Abstract
Introduction: Autologous transplantation (ASCT) in myeloma (MM) is standard consolidative therapy in first line therapy in eligible patients. We have shown definitely that a salvage ASCT in relapse setting can induce superior durability of responses (time-to-progression; TTP) over non-transplant consolidation with oral cyclophosphamide after a proteasome inhibitor-based re-induction schedule (ISRCTN601231201). The secondary end point of this multi-centre phase III randomised controlled trial was to evaluate the impact of salvage ASCT on the overall survival (OS_ of patients relapsing after a prior ASCT and delineate patient subgroups that may benefit the most.
Patients and Methods: Eligible patients with MM relapsing after a prior ASCT were enrolled. All patients were re-induced with Bortezomib, Doxorubicin and Dexamethasone (PAD) therapy delivered in 2-4 21-day cycles before 1:1 randomization to either a second ASCT (melphalan 200mg/m2 iv; ASCT2 supported by either stored or remobilized stem cells) or low dose consolidation with weekly cyclophosphamide 400mg/m2 PO for 12 weeks (Non-Transplant Consolidation; NTC). Response was assessed (by IMWG criteria) after re-induction and 100 days post-randomization with TTP being determined as the primary end-point. Patients were stratified by β2microglobulin (β2M) at trial entry, ASCT1 TTP and response to re-induction, analyzed according to cytogenetic abnormalities by iFISH (unfavorable: t(4;14), t(14;16) and del17p) with OS was a key secondary endpoint.
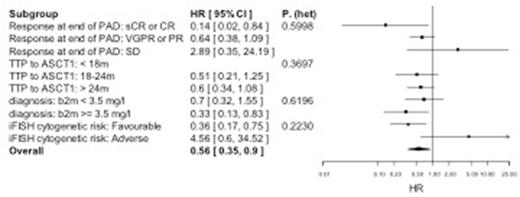
Results: 297 patients were entered into the study and 174 randomized from April 2008 to November 2012: ASCT2 n=89, NTC n=85. Median age was 61 (range 38-75) with 73.6% of patients relapsing more than 24 months from first ASCT. ORR to re-induction therapy was 79.4% with a 16.0% sCR/CR rate. Post-randomization, sCR/CR was significantly higher after ASCT2 (39.3% [95% CI 29.1,50.3] vs 22.4% [95% CI 14.0,32.7]; p=0á012). The median follow-up is 52 months (IQR range 41, 62) and the up-dated TTP demonstrates continued advantage in ASCT2 cohort compared to NTC (19 months [95% CI 16,26] vs 11 months [95% CI 9,12]; Log Rank p<0.0001). 75 patients (43.1%) have died since randomization, primarily from disease progression (59.4%). The median survival was 67 months (95% CI 55, °) in the ASCT2 cohort compared with 52 months (95% CI 42,60) in the NTC cohort (Log Rank p=0.022). Cox proportional hazards regression (adjusted for stratification factors including whether PBSC was remobilized) showed a reduced hazard of death in the ASCT2 group compared to NTC (HR=0.56, 95%CI [0.35, 0.90], p=0.0169). CR/sCR to re-induction therapy (HR 0.14, p=0.032), ASCT1 TTP > 24m (HR0.60, p=0.089), β2M level <3.5mg/L (HR 0.35, p=0.039) and the absence of high risk iFISH (HR 0.36, p=0.007) were associated with improved OS in favour of ASCT2 (Fig. 1A). To-date, following progression on protocol, 88.7% in the ASCT2 and 84% in the NTC cohorts have received 3rd line therapy, primarily consisting of a lenalidomide based combination (88.9% in the ASCT2 and 81% in the NTC cohorts). 20 patients (26.7%) in the NTC cohort underwent salvage ASCT in 3rd/4th line (NTC/ASCT2), with 1 patient in each cohorts proceeding to allogeneic SCT. The PFS2 was significantly better in the ASCT2 compared with both NTC/ASCT2 and NTC cohorts (ASCT2: 67m, [95%CI 52,°] vs NTC/ASCT2: 31m, [95%CI 23,42] vs NTC 39m, [95%CI 32,47]; p<0.0001). Consequently, the 4-year OS demonstrated a superiority of a salvage ASCT in second line over 3rd line or not (ASCT2: 69% [95%CI 58,79] vs NTC/ASCT2: 61% [95%CI 52,69] vs NTC 50% [95%CI 36,64]) where the OS in NTC groups split by 3rd line ASCT were not significantly different (p = 0.139, Fig. 1b).
Conclusion: This long-term follow-up analysis demonstrates a clear advantage in terms of OS when salvage ASCT consolidates bortezomib-based re-induction therapy in patients with MM at first relapse. The delay of salvage ASCT to third line, though being suggestive of benefit over no salvage ASCT, does not confer the same degree of OS advantage as shown with a salvage transplant in second line. This data is key for patient-centered clinical decision-making.
1. G Cook, et al.. The Lancet Oncology, Vol. 15, No. 8, p874-885
Forest plots showing (a) heterogeneity of effect of randomised treatment on OS and (b) effect on OS of randomised treatment followed by ASCT at second relapse (NTC/ASCT2)
Forest plots showing (a) heterogeneity of effect of randomised treatment on OS and (b) effect on OS of randomised treatment followed by ASCT at second relapse (NTC/ASCT2)
Cook:Amgen: Consultancy, Speakers Bureau; Celgene: Consultancy, Research Funding, Speakers Bureau; BMS: Consultancy; Sanofi: Consultancy, Speakers Bureau; Takeda Oncology: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau. Williams:Celgene: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau. Cavenagh:Amgen: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau. Snowden:MSD: Consultancy, Other: Educational support, Speakers Bureau; Janssen: Other: Educational support, Speakers Bureau; Celgene: Other: Educational support, Speakers Bureau; Sanofi: Consultancy. Parrish:Janssen: Speakers Bureau; Celgene: Speakers Bureau. Yong:Takeda: Honoraria; Autolous: Consultancy; Janssen: Honoraria; Novartis: Consultancy; BMS: Honoraria; Amgen: Honoraria. Cavet:Celgene: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau. Bird:Janssen: Other: Educational support; Celgene: Speakers Bureau; Amgen: Consultancy; Novartis: Consultancy; Pfizer: Consultancy. Heartin:Celgene: Speakers Bureau; Janssen: Consultancy. O'Connor:Celgene: Research Funding. Ashcroft:Janssen: Consultancy, Other: Educational support. Brown:Janssen: Research Funding; Roche: Research Funding; Celgene: Research Funding; Bayer: Research Funding. Morris:Janssen: Other: Meeting support; Celgene: Other: Meeting support.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal