To the editor:
In immunoglobulin light chain (AL) amyloidosis, amyloid fibril deposits, derived from immunoglobulin light chains produced by a clonal plasma cell dyscrasia, accumulate in extracellular tissues and damage vital organs.1 High-dose melphalan and autologous stem cell transplantation (HDM/SCT) have been shown to induce both hematologic and clinical remissions in selected patients with AL amyloidosis, and it prolongs survival substantially when hematologic remissions are achieved.
We report on the outcome for AL amyloidosis patients treated with HDM/SCT for a >20-year period to address remission durability and long-term results. We previously reported on our experience of 312 patients treated with HDM/SCT in 2004,2 and this series was updated in Blood in 2011 with 421 patients.3
The first HDM/SCT for AL amyloidosis was performed in July 1994 at the Amyloidosis Center at Boston University School of Medicine. Patients have been enrolled in several sequential institutional review board–approved protocols during the 20-year study period. Eligibility criteria for all protocols required biopsy proof of amyloid disease; evidence of a plasma cell dyscrasia and exclusion of other types of amyloidosis as appropriate; ≥1 major affected organ; and adequate measures of cardiac and pulmonary function and performance status. Inclusion criteria included cardiac ejection fraction ≥40%, absence of symptomatic pleural effusions, absence of uncompensated heart failure or arrhythmias resistant to medical management, oxygen saturation ≥95% on room air, lung diffusion capacity ≥50% predicted, supine systolic blood pressure ≥90 mm Hg, and Southwest Oncology Group performance status score ≤2 unless limited by peripheral neuropathy. End-stage renal disease requiring dialysis was not an absolute contraindication if other eligibility criteria were met. Age, renal function, time from diagnosis, prior therapy, and details of the conditioning regimen varied among the trials. A modified dose of melphalan at 100 or 140 mg/m2 was administered to patients >65 years of age, with a left ventricular ejection fraction between 40% and 45%, stem cell collection of 2 to <2.5 × 106 CD34+ cells/kg, or with poor performance status.
Between 1994 and 2014, 629 patients with AL amyloidosis were treated with HDM/SCT. The median age was 57 years (range, 28-80 years). A total of 350 patients (55.6%) received full-dose melphalan at 200 mg/m2, whereas 279 (44.4%) received modified-dose melphalan at 100 to 140 mg/m2, based on age and organ function. All patients underwent stem cell mobilization with growth factor alone. Treatment-related mortality (TRM) defined as death occurring within 100 days after SCT occurred in 47 patients, leading to an overall TRM of 7.5% (n = 47 of 629). Additionally, there were 11 deaths during stem cell mobilization and collection phase prior to initiating HDM. After 2005, no deaths have occurred during stem cell mobilization and collection, and TRM has improved to 3.4% (n = 10 of 292).
Hematologic responses, based on international consensus criteria,4,5 were assessed in 543 (86.3%) patients at 6 to 12 months following treatment. Of evaluable patients, 40.3% (n = 219 of 543) achieved a hematologic complete response (CR) after SCT, and by intention to treat, the CR rate was 34.8%. Hematologic CR occurred in 44.9% (n = 142 of 316) patients who received 200 mg/m2 HDM compared with 33.8% (n = 76 of 225) patients who received 100 to 140 mg/m2 HDM (χ2, P = .0091). Hematologic relapse occurred in 40 patients (18.2%) with CR at a median of 3.97 years (range, 1.89-12.45 years). Of these patients with hematologic relapse, 16 (40%) had received 100 to 140 mg/m2 HDM, whereas 24 (60%) had received 200 mg/m2 HDM.
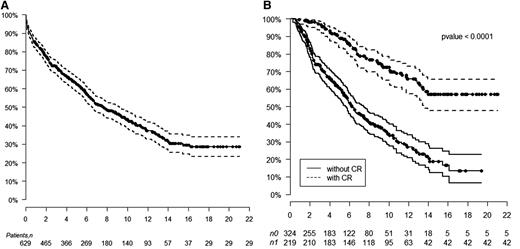
The median overall survival (OS) is 7.63 years, with a median follow-up of 8 years. The median OS has not been reached for patients achieving a hematologic CR compared with 6.3 years for those not achieving CR (log-rank, P < .0001). The median OS for patients receiving 200 mg/m2 melphalan is 10.47 years compared with 5.15 years for those receiving 100 to 140 mg/m2 HDM (log-rank, P < .0001). Kaplan-Meier estimates of survival are shown in Figure 1. OS at 1, 5, 10, and 15 years for those with hematologic CR is 100%, 88%, 72%, and 57%, respectively, compared with those without CR of 94%, 60%, 34%, and 18%, respectively. The median OS for patients following hematologic relapse is 4.3 years. Forty patients who achieved CR died without evidence of hematologic relapse. Their causes of death included sudden death (7), sepsis (4), metastatic malignancy (6), stroke (3), heart failure (5), renal failure (5), therapy-related myelodysplastic syndrome/acute myeloid leukemia (4), bleeding complications (2), and unknown (4).
Kaplan-Meier estimates of overall survival. (A) Overall survival after HDM/SCT for AL amyloidosis. (B) Overall survival based on achievement of hematologic CR after HDM/SCT.
Kaplan-Meier estimates of overall survival. (A) Overall survival after HDM/SCT for AL amyloidosis. (B) Overall survival based on achievement of hematologic CR after HDM/SCT.
It is important to note that these 629 patients are a highly selected group of all new patients with AL amyloidosis seen at our center.
In summary, long-term survival up to 20 years was achieved in 28.6% of patients with AL amyloidosis treated with HDM/SCT. Although survival is strongly dependent on achieving a hematologic CR, the survival of patients who do not achieve a CR and of those who relapse after CR is also notable, suggesting a benefit of aggressive treatment. Strategies to improve risk stratification of patients and reduce TRM, as well as using sequential or combination therapies with novel agents to increase the CR rate, will likely improve outcomes in the future for patients who just a few years ago were considered to have a rapidly fatal diagnosis.
Authorship
Acknowledgments: The authors acknowledge the numerous colleagues in the Amyloidosis Center, Cancer Clinical Trials Office, and Center for Cancer and Blood Disorders at Boston Medical Center, who assisted with the multidisciplinary evaluation and treatment of the patients with AL amyloidosis.
Contribution: V.S. designed research, performed research, analyzed data, and wrote the manuscript; F.S. performed statistical analysis; K.Q. designed research, performed research, analyzed data, and critically reviewed the manuscript; J.M.S. critically reviewed the manuscript; J.L.B. performed research and critically reviewed the manuscript; D.C.S. designed research, performed research, analyzed data, and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
David C. Seldin died on June 27, 2015.
Correspondence: Vaishali Sanchorawala, Section of Hematology/Oncology, FGH 1007, 820 Harrison Ave, Boston, MA 02118; e-mail: vaishali.sanchorawala@bmc.org.