutional Research Funding; Array Biopharma: Consultancy, Other: Institutional Research Funding, Research Funding; Onyx: Consultancy, Research Funding. Jagganath:Millennium: Honoraria; Celgene: Honoraria. Jakubowiak:Sanofi-Aventis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Onyx: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: institutional funding for support of clinical trial conduct, Speakers Bureau; SkylineDx: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SkylineDx: Membership on an entity's Board of Directors or advisory committees; Onyx: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi-Aventis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Millennium: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Millennium: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Kumar:Celgene, Millenium, Sanofi, Skyline, BMS, Onyx, Noxxon,: Other: Consultant, no compensation,; Janssen: Research Funding; AbbVie: Research Funding; Sanofi: Research Funding; Millenium/Takeda: Research Funding; Celgene: Research Funding; Onyx: Research Funding; Skyline, Noxxon: Honoraria. Lebovic:Onyx: Speakers Bureau; Celgene: Speakers Bureau. Lonial:Millennium: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Onyx: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Celgene: Consultancy, Research Funding. Reece:Onyx: Honoraria; Novartis: Honoraria; Millennium: Research Funding; Merck: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; BMS: Research Funding. Richardson:Millennium Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gentium S.p.A.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees. Siegel:Celgene Corporation: Consultancy, Speakers Bureau; Amgen: Speakers Bureau; Takeda: Speakers Bureau; Novartis: Speakers Bureau; Merck: Speakers Bureau. Stewart:SkylineDx: Membership on an entity's Board of Directors or advisory committees. Vij:Onyx: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Millennium: Honoraria, Speakers Bureau; BMS: Consultancy; Takeda: Consultancy, Research Funding; Novartis: Consultancy; Sanofi: Consultancy; Janssen: Consultancy; Merck: Consultancy. Zimmerman:Celgene: Honoraria, Speakers Bureau; Millennium: Honoraria, Speakers Bureau; Onyx: Honoraria; Amgen: Honoraria, Speakers Bureau. Fonseca:Onyx/Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Binding Site: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Millennium: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Applied Biosciences: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal