Abstract
Introduction: Most pts with CLL do not require anti-leukemic therapy. However, many pts experience fatigue and/or other disease related symptoms and their quality of life (QoL) is significantly impaired. None of the currently approved supportive care drugs has significantly improved symptoms or QoL in CLL. Therefore, as per iWCLL guidelines, systemic therapy is offered to pts with severe symptoms. Because we found1 that stimulation of the B-cell receptor activates the JAK/STAT3 pathway in CLL cells and since the JAK1/2 inhibitor ruxolitinib significantly reduced inflammatory cytokine levels and abolished disease related symptoms in pts with myelofibrosis, we designed a phase II clinical trial to explore the effect of ruxolitinib on disease related symptoms in pts with CLL.
Methods: Primary objective was to assess improvement in the brief fatigue inventory (BFI) score, and secondary objective was to assess improvement in symptom associated interference in daily activities (interference score; IS) and changes in CLL related symptoms using the CLL module of the MD Anderson symptom inventory (MDSAI)2. Ruxolitinib was administered orally at 10 mg BID and dose adjustments were allowed. Symptom scores (SS) and plasma cytokine/chemokine levels (C/CL) were assessed at baseline and at month 3 of treatment. Levels were measured using a multiplex cytokine array. Changes in C/CL in the scale of log2 fold change were analysed and a linear regression analysis was performed to estimate the association between changes in C/CL and % changes in SS.
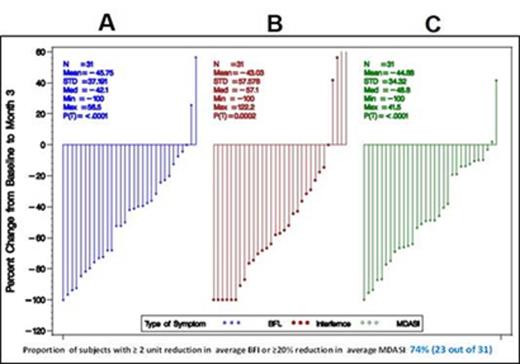
Results: Thirty six CLL pts [untreated; n=21 and previously treated; n=15] were enrolled into the study since September 2014. Thirty one of these pts [untreated; n=19 and previously treated; n=15] were followed-up for ≥3 months and were evaluable. Ten pts came off study (3 for disease progression, 3 for lack of response, 2 due to new ruxolitinib-unrelated symptoms, one with glioblastoma, and one with myasthenia gravis). Among these 31 pts, the average SS were calculated and % change from baseline to 3 months was assessed. Overall, the proportion of subjects with ≥20% reduction in average MDASI or ≥2 units reduction in the average BFI score was 74% (23 out of 31). The mean % change in the average BFI score was 46%, (Figure 1A; p<0.0001), the mean % change in the IS was 43% (Figure 1B; p<0.0002), and the mean % change in the MDSAI score was 45% (Figure 1C; p<0.0001), indicating a significant improvement in the severity of fatigue, symptoms interfering with daily activities, and a decrease in severity of CLL-related symptoms compared to baseline. The mean %change in individual symptoms at 3 months of ruxolitinib treatment compared to baseline significantly correlated with a reduction in C/CL (Figure 2). When BFI score, IS, and MDASI score were considered, the changes of 8 out of 10 C/CL (BCAC1/CXCL13, IL-1RA, ICAM-1/CD54, VCAM-1/CD106, IP-10/CXCL10, MIP1-β/CCL4, osteopontin, and IL-6) were associated to the symptom changes with the highest R2 of 0.6398 at 3 months of treatment. The linear composite of the 8 C/CL changes using the regression slopes as the coefficients was derived, and was then associated with different symptoms using the simple regression analysis. The decrease in composite variable correlated with a significant reduction of BFI scores, IS, and MDASI symptom scores with p-values consistently < 0.05 (Figure 2A-C). Because C/CL correlate with disease burden, we also assessed changes in the levels of β2M, white blood cell count (WBC) and absolute lymphocyte count (ALC) at the same time points. In most pts β2M decreased at 3 months of therapy and the WBC and ALC increased and then decreased below baseline levels.
Conclusions: Ruxolitinib significantly reduces fatigue and other disease related symptoms in pts with CLL that do not require systemic therapy. Improvements in clinical SS significantly correlated with a reduction in C/CL. The reduction in β2M levels and WBC and ALC counts suggest that ruxolitinib may reduce tumor burden in pts with CLL.
Ref:-
Rozovski U et al. Blood 2014; 123:3797-802.
Cleeland C et al. Cancer 89:1634-1646, 2000.
Wierda:Glaxo-Smith-Kline Inc.: Research Funding; Celgene Corp.: Consultancy. DiNardo:Novartis: Research Funding. Pemmaraju:incyte: Consultancy. Burger:Pharmacyclics LLC, an AbbVie Company: Research Funding. Estrov:incyte: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal