Abstract
Introduction: Acute promyelocytic leukemia (APL) is biologically distinct subtype of acute myeloid leukemia characterized by PML-RARA fusion transcripts, and its survival has significantly improved with all-trans retinoic acid (ATRA). Monitoring PML-RARA fusion transcripts with quantitative real-time polymerase chain reaction (RT-PCR) is the standard of care for the evaluation of response in patients with acute promyelocytic leukemia. We sought to determine the correlation between peripheral blood (PB) and bone marrow (BM) samples in patients treated on our frontline studies.
Methods: We correlated results from BM and PB samples obtained from patients with newly diagnosed APL treated on three consecutive prospective clinical trials of combination of arsenic trioxide (ATO) and ATRA with or without gemtuzumab ozogamycin (ID01-014; NCT01409161; and NCT00413166) at our institution. Qualitative RT-PCR was performed on reverse-transcribed RNA from PB and BM samples for the short and long isoforms of PML-RARA, and the percent ratios of PML-RARA to ABL1 transcript levels were calculated. The sensitivity of detection with RT-PCR was 1 in 100,000. For this analysis, we selected samples with the time interval of collection within 1 day. Qualitative correlation was assessed using Kendall tau rank correlation coefficient test. Spearman's correlation coefficient was computed for quantitative variables to measure the extent of the association between samples. P values were two-sided and a p value of <0.05 was considered as statistically significant.
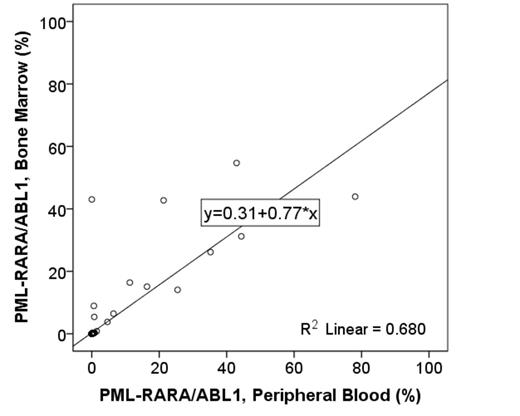
Results: From July 2002 to May 2015, 184 patients were enrolled in the clinical trials. RT-PCR for PML-RARA was performed in 2077 samples including 1261 BM samples and 816 PB samples. In total, 584 samples (292 sample pairs) from BM and PB were identified within 1-day time interval. PML-RARA levels from PB samples with RT-PCR were strongly qualitatively and quantitatively correlated with those from BM samples (r= 0.831, p<0.001; τ= 0.792, p<0.001, respectively). Minimal qualitative discrepancy was observed in 13 samples from 11 patients: 8 BM samples from 8 patients with PML-RARA/ABL1 ≤0.01% (4 short form; 4 long form), with undetectable PML-RARA from concurrent PB; and 5 PB samples from 4 patients with PML-RARA/ABL1 ≤0.01% (2 short form; 3 long form), with undetectable PML-RARA from concurrent BM samples. Of 8 BM samples, 4 BM samples were obtained after 2 cycles, 3 cycles, 3 cycles, and 4 cycles of ATRA + ATO, respectively; 4, 1 month, 9 months, 9 months, and 10 months after completion of ATO + ATRA. The discrepancy of 4 BM samples during ATRA + ATO was resolved with undetectable PML-RARA level from peripheral blood samples and bone marrow samples after 1 additional cycle of ATO + ATRA. Of 4 BM samples after completion of ATO + ATRA, previous PML-RARA showed undetectable PML-RARA level, and repeat PML-RARA showed undetectable PML-RARA level from bone marrow and peripheral blood samples without intervention. Of 5 PB samples from 4 patients, 3 PB samples from 2 patients were obtained after 3 cycles, 3 cycles, and 4 cycles of ATRA. One patient had persistent PML-RARA/ABL1 <0.01% (short form) from peripheral blood samples with undetectable PML-RARA from concurrent BM samples after 3 cycles and 4 cycles of ATO + ATRA. Two PB samples from 2 patients were obtained 1 month after completion of ATO + ATRA.
Conclusions: Strong correlation between PB and BM results indicate that PML-RARA fusion transcript levels in patients with APL can be monitored using PB samples.
The correlation between bone marrow and peripheral blood samples for detection of PML-RARA
The correlation between bone marrow and peripheral blood samples for detection of PML-RARA
Verstovsek:Incyte Corporation: Research Funding. Estrov:incyte: Consultancy, Research Funding. Cortes:Novartis: Consultancy, Research Funding; Teva: Research Funding; BerGenBio AS: Research Funding; BMS: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Ariad: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Ambit: Consultancy, Research Funding; Arog: Research Funding; Celator: Research Funding; Jenssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal