In this issue of Blood, Da et al report that, in a high shear stress model, free hemoglobin (Hb) binding to von Willebrand factor (VWF) elicits the formation of platelet aggregates on surfaces coated with fibrinogen. This defines a novel paradigm for understanding the development of thrombosis in mechanical circulatory support devices.1
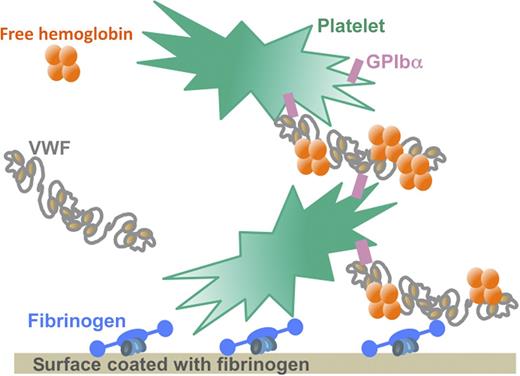
Under the high shear stress conditions characteristic of mechanical circulatory support devices, free Hb binds to VWF and increases the affinity of the VWF multimers for the GPIb receptor on the surface of platelets. This interaction allows a massive binding of platelets to surfaces coated with fibrinogen or VWF. Because free Hb levels are elevated in the blood of patients with mechanical circulatory support devices as a result of hemodynamic perturbations, the interaction between free Hb and VWF may contribute to the development of thrombi by boosting platelet binding and aggregation at the surface of the circulatory support device that is coated with adsorbed fibrinogen. Illustration by Sart-Bernard Studios.
Under the high shear stress conditions characteristic of mechanical circulatory support devices, free Hb binds to VWF and increases the affinity of the VWF multimers for the GPIb receptor on the surface of platelets. This interaction allows a massive binding of platelets to surfaces coated with fibrinogen or VWF. Because free Hb levels are elevated in the blood of patients with mechanical circulatory support devices as a result of hemodynamic perturbations, the interaction between free Hb and VWF may contribute to the development of thrombi by boosting platelet binding and aggregation at the surface of the circulatory support device that is coated with adsorbed fibrinogen. Illustration by Sart-Bernard Studios.
Mechanical circulatory support devices may contribute to the development of thrombosis not only by contact of their artificial surfaces with blood but also through hydrodynamic forces. This high shear stress induces the transition of VWF from a globular state to an extended chain conformation,2 potentially facilitating its interaction with platelets. Hydrodynamic forces may also indirectly contribute to the development of thrombosis through the destruction of red blood cells. Hemolysis is indeed often associated with platelet activation3 and several mechanisms involving free Hb may account for this, including direct platelet activation by Hb, the production of reactive oxygen species, and the scavenging of nitric oxide.4,5
Using in vitro conditions representative of those occurring on the surface of extracorporeal membrane oxygenation devices, Da et al1 now demonstrate that free Hb binds directly to VWF, thereby increasing the affinity of the VWF A1 domain for the glycoprotein Ib (GPIb) receptor on the surface of platelets (see figure). The increased interaction between platelets and VWF then allows a massive binding of platelets to insolubilized fibrinogen or to components of the extracellular matrix such as collagen.1 This qualitative and quantitative alteration of platelet binding and aggregation induced by free Hb may contribute to the development of thrombi in circulatory devices despite strong anticoagulation with heparin.
The role of VWF in thrombosis in mechanical circulatory support devices may appear paradoxical. Indeed, VWF is secreted by endothelial cells into plasma as a large multimeric glycoprotein and the size of the multimers determines their ability to mediate platelet aggregation. However, in mechanical circulatory support devices, the hydrodynamic forces induce the loss of the high molecular weight multimers of VWF, a so-called acquired von Willebrand syndrome, predisposing patients to bleeding complications.6,7 It remains to be determined whether the interaction of free Hb with low and intermediate molecular weight multimers increases VWF activity, which might reduce the risk of bleeding yet promote the development of thrombosis in circulatory support devices. Alternatively, the interaction of free Hb with VWF may contribute to the disappearance of the largest multimers trapped in platelet aggregates.
The study of Da et al was carried out under experimental conditions representative of the circulation in mechanical circulatory support devices. However, it is tempting to speculate that the modulation of platelet interaction with VWF that is mediated by free Hb may also play a role in other clinical situations. Microangiopathic hemolytic anemia of diverse origins is characterized by thrombosis in small vessels, which results in the destruction of red blood cells and increased free Hb levels. In such diseases, free Hb may thus contribute to a vicious circle, accelerating the formation of platelet aggregates and the subsequent clinical thrombotic and hemorrhagic complications. Such a mechanism may play an even more important role in cases of thrombotic thrombocytopenic purpura, a disease caused by a defect in the cleavage of ultralarge VWF molecules by the VWF protease a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13).8 It has been previously reported that free Hb can inhibit the cleavage of ultralarge VWF strings by ADAMTS13,9 so it will be interesting to determine whether free Hb also significantly increases the interaction between ultralarge VWF strings and platelets.
The observations by Da et al are important not only because they define a novel paradigm explaining the prothrombotic action of free Hb, but also because they provide a rationale for the development of novel strategies to prevent thrombosis in extracorporeal circulation devices and possibly in microangiopathic hemolytic anemias. In their study, Da et al were able to successfully neutralize the effect of free Hb on platelets using an antibody blocking GPIb. The usefulness of other antibodies or small molecules targeting VWF10 may also be worth investigating.
Conflict-of-interest disclosure: The authors declare no competing financial interests.