Abstract
Purpose: The use of rituximab, a chimeric monoclonal antibody to CD20, has led to significant improvement in the treatment of B-cell non-Hodgkin's lymphoma and mature B-cell ALL. CD20 is expressed in 30 to 50% of adult BCP-ALL patients. Although some single arm studies suggested that adding rituximab to chemotherapy could improve the outcome of these patients, no randomized study has been reported so far.
Methods: To evaluate the potential benefit of adding rituximab, we conducted a multicenter randomized trial comparing the pediatric-inspired GRAALL protocol to the same regimen plus rituximab, in patients aged 18-59 years old with newly diagnosed CD20-positive Ph-negative BCP-ALL enrolled in the GRAALL 2005 trial. CD20 positivity was defined as expression of CD20 in more than 20% of leukemia blasts. Rituximab (375 mg/m2) was given during induction (day 1 and 7), salvage reinduction when needed (day 1 and 7), consolidation blocks (6 infusions), late intensification (day 1 and 7) and first year of maintenance (6 infusions) for a total of 16 to 18 infusions. Allogeneic stem cell transplantation (SCT) was offered in first complete remission (CR) to patients with one or more conventional high-risk criteria and a donor. The primary study objective was event-free survival (EFS). A study sample size of 220 patients was estimated in order to detect a 20% gain in EFS at 2 years (two-sided test, power 85%, type 1 error 5%). A sensitivity analysis was performed after censoring patients allografted in first CR at transplant time. This trial was registered at http://www.clinicaltrials.gov as #NCT00327678.
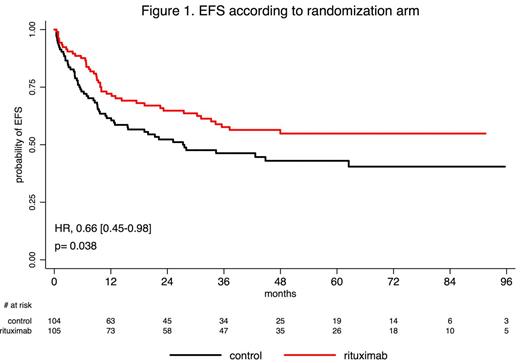
Results: From 2005 to 2014, 220 patients from 56 centers were randomized. Eleven patients had non-eligibility criteria (n=5 Ph+ ALL; n=3 CD20-negative ALL; n=1 HIV infection) or withdrew their consent (n=2) and were accordingly excluded from this modified ITT analysis that dealt with 209 patients (105 in the rituximab arm and 104 in the control arm). Median age was 40 years. Both randomization arms were well balanced for pretreatment characteristics including age, ECOG status, WBC, and central nervous system (CNS) involvement (6% of the whole cohort). After induction ± salvage reinduction, CR rate was 92% and 91% in rituximab and control arm, respectively. In patients who reached CR after first induction and were evaluated for Ig/TCR minimal residual disease level (MRD), the rates of patients with MRD<10-4 in the rituximab vs control arm were 65% vs 61% (p=0.82) and 91% vs 82% (p=0.31), after induction and first 3 consolidation blocks respectively. A higher proportion of patients received allogeneic SCT in first CR in the rituximab arm (34% vs 20% in the control arm; p=0.029). With a median follow-up of 30 months, patients treated in the rituximab arm had a lower cumulative incidence of relapse (CIR) (2-year CIR, 18% [95% CI, 10-26] vs 30.5% [95% CI, 21-40] in control arm; p=0.02), while no significant difference was observed regarding non-relapse mortality (NRM) between both arms (2-year NRM, 12% [95%CI, 5-18] vs 12% [95%CI, 5-18] in control arm; p=0.80). This translated into longer EFS in patients treated in the rituximab arm (2-year EFS, 65% [95% CI, 56-75] vs 52% [95% CI, 43-63] in control arm; HR= 0.66 [0.45-0.98]; p=0.038; Figure 1) but no longer overall survival (OS) (2-year OS, 71% [95% CI, 62-80] vs 64% [95%CI, 55-74] in control arm; HR= 0.70 [0.46-1.07]; p=0.095). Other factors impacting EFS were age, CNS involvement and WBC at diagnosis. Together with the randomization arm, all these factors remained significantly associated with EFS in multivariate analysis, even when adjusting on allogeneic SCT in first CR as a time-dependent covariate. When censoring patients who received allogeneic SCT in first CR at transplant time, EFS and OS were longer in the rituximab arm (2-year EFS, 66% [95% CI, 56-78] vs 53% [95% CI, 44-65] and 2-year OS, 74% [95% CI, 65-85] vs 63% [95%CI, 54-74]; HR= 0.59 [0.37-0.93] and 0.55 [0.34-0.91] and p=0.021 and 0.018, respectively). Finally, 71 and 55 infection-related SAEs were reported in non-transplanted patients in the rituximab and control arm, respectively, without significantly higher incidence in the rituximab arm, at any treatment phase.
Conclusions: In adults with CD20-positive, Ph-negative, BCP-ALL, the addition of rituximab to the pediatric-inspired GRAALL protocol improves EFS; it also prolongs OS when ignoring patient's outcome after transplantation in first CR.
Off Label Use: Rituximab is not currently approved for this indication.. Chalandon:Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal