Abstract
Idiopathic aplastic anemia (IAA) is an acquired bone marrow disease probably caused by an auto-immune attack against hematopoietic stem cells (HSCs), which leads to bone marrow failure. Many abnormalities have been observed in the T cell compartment, but the putative auto-antigen(s) remain elusive. A large body of evidence links paroxysmal nocturnal hemoglobinuria (PNH) to IAA, supporting the notion that autoimmunity is a key pathogenic mechanism in both diseases. In PNH, auto-reactive T cells may be the 'noxious agent' capable of killing GPI positive HSCs while sparing GPI negative HSCs. Recently, CD1d restricted, GPI-specific T-cells have been demonstrated in PNH patients. Here, we investigate whether CD1d restricted, GPI-specific T cells are also present in IAA patients.
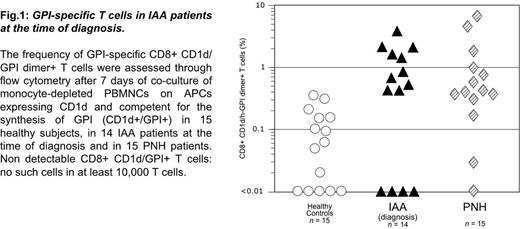
When peripheral blood mononuclear cells (PBMNCs) from 14 newly diagnosed IAA patients [12 of whom had a small percentage (between 0.003% and 5%) of GPI-negative granulocytes] were co-cultured with antigen presenting cells (APCs) expressing CD1d and competent for the synthesis of GPI, we detected GPI specific T cells (CD8+CD1d/GPI dimer+ T cells) in 10 out of 14 patients (71%) at a significantly higher abundance than in co-culture experiments performed with PBMNCs from healthy controls (Fig. 1). In fact, the frequency of CD8+CD1d/GPI dimer+ T cells was below the cutoff value of 0.35% in all the 15 healthy controls but only in 4 out 14 IAA patients (Fisher test, P <0.00005). The frequency of GPI specific T cells in IAA patients was similar to that previously found in PNH patients (Fig.1). In the same experimental setting, in IAA we found increased frequency of INFγ producing T cells (CD8+INFγ+ T cells) than in healthy controls: interestingly, in the 4 patients with no detectable CD8+CD1d/h-GPI dimer+ T cells, we found INFγ producing T cells regardless of the GPI stimulus. Furthermore, we have observed a trend towards the association of higher frequency of CD8+CD1d/GPI dimer+ T cells and severe IAA versus non-severe IAA.
In 5 of the 10 IAA patients with detectable CD8+CD1d/GPI dimer+ T cells we tested sequential samples; we observed that response to immunosuppressive treatment (IST) was associated with significant decrement of both GPI specific and INFγ producing T cells. In keeping with these data, GPI specific T cells were absent in 2 AA patients in remission after engraftment of HSC transplants.
We then similarly analyzed 27 IAA patients who were already receiving IST. 8 of these patients did not have any GPI-negative granulocyte population, 10 had a small PNH clone (between 0.003% and 5%) and 9 had more than 5% of GPI-negative granulocytes. GPI specific T cells were detected in 20 out of these 27 (74%) patients, regardless of the presence/absence of a PNH (GPI-negative) population; the frequency of GPI specific T cells was lower than in patients at diagnosis. In these 20 patients, INFγ producing T cells were detected only when co-cultured in presence of GPI on CD1d+ APC; in the remaining 7 patients with no detectable CD8+CD1d/h-GPI dimer+ T cells, INFγ producing T cells were present regardless of any GPI stimulus.
In one patient with a congenital form of mild AA due to a TERC mutation, GPI specific T cells were present. This finding might signify that although congenital AA patients are innately predisposed to AA, an auto-immune attack may be still needed to produce full-blown AA.
We conclude that GPI specific T cells are present in about 70% of IAA patients regardless of the presence/absence of a PNH (GPI-negative) population; and that the presence and size of a PNH cell population results from the existence of a PIG-A mutant HSC and from its degree of 'stemness'. The presence of GPI specific T cells in the large majority of AA patients suggests that in most of these cases, GPI is the target of the auto-immune attack potentially resulting in the suppression of hematopoiesis. In the remaining IAA patients, different and still unknown auto-immune targets are presumably involved in the T-cell attack. These data strongly support the hypothesis that AA and PNH could be different clinical expression of the same pathogenetic mechanism, namely the auto-immune suppression of the GPI+ (normal) HSCs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal