Key Points
Extracellular Hb alters the GPIbα-VWF interaction.
Abstract
Intravascular hemolysis occurs in patients on extracorporeal membrane oxygenation. High levels of free acellular adult hemoglobin (free HbA) are associated with clotting in this mechanical device that can result in thrombotic complications. Adsorption of fibrinogen onto the surface of biomaterial correlates with platelet adhesion, which is mediated by von Willebrand factor (VWF). Because free Hb interacts with VWF, we studied the effect of hemoglobin (Hb) on platelet adhesion to fibrin(ogen) under conditions of different hydrodynamic forces. This effect was investigated using purified human HbA and fibrinogen, extracellular matrix, collagen, or purified plasma VWF as surface-coated substrates to examine flow-dependent platelet adhesion. Antibodies and VWF-deficient plasma were also used. Free Hb (≥50 mg/dL) effectively augmented platelet adhesion, and microthrombi formation on fibrin(ogen), extracellular matrix, and collagen at high shear stress. The effect of free Hb was effectively blocked by anti-glycoprotein Ibα (GPIbα) antibodies or depletion of VWF. Unexpectedly, free Hb also promoted firm platelet adhesion and stable microthrombi on VWF. Lastly, we determined that Hb interacts directly with the A1 domain. This study is the first to demonstrate that extracellular Hb directly affects the GPIbα-VWF interaction in thrombosis, and describes another mechanism by which hemolysis is connected to thrombotic events.
Introduction
The excessive release of hemoglobin (Hb) from erythrocytes into the circulation of patients on mechanical circulatory support devices is a well-recognized major clinical complication.1 Increasing incidence of hemolysis and thrombosis is associated with morbidity and mortality in patients on extracorporeal membrane oxygenation (ECMO).2 Prevention of circuit clotting in ECMO can improve clinical outcome.
von Willebrand factor (VWF) is a multimeric plasma glycoprotein that mediates platelet adhesion, activation, and aggregation under high flow conditions.3-7 Plasma VWF mediates platelet adhesion to surfaces coated with fibrin(ogen),8,9 which is adsorbed onto surfaces of many materials used in biomedical instruments, including ECMO.10,11 Previously, we reported that free Hb interacts with the A2 domain of VWF12 and, moreover, we and many others have described that the A2 domain regulates the binding of its neighboring A1 domain in VWF to platelet receptor glycoprotein Ibα (GPIbα).13-15 Thus, in this study, we examined the effect of the free Hb-VWF interaction on mediating platelet adhesion to immobilized fibrin(ogen) at high shear stress; a mechanism not previously investigated.
Study design
Reagents
Purified Hb and plasma VWF were obtained using established methods.13,16 Human collagen type III was purchased from Advanced BioMatrix, human fibrinogen from Calbiochem, and extracellular matrix (ECM) from Sigma-Aldrich. Anti-GPIbα antibody 6D1 was a gift from Dr Barry Coller (The Rockefeller University, New York, NY). Antibodies, AN51 and SZ2, were purchased from ThermoScientific. Heparin was purchased from APP Pharmaceuticals LLC. VWF-deficient plasma was obtained from Aniara Diagnostica. Recombinant A1A2A3 variants of VWF, and the single A1 domain, were purified as described previously.13,14
Binding assays
The dissociation constant for the binding of acellular adult Hb (HbA) to the A1 domain of VWF coupled onto a CM5 chip was determined by using surface plasmon resonance (Biacore 3000).17 We used the gain-of-function A1(R1450E)A2A3 mutant to analyze the effect of free Hb on VWF-GPIbα binding.18 Platelet-rich plasma containing the mutant and purified Hb were mixed with rabbit anti-VWF antibody (10 μg/mL; Dako) followed by a goat anti-rabbit-conjugated Alexa Flour 647 (20 μg/mL). The platelets were fixed with 1% formaldehyde and analyzed using Image Stream.
Flow assays
To obtain blood, informed consent was provided according to the recommendations of the Declaration of Helsinki. Approval was obtained from the Baylor College of Medicine Institutional Review Board for these studies. We used a microfluidic BioFlux System, and plates coated with ECM (25 μg/mL), collagen (50 μg/mL), and fibrinogen (100 μg/mL).14,17 Platelets were labeled with the fluorescent dye.17 Three hundred microliters of citrated whole blood containing buffer, acellular Hb, antibodies, and/or heparin were perfused over the coated plate at shear stress 60 dyne/cm2. Experiments were performed in duplicate using 4 different blood donors. To analyze the role of VWF, whole blood was centrifuged at 2500g for 15 minutes at room temperature. The supernatant plasma was removed and the blood cells were resuspended to their original volume with either VWF-deficient plasma or normal plasma as a positive control. The fluorescently labeled platelets that adhered and aggregated on the ligand-coated surface were analyzed using the system’s software.
Results and discussion
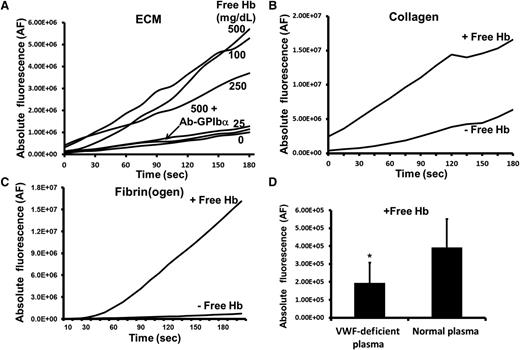
Acellular Hb has a threshold level of ≥50 mg/dL (∼30 μM heme) for initiation of platelet adhesion to ECM that contains proteins which capture flowing platelets (Figure 1A; supplemental Figure 1A, see supplemental Data available at the Blood Web site). Similarly, free Hb significantly increases VWF-mediated platelet adhesion to collagen and fibrin(ogen) (Figures 1B-C; supplemental Figure 1B). Acellular Hb increases the formation of large stable microthrombi on those substrates (supplemental Figure 1C). We examined 3 anti-GPIbα antibodies, and each one effectively blocked platelet deposition in the presence of free Hb (Figure 1A). Moreover, platelet adhesion to collagen was reduced 50% when ∼50% to 60% of plasma VWF was depleted from the blood mixed with purified Hb (Figure 1D). These results indicate that free Hb can directly affect GPIbα-VWF binding, which is the first step in platelet adhesion at high shear stress.
Free Hb increases platelet adhesion to ECM, collagen, and fibrin(ogen) at high shear stress. (A) Citrated whole blood containing purified human HbA (0.0-500 mg/dL) was perfused over a surface coated with ECM at high shear stress (60 dyne/cm2). Each anti-GPIbα antibody (SZ2, AN51, or 6D1, 20 μg/mL) effectively blocked (85%-95%) platelet adhesion in the presence of acellular Hb. Under similar flow conditions, blood mixed with 500 mg/dL (∼300 μM heme) free Hb was perfused over (B) human collagen type III, or (C) human fibrinogen. Buildup of the fluorescently labeled platelets was recorded during the 3-minute perfusion. The graphs depict adhered/accumulated fluorescently labeled platelets (AF) as a function of time, and are representative of 4 to 10 separated experiments. (D) The enhancement of platelet adhesion to collagen by free Hb (500 mg/mL) was significantly reduced when VWF was diminished in the perfused whole blood. The bar graph shows the adhesion of platelets after 3-minute perfusion (mean ± SD) (*P < .0001).
Free Hb increases platelet adhesion to ECM, collagen, and fibrin(ogen) at high shear stress. (A) Citrated whole blood containing purified human HbA (0.0-500 mg/dL) was perfused over a surface coated with ECM at high shear stress (60 dyne/cm2). Each anti-GPIbα antibody (SZ2, AN51, or 6D1, 20 μg/mL) effectively blocked (85%-95%) platelet adhesion in the presence of acellular Hb. Under similar flow conditions, blood mixed with 500 mg/dL (∼300 μM heme) free Hb was perfused over (B) human collagen type III, or (C) human fibrinogen. Buildup of the fluorescently labeled platelets was recorded during the 3-minute perfusion. The graphs depict adhered/accumulated fluorescently labeled platelets (AF) as a function of time, and are representative of 4 to 10 separated experiments. (D) The enhancement of platelet adhesion to collagen by free Hb (500 mg/mL) was significantly reduced when VWF was diminished in the perfused whole blood. The bar graph shows the adhesion of platelets after 3-minute perfusion (mean ± SD) (*P < .0001).
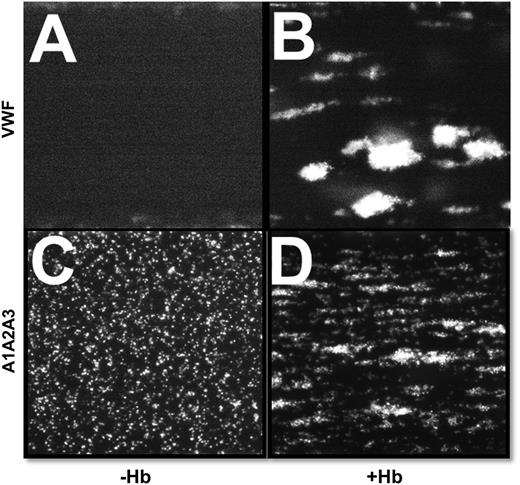
We examined the GPIbα-A1 domain interaction in the presence of free Hb by perfusing whole blood over a surface coated with purified VWF. Normally, platelets moderately stick over VWF-coated surfaces,13 but surprisingly, free Hb induced the flowing platelets to quickly adhere and form large stable microthrombi (Figure 2B). Similar results were observed when blood was perfused over the fragment A1A2A3 domain protein, which functions like VWF but lacks the site for the GPIIb/IIIa receptor.19 Contrary to the rolling platelets with whole blood (Figure 2C),18 free Hb induced the formation of both firmly stable microthrombi and rolling microaggregates (Figure 2D and supplemental Figure 2A, respectively). Free Hb also increased the adhesion of flowing fixed platelets to the VWF-coated surface at high shear stress (supplemental Figure 2B). Thus, free Hb clearly alters the strength of the binding interaction between the A1 domain and GPIbα.
Free Hb enhances the interaction between platelet GPIbα and purified plasma VWF at high shear stress. (A,C) Citrated whole blood with buffer or (B,D) purified human HbA (500 mg/dL) was perfused over a surface coated with purified plasma VWF or wild-type A1A2A3 protein at high shear stress (60 dyne/cm2). A magnification of ×10 was used for VWF, whereas ×20 was used for A1A2A3 protein. Platelets from blood containing free Hb were quickly arrested and capable of forming stable microthrombi.
Free Hb enhances the interaction between platelet GPIbα and purified plasma VWF at high shear stress. (A,C) Citrated whole blood with buffer or (B,D) purified human HbA (500 mg/dL) was perfused over a surface coated with purified plasma VWF or wild-type A1A2A3 protein at high shear stress (60 dyne/cm2). A magnification of ×10 was used for VWF, whereas ×20 was used for A1A2A3 protein. Platelets from blood containing free Hb were quickly arrested and capable of forming stable microthrombi.
Collagen and snake venom protein botrocetin bind to the A1 domain of VWF and increase its affinity for GPIbα, decreasing the GPIbα dissociation rate.20-22 Thus, we explored whether free Hb also directly interacts with the A1 domain, and determined that binding occurs with an equilibrium dissociation constant (KD) = 14.8 ± 8.8 μM (supplemental Figure 3), possibly explaining the increase of VWF-mediated platelet adhesion when the level of free Hb is ≥50 mg/dL (or 30 μM). A recent study reported that levels of free Hb ≥50 mg/dL may predict mortality in patients on ECMO,23 and this level correlates with the threshold observed for platelet adhesion in our flow-dependent assay (supplemental Figure 1A). It appears possible that free Hb binds to the A1 domain when the A1A2 interaction in VWF is dissociated as a consequence of stretching forces or immobilization onto various surfaces.13-15 Thus, A1 binding to Hb, like collagen and botrocetin, may induce a conformational change that increases the surface available for interaction with GPIbα,20 and/or the Hb bound to the A1 domain forms a new and stronger interface for GPIbα binding.22 In fact, the binding of the more active A1(R1450E)A2A3 mutant18 to GPIbα was slightly increased by free Hb (supplemental Figure 4).
Free Hb may mediate platelet activation via multiple mechanisms, one example being its ability to scavenge nitric oxide, which inhibits platelet activation in circulation.24 Our new study shows that acellular Hb increases platelet adhesion and thrombosis by interacting with the A1 domain of VWF, and not via GPIbα, because free Hb (∼30 μM) did not bind to platelets (not shown). Flow through the circuit and oxygenator in ECMO generates a shear stress that causes hemolysis.25 This shear could fluctuate in ECMO, and we have shown that free Hb also increases platelet adhesion at lower hydrodynamic forces (supplemental Figure 5). However, our data indicate that the presence of a high level of free Hb in circulation has a much greater effect on promoting the Hb-VWF-GPIb interactions at high shear stress. This effect would explain the increased buildup of platelet clots on the surfaces of the extracorporeal circuit for patients with higher levels of hemolysis. Finally, heparin only weakly inhibited the action of free Hb (supplemental Figure 6), and this lack of effect could be a reason for the formation of clots in the circuit despite the continuous infusion of heparin. Thus, blocking Hb-VWF interactions could be an effective way to prevent circuit clotting, and further investigation is needed to understand the pathophysiological role of the GPIbα-Hb-VWF complex in thrombosis in mechanical circulatory support devices.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant P01-HL110900 from the National Heart, Lung, and Blood Institute (NHLBI) (J.S.O.) and grant C-0612 from the Robert A. Welch Foundation (J.S.O.). This work was also supported by the Mary Gibson Foundation, the Alkek Foundation, the Fondren Foundation, and NIH grant R01-GM112806 from the National Institute of General Medical Sciences (NIGMS) (M.A.C.).
Authorship
Contribution: Q.D. and M.T. performed most of the experiments and analyzed data; P.G. performed experiments; J.T. and J.S.O. contributed ideas for experiments and reagents, and helped write the paper; and M.A.C. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Miguel A. Cruz, Section of Cardiovascular Research, Baylor College of Medicine and MEDVAMC, 2002 Holcombe, Building 109, R-146, Houston, TX 77030; e-mail: miguelc@bcm.edu.
References
Author notes
Q.D. and M.T. equally contributed to the study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal