Abstract
Background: Early T-cell precursor (ETP) acute lymphoblastic leukemia/lymphoma (ALL) is a recently recognized high-risk T-ALL subgroup. The optimal therapeutic approaches to adult patients with ETP-ALL are poorly characterized. In this study, we compared the outcomes of adults with ETP-ALL who received treatment on frontline regimens to those of patients with other T-ALL immunophenotypic subtypes.
Methods: Patients with newly-diagnosed T-ALL who received frontline chemotherapy between the years 2000 and 2014 at The University of Texas MD Anderson Cancer Center (MDACC) were identified and immunophenotypically categorized into early, thymic, and mature per the European Group for the Immunologic Classification of Leukemia (EGIL)/WHO classification. Patients with ETP-ALL were identified on the basis of the following immunophenotype: CD1a(-), CD8(-), CD5(-/dim), and positivity for one or more stem cell or myeloid antigens. Patients received frontline treatment with the following chemotherapy regimens: hyper-CVAD alone (n=43), hyper-CVAD + nelarabine (n=44) or augmented BFM regimen (n=24).
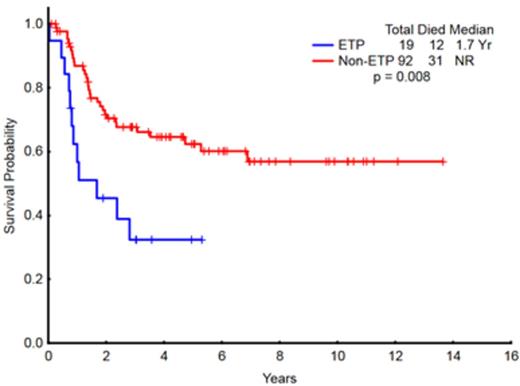
Results: A total of 111 patients with T-ALL with adequate immunophenotype data were identified. There was no difference in the outcomes of patients based on the EGIL/WHO subtypes (Fig 1). A total of 19 patients (17%) had ETP-ALL. The complete remission rate (CR)/CR with incomplete platelet recovery (CRp) rate in patients with ETP-ALL was significantly lower than that of non-ETP-ALL patients (73% vs. 91%; p=0.03). The median overall survival for patients with ETP-ALL was 20 months vs. not reached for the non-ETP-ALL patients (p = 0.008) (Fig 2). ETP-ALL remained a high-risk subgroup within the WHO 'Early' group (Fig 3). A subset of patients with early T-ALL had an immunophenotype that resembled that of ETP-ALL except for having ≥75% CD5 expression (ETP+CD5). The OS of patients with ETP+CD5 (n=19) was similar to that of non-ETP-ALL patients and differed from that of ETP-ALL patients (p=0.059).
By univariate analysis, the following variables were significant for survival: age, WBC count (<50 vs. ≥50 x109 /L), platelet count (<100 vs. ≥100 x109 /L), LDH (<600 vs. ≥600 IU/L) and ETP-ALL (Table 1). By multivariate analysis, only age (HR: 2.862; 95%CI: 1.140-7.183; p=0.025) and ETP-ALL (HR: 2.275; 95%CI: 1.117-4.631; p=0.023) were significant.
Conclusions: ETP-ALL represents a high-risk disease subtype of adult ALL. Allogeneic stem cell transplant in CR1 should be considered. Novel treatment strategies are needed to improve treatment outcomes in this T-ALL subset.
Univariate and multivariate analysis for survival
| Parameter . | Survival . | |||
|---|---|---|---|---|
| UVA . | MVA . | |||
| P . | P . | HR . | 95%CI . | |
| Age ≥60 | 0.013 | 0.025 | 2.862 | 1.140-7.183 |
| Gender | 0.24 | - | - | - |
| Diagnosis (ALL vs. LBL) | 0.13 | - | - | - |
| WBC < 50.0 (x 109 /L) | 0.009 | - | - | - |
| Hemoglobin <10 (g/dL) | 0.36 | - | - | - |
| Platelet <100 (x 109 /L) | 0.036 | - | - | - |
| LDH <600 (IU/L) | 0.045 | - | - | - |
| CNS involvement at Dx | 0.18 | - | - | - |
| WHO classification (early, thymic, mature) | 0.101 | - | - | - |
| ETP-ALL | 0.008 | 0.023 | 2.275 | 1.117-4.631 |
| Treatment received | 0.43 | - | - | - |
| Parameter . | Survival . | |||
|---|---|---|---|---|
| UVA . | MVA . | |||
| P . | P . | HR . | 95%CI . | |
| Age ≥60 | 0.013 | 0.025 | 2.862 | 1.140-7.183 |
| Gender | 0.24 | - | - | - |
| Diagnosis (ALL vs. LBL) | 0.13 | - | - | - |
| WBC < 50.0 (x 109 /L) | 0.009 | - | - | - |
| Hemoglobin <10 (g/dL) | 0.36 | - | - | - |
| Platelet <100 (x 109 /L) | 0.036 | - | - | - |
| LDH <600 (IU/L) | 0.045 | - | - | - |
| CNS involvement at Dx | 0.18 | - | - | - |
| WHO classification (early, thymic, mature) | 0.101 | - | - | - |
| ETP-ALL | 0.008 | 0.023 | 2.275 | 1.117-4.631 |
| Treatment received | 0.43 | - | - | - |
Overall survival of patients with T-ALL (N=111) categorized as Early, Thymic and Mature per EGIL/WHO Classification
Overall survival of patients with T-ALL (N=111) categorized as Early, Thymic and Mature per EGIL/WHO Classification
Overall survival of patients with ETP-ALL (N=19) compared to non-ETP ALL (N=92)
Overall survival of patients with WHO 'early' subcategorized as ETP vs. non-ETP, WHO 'thymic', and WHO 'mature' (N=111)
Overall survival of patients with WHO 'early' subcategorized as ETP vs. non-ETP, WHO 'thymic', and WHO 'mature' (N=111)
Konopleva:Novartis: Research Funding; AbbVie: Research Funding; Stemline: Research Funding; Calithera: Research Funding; Threshold: Research Funding. Cortes:Astellas: Consultancy, Research Funding; BerGenBio AS: Research Funding; Novartis: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Ariad: Consultancy, Research Funding; Teva: Research Funding; Ambit: Consultancy, Research Funding; Arog: Research Funding; Celator: Research Funding; Jenssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal