Abstract
Aberrant activation of the FMS-like tyrosine kinase-3 (FLT3) is driven by internal tandem duplication (ITD) mutations in the FLT3 gene, which are commonly observed in patients with acute myeloid leukemia (AML). Hence, FLT3 represents an attractive therapeutic target in AML (Weisberg et al., 2002). Indeed, several small molecule FLT3 inhibitors including sorafenib have showed encouraging efficacy in reducing leukemia blasts in the peripheral blood in FLT3 mutated AML patients. However, these agents have little effect on leukemic stem cells in the bone marrow (BM) microenvironment (Borthakur et al., 2011; Fathi and Chabner, 2011; Zhang et al., 2008).
The BM microenvironment is enriched with cytokines and adhesion molecules, such as CXCR4 and E-selectin, which are believed to provide AML cells protection against chemotherapeutic agents (Horacek et al., 2013; Peled and Tavor, 2013). In fact, treatment with sorafenib markedly upregulated CXCR4 levels in FLT3 -mutated cells. In addition, leukemia cells can activate endothelial cells (EC) that induce adhesion of a sub-set of the leukemia cells through E-selectin. The adherent AML cells are sequestered in a nonproliferative state that further protects them from chemotherapy (Pezeshkian et al., 2013). Therefore, blocking CXCR4 and E-selectin in parallel could theoretically eliminate the protection provided by the interaction of leukemic cells with their BM microenvironment and enhance effectiveness of chemotherapy in FLT3-mutant AML patients. In the present study, we evaluated the effectiveness of a dual CXCR4 and E-selectin antagonist, GMI-1359 (GlycoMimetics, Inc., Rockville, MD), in targeting FLT3-ITD-mutant AML in vitro and in vivo. High levels of CXCR4 expression were observed in several human and murine AML cell lines, which was further increased in hypoxic (i.e., 1% oxygen) conditions that mimic the BM microenvironment. These FLT3 -ITD leukemic cell lines also expressed hypoxia-responsive, functional E-selectin ligands identified by reactivity with an antibody (HECA452) that binds the same carbohydrate epitope required for binding to E-selectin. One such E-selectin ligand CD44 increased in FLT3 -ITD cells cultured in hypoxia compared to those cultured in normoxia (i.e. 21% oxygen). In addition, hypoxia also enhanced CXCR4 expression on mesenchymal stem cells (MSC) and EC such as HUVEC. In hypoxic co-cultures of the FLT3 -ITD-mutant leukemia cells MV4-11 or MOLM14 with MSCs and ECs (i.e., HUVEC or TeloHAEC), the presence of the dual E-selectin/CXCR4 inhibitor GMI-1359 effectively reduced leukemic cell adhesion by ~ 50% to the MSC/EC feeder layer compared to the PBS-treated control (p<0.05), even in the presence of TNFa, which induces E-selectin expression in EC. However, an E-selectin specific inhibitor only reduced adhesion of MV4-11 and MOLM14 by ~ 20%. GMI-1359 markedly abrogated the protection provided by the BM microenvironment (i.e., hypoxia and/or MSC and EC) of Baf3-FLT3 -ITD leukemic cells treated with the FLT3 inhibitor sorafenib. Apoptosis was induced in 36.6%, 35.6% and 48.9% of leukemic cells cultured with sorafenib alone, sorafenib and an E-selectin inhibitor or sorafenib and GMI-1359, respectively.
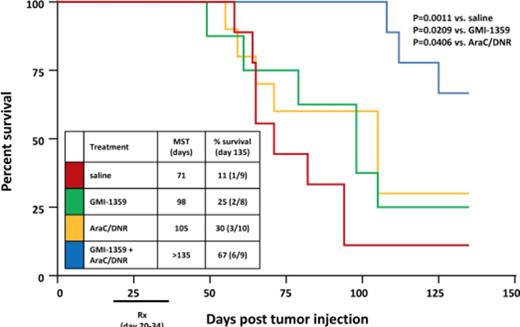
The significance of these in vitro findings were studied in vivo. Female SCID beige mice were injected iv with MV4-11 and followed for survival. Beginning 14 days post tumor injection, cohorts of mice (n=10/group) were treated with saline, GMI-1359 (40 mg/kg), standard chemotherapy cytarabine plus daunorubicin, or a combination of GMI-1359 and chemotherapy. Combined treatment of mice with GMI-1359 (40 mg/kg) and chemotherapy demonstrated a profound survival benefit compared to controls or chemotherapy alone at day 135 after leukemia cell injection (i.e., 67% vs. 11% or 30%, p=0.0011 and 0.0406, respectively). Single agent treatment with GMI-1359 was statistically indistinguishable from saline alone or chemotherapy alone. In a separate cohort of MV4.11-engrafted mice, the single administration of GMI-1359 increased circulating WBC and leukemic MV4-11cells, which persisted for at least 8 hrs. This effect was consistent with GMI-1359 disrupting the protective effects of the tumor microenvironment and mobilizing MV4-11 cells from the BM niche.. These findings provide the pre-clinical basis for the evaluation of GMI-1359 in patients with FLT3 -mutant AML.
Zhang:Karyopharm: Research Funding. Fogler:GlycoMimetics, Inc.: Employment. Magnani:GlycoMimetics: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal