Key Points
Plasmin inactivates the enzyme FXIIIa, but not the zymogen FXIII.
FXIIIa is inactivated during clot lysis.
Abstract
Coagulation factor XIIIa (FXIIIa) is a transglutaminase that covalently cross-links fibrin and other proteins to fibrin to stabilize blood clots and reduce blood loss. A clear mechanism to describe the physiological inactivation of FXIIIa has been elusive. Here, we show that plasmin can cleave FXIIIa in purified systems and in blood. Whereas zymogen FXIII was not readily cleaved by plasmin, FXIIIa was rapidly cleaved and inactivated by plasmin in solution (catalytic efficiency = 8.3 × 103 M−1s−1). The primary cleavage site identified by mass spectrometry was between K468 and Q469. Both plasma- and platelet-derived FXIIIa were susceptible to plasmin-mediated degradation. Inactivation of FXIIIa occurred during clot lysis and was enhanced both in plasma deficient in fibrinogen and in plasma treated with therapeutic levels of tissue plasminogen activator. These results indicate that FXIIIa activity can be modulated by fibrinolytic enzymes, and suggest that changes in fibrinolytic activity may influence cross-linking of blood proteins.
Introduction
The coagulation system controls a polymerization process that is required to seal vascular damage. Fibrin is quickly generated and cross-linked into a mesh of insoluble fibers by the transglutaminase factor XIIIa (FXIIIa). Mechanisms of synthesis and activation of FXIIIa are known,1 however, the physiological mechanism of inactivation remains unclear.
Plasma FXIII (pFXIII-A2B2) consists of 2 catalytic A subunits and 2 regulatory B subunits arranged as a heterotetramer, whereas platelets and monocytes express intracellular FXIII-A2 without the B subunits (cFXIII-A2).1 During clotting, thrombin cleaves a 37-residue activation peptide from the A subunit of pFXIII-A2B2. Then, in the presence of calcium, the A subunits disassociate from the B subunits and undergo a conformational change to become catalytically active. The active FXIII-A2*, also referred to as FXIIIa, catalyzes the formation of a pseudopeptide bond between a glutamine residue of a protein and a lysine residue of another protein, releasing ammmonia.1 Fibrin is the major substrate of FXIII-A2*. Self-assembled fibrin becomes stabilized when it is covalently cross-linked to itself, the vessel wall, and to antifibrinolytic proteins by FXIII-A2*. In plasma, pFXIII-A2B2 circulates bound to fibrinogen.2 Although most FXIIIa remains bound to fibrin during clot formation,3 active FXIIIa released from the clot, such as during clot lysis, would presumably circulate systemically. Although it has been reported that FXIIIa can be inactivated by thrombin4 or proteolytic enzymes released by granulocytes,5 it remains unclear whether these mechanisms extend to all the intracellular, intravascular, and extravascular FXIIIa. These reports have led to the question: are there other mechanisms of inactivation of FXIIIa?
A long-standing hypothesis is that FXIIIa is inactivated by the fibrinolytic system, which normally degrades the fibrin clot and associated proteins.6 The fibrinolytic enzyme plasmin is generated from plasminogen by activators such as tissue plasminogen activator (tPA). Previous reports found that cFXIII-A2, cFXIII-A2*, and pFXIII-A2B2 were resistant to plasmin degradation.7 Here, we show that zymogen FXIII is resistant to degradation by fibrinolytic enzymes at physiological concentrations. However, contrary to previous reports, the enzymatically active FXIII-A2* is readily degraded by endogenous plasmin during clot lysis.
Methods
Activating and degrading FXIII A and B subunits in purified systems and in blood
To generate FXIII-A2*, human pFXIII-A2B2 (Haematologic Technologies Inc) was incubated with bovine thrombin (400 nM, 2 U/mL; Sigma-Aldrich) and calcium chloride (4 mM) for 30 minutes in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)-buffered saline (HBS; 10 mM HEPES, 140 mM NaCl, pH 7). Thrombin was then inhibited by hirudin (400 anti-thrombin units [ATU]/mL; EMD Millipore), and plasmin (Haematologic Technologies Inc) was added to pFXIII-A2B2 or pFXIII-A2* (30 nM, 0.6 U/mL). For platelet experiments, platelets (1 × 1011/L) were activated with bovine thrombin (400 nM, 2 U/mL) before incubation with plasmin or tPA (Genentech). For plasma-based experiments, citrated plasma was recalcified (10 mM CaCl2) and then activated with diluted Innovin (1 pM; Dade Behring) for 30 minutes. Fibrinogen-deficient plasma was activated with thrombin (200 nM, 1 U/mL) to fully activate FXIII. In control samples that were not activated, hirudin (100 ATU/mL) was added to inhibit endogenous thrombin and thus FXIIIa generation. Tranexamic acid (TXA) (7.5 mM; Sigma-Aldrich), an inhibitor of plasmin and tPA,8 was added to specified samples before plasmin or tPA was added. All samples were incubated at 37°C. Each experiment was repeated at least 3 times. All P values were calculated by the 2-tailed Student t test.
Preparing platelets
This study was approved by the research ethics boards of the University of British Columbia, and informed consent was obtained from all healthy volunteers before whole-blood donation. Whole blood was collected into tubes containing sodium citrate (12 mM) and was centrifuged at 100g for 20 minutes. The top 75% of the platelet-rich plasma fraction was collected. Platelets were pelleted by centrifugation at 250g for 10 minutes, and washed in CGS buffer (120 mM NaCl, 30 mM d-glucose, 11 mM trisodium citrate, pH 6.5) and then in Tyrode buffer (1.8 mM CaCl2,137 mM NaCl, 2.7 mM KCl, 0.4 mM NaH2PO4, 10 mM HEPES, 5.6 mM monohydrate d-glucose, 1.1 mM MgCl2, pH 6.5). Platelets were then resuspended in Tyrode buffer at a final concentration of 2 × 1011 platelets/L.
Western blotting
Samples were prepared as described previously.9 All samples were reduced and boiled prior to electrophoresis on 4% to 15% Mini-PROTEAN TGX gel (Bio-Rad) and transferred to a nitrocellulose membrane (Pall Life Sciences). Boiling the samples did not induce autodegradation of FXIII-A* (supplemental Figure 1, see supplemental Data available at the Blood Web site). After blocking with Odyssey Blocking Buffer (Li-Cor), the membrane was incubated overnight at 4°C with the primary antibody: sheep or rabbit anti-human FXIII A (Affinity Biologicals or Thermo, respectively, diluted 1/1000), rabbit anti-human FXIII B (Sigma-Aldrich, diluted 1/1000), or rabbit anti-human fibrinogen (Dako, diluted 1/50 000). The membrane was washed with phosphate-buffered saline with Tween 20 (8 mM Na2HPO4, 150 mM NaCl, 2 mM KH2PO4, 3 mM KCl, 0.05% Tween 20, pH 7.4). After incubation with the preadsorbed secondary antibody (Abcam, diluted 1/10 000), the membrane was washed and antibody detected with ECL substrates (Bio-Rad). Membranes were stripped with a solution of 62.5 mM Tris, pH 6.8, 2% sodium dodecyl sulfate, and 0.7% β-mercaptoethanol for 1 hour at 60°C with occasional agitation and then reprobed. The statistical significance was calculated using a 2-tailed Student t test.
Identifying the plasmin-mediated cleavage site of FXIIIa
FXIII-A2* (10 μM) was incubated with plasmin (2.7 μM) at 37°C for 2 hours. The reaction was stopped by heat-inactivating the sample at 95°C for 5 minutes. The samples were labeled on the newly exposed N termini using reductive methylation, trypsin-digested, and cleaned.10-12 Peptide samples were purified by solid-phase extraction and analyzed using a linear-trapping quadrupole-Orbitrap mass spectrometer online coupled to an Agilent 1290 Series high-performance liquid chromatography using a nanospray ionization source. Centroided fragment peak lists were processed with Proteome Discoverer version 1.2 and searched with Mascot algorithm. The peptides identified as cleavage products were those that had IonScores over the 99% confidence limit. This experiment was repeated twice with similar results.
Measuring the kinetics of inactivation of FXIII-A2* by plasmin
Plasmin-mediated inactivation of FXIII-A2* was evaluated using steady-state kinetics at 37°C. The rate of inactivation of FXIIIa was determined by measuring the transglutaminase activity of FXIII-A2* via the production of ammonia. First, a calibration curve was generated that related the concentrations of ammonia to A570 using a colorimetric detection assay for ammonia (BioVision Inc), monitored with a Tecan M200 plate reader. A second calibration curve was generated that related the concentration of FXIII-A2* (1.6 to 78.1 nM) to the velocity of ammonia generation. This transglutaminase assay was performed by mixing FXIII-A2* with an amine donor (glycine ethyl ester, 2.5 mM), a glutamine-containing peptide (NQEQVSPLTLLK, 1 mM), dithiothreitol (40 μM), and CaCl2 (3 mM). Aliquots from the transglutaminase reaction mixture were removed and quenched every 15 minutes with EDTA (15 mM). Plasma FXIII-A2B2 (7.8-62.5 nM) was converted to FXIII-A2* by pretreating it with thrombin (4 U/mL) and CaCl2 (3 mM) for 1 hour to distinguish the rate of degradation by plasmin from the rate of activation by thrombin. Thrombin was then inhibited with excess hirudin (13 ATU/mL). To this mixture, plasmin (300 nM) was added. At various time points, aliquots were removed and plasmin activity was quenched with a cocktail of protease inhibitors (Sigma-Aldrich). The velocity of inactivation of FXIII-A2* was determined by measuring the residual amount of FXIII-A2* activity at each time point using the transglutaminase assay with the 2 calibration curves. Kinetic parameters were calculated using graphing software (OriginPro 9.1).
Measuring elastic moduli by TEG
Studies were performed using normal, plasminogen-deficient, α2-antiplasmin–deficient, or fibrinogen-deficient plasma (Haematologic Technologies Inc) and shear elastic moduli were evaluated at 37°C using a thromboelastography (TEG) Hemostasis Analyzer System 5000 (Haemoscope Corporation). Inactivation of FXIII-A2* was evaluated during clot formation by combining 230 μL of plasma with CaCl2 (10 mM), Innovin (2 pM), and tPA (200 pM), with or without T101 (0.8 mM). FXIIIa inactivation was evaluated during clot lysis by initiating clotting of plasma with CaCl2 (10 mM) and bovine thrombin (400 nM, 2 U/mL), allowing the clot to form for 30 minutes and then adding tPA (800 nM). Fibrinolysis was inhibited by TXA (10 mM) at either 1 or 3 hours after addition of tPA and the resulting plasma was added to a TEG cup containing fibrinogen (FXIII-free 1.4 mg/mL; Enzyme Research Laboratories), thrombin (2 U/mL), and either HBS, T101 (0.8 mM), or FXIII (200 nM), upon which the TEG analysis began. FXIII-A2* inactivation was evaluated during clot formation under conditions mimicking thrombolysis by combining CaCl2 (10 mM), Innovin (2 pM), and each plasma containing either 5 nM tPA (α2-antiplasmin–deficient plasma) or 50 nM tPA (normal and plasminogen-deficient plasma). Activation of clotting progressed for 4 minutes before adding TXA (10 mM), followed by adding HBS, T101 (0.8 mM), or FXIII (200 nM), then fibrinogen (1.4 mg/mL; Haematologic Technologies Inc), before commencing TEG analysis.
Results
Activated FXIII-A2*, but not zymogen pFXIII-A2B2, is proteolytically inactivated by plasmin
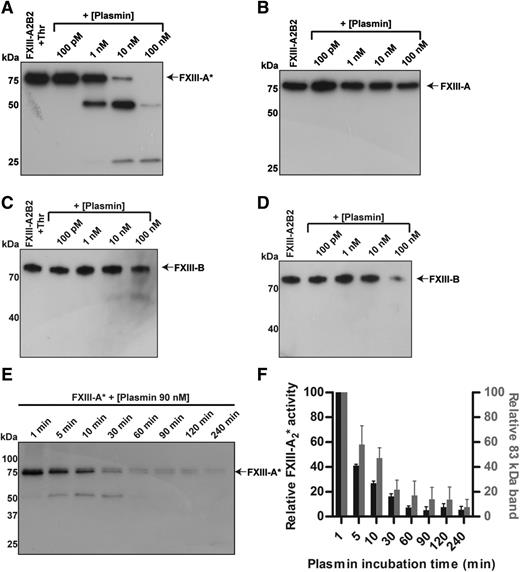
To evaluate the degradation of plasma-derived FXIII by plasmin, pFXIII-A2B2 and FXIII-A2* were each treated with varying concentrations (100 pM to 100 nM) of plasmin for 3 hours, and analyzed by western blot. During the 3 hours, FXIII-A2* was cleaved by concentrations as low as 1 nM of plasmin (Figure 1A). Cleavage products of ∼50 and 25 kDa were visible, but were transient. However, the A subunit of the zymogen was more resistant to cleavage than the enzyme (Figure 1B, supplemental Figure 2). Degradation of the B subunit from both activated and unactivated pFXIII-A2B2 also occurred (Figure 1C-D). Activation of FXIII by plasmin was not observed. Cleavage products of the B subunit, and possibly other cleavage products of the A subunit, may not have been detected due to a faster rate of degradation relative to that of intact FXIII, or a low abundance or absence of antigens identified by the antibody (supplemental Figure 3). To evaluate whether the cleavage products were enzymatically active, the cleavage and activity of FXIII-A2* were monitored over time. Degradation of FXIII-A2* by plasmin (90 nM) was evident within 5 minutes and the transglutaminase activity of FXIII-A2* decreased rapidly over 30 minutes (Figure 1E-F). The loss of FXIII-A2* activity correlated with the loss of fully intact FXIII-A2*, indicating that cleavage products of FXIII-A2* were enzymatically inactive.
FXIII-A2* is cleaved and inactivated by plasmin. FXIII (100 nM), with or without prior activation by thrombin (400 nM), was mixed with varying concentrations of plasmin (100 pM to 100 nM) for 3 hours, and analyzed by western blot against FXIII A and B subunits. (A) Blot against the A subunit of pFXIII-A2*, with thrombin activation. (B) Blot against the A subunit of pFXIII-A2B2. (C) Blot against the B subunit of pFXIII-A2*, with thrombin activation. (D) Blot against the B subunit of pFXIII-A2B2. (E) Time course of cleavage of FXIII-A2* by plasmin (90 nM). (F) Transglutaminase activity (left axis) of FXIII-A2* after incubation with plasmin (90 nM) and the relative amount of intact FXIII-A2* (right axis), determined by quantifying the intensity of the band at 83 kDa. FXIII-A* was calculated as a percentage of total signal in the lane using densitometry. The error bars represent standard error of the mean (SEM). n = 3 for all experiments. Thr, thrombin.
FXIII-A2* is cleaved and inactivated by plasmin. FXIII (100 nM), with or without prior activation by thrombin (400 nM), was mixed with varying concentrations of plasmin (100 pM to 100 nM) for 3 hours, and analyzed by western blot against FXIII A and B subunits. (A) Blot against the A subunit of pFXIII-A2*, with thrombin activation. (B) Blot against the A subunit of pFXIII-A2B2. (C) Blot against the B subunit of pFXIII-A2*, with thrombin activation. (D) Blot against the B subunit of pFXIII-A2B2. (E) Time course of cleavage of FXIII-A2* by plasmin (90 nM). (F) Transglutaminase activity (left axis) of FXIII-A2* after incubation with plasmin (90 nM) and the relative amount of intact FXIII-A2* (right axis), determined by quantifying the intensity of the band at 83 kDa. FXIII-A* was calculated as a percentage of total signal in the lane using densitometry. The error bars represent standard error of the mean (SEM). n = 3 for all experiments. Thr, thrombin.
FXIII-A2* is cleaved by plasmin at multiple sites
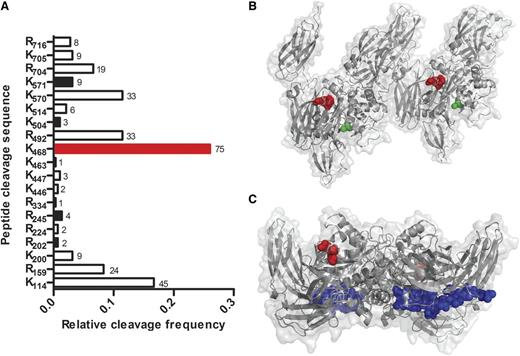
To identify which sites of FXIII-A2* were cleaved by plasmin, purified FXIII-A2* was incubated with plasmin and analyzed by mass spectrometry. Nineteen cleavage sites were identified (8 at arginines and 11 at lysines) of the 43 arginines and 34 lysines found in FXIII-A, with detection frequencies between 0.3% and 26% (Figure 2A). The most prominent cut site was between K468 and Q469, the product of which corresponds in size to the 25- and 50-kDa cleavage products observed in Figure 1A. To determine the spatial location of cleavage, the site was mapped onto 3-dimensional structures of FXIII-A2* and FXIII-A2.13,14 In the FXIII-A2* structure, the K468-Q469 cleavage site is near the surface, consistent with accessibility to plasmin-mediated cleavage (Figure 2B). Interestingly, this K468-Q469 bond also appears surface-accessible in the FXIII-A2 (unactivated) structure (Figure 2C). Because FXIII-A2 is not readily cleaved by plasmin, as shown in Figure 1B, this bond may be protected in the zymogen conformation by the B subunit, which has not been cocrystalized with the A subunit. Five other surface-exposed cleavage sites were identified, but detected eightfold to 38-fold less frequently.
Plasmin cleaves FXIII-A2* at multiple sites. (A) FXIII-A2* cleavage sites were identified by matrix-assisted laser desorption ionization time-of-flight (MALDI/TOF) mass spectrometry after purified FXIIIa (10 μM) was incubated with plasmin (2.7 μM) for 2 hours. Surface-exposed sites are represented with black bars, and the primary cleavage site with a red bar. The frequency of detection of cut sites is indicated beside the respective bars (n = 2). (B) A reported structure of FXIII-A2*,14 showing the surface-exposed K468-Q469 cleavage site (red) and the catalytic cysteine (green). The distance between the cleavage site and the catalytic cysteine is 18 Å. (C) A reported structure of FXIII-A2,13 showing K468-Q469 (red) and the activation peptide (blue).
Plasmin cleaves FXIII-A2* at multiple sites. (A) FXIII-A2* cleavage sites were identified by matrix-assisted laser desorption ionization time-of-flight (MALDI/TOF) mass spectrometry after purified FXIIIa (10 μM) was incubated with plasmin (2.7 μM) for 2 hours. Surface-exposed sites are represented with black bars, and the primary cleavage site with a red bar. The frequency of detection of cut sites is indicated beside the respective bars (n = 2). (B) A reported structure of FXIII-A2*,14 showing the surface-exposed K468-Q469 cleavage site (red) and the catalytic cysteine (green). The distance between the cleavage site and the catalytic cysteine is 18 Å. (C) A reported structure of FXIII-A2,13 showing K468-Q469 (red) and the activation peptide (blue).
The rate of inactivation of FXIII-A2* can occur on a physiologically relevant timescale
To determine the kinetic parameters of plasmin-mediated inactivation of purified FXIII-A2*, the loss of FXIII-A2* activity with plasmin treatment was measured. Plasmin-mediated inactivation of FXIII-A2* had an apparent Michaelis constant (Km) of 0.49 ± 0.02 µM and turnover number (kcat) of 4.2 ± 1.1 × 10−3 s−1, resulting in a catalytic efficiency (kcat/Km) of 8.3 ± 1.7 × 103 M−1s−1. The Km was ∼10-fold higher and the kcat was ∼10-fold lower than reported parameters for fibrin.15 The half-life of FXIIIa degradation by plasmin, in the absence of fibrin, is estimated to be 34 seconds, using these experimentally determined kinetic parameters and the physiological concentrations of the zymogens (200 nM for plasma- and platelet-derived FXIII[a], and 2.4 µM for plasmin[ogen]) in circulating blood. The maximal rate (Vmax) and Km were calculated by nonlinear fitting of the measured initial velocities to the Michaelis-Menten equation, which produced similar values to those obtained from a Lineweaver-Burke plot (Figure 3A-B). The rate of inactivation was calculated from the loss of FXIII-A2* activity, determined using an ammonia production assay (Figure 3C). The activity of FXIII-A2* decreased as it was cleaved by plasmin, resulting in a lower rate of ammonia generation (Figure 3D). These data indicate that the rate of FXIII-A2* degradation can occur on a physiologically relevant timescale.
The rate of inactivation of FXIII-A2* can occur on a physiologically relevant timescale. (A) Lineweaver-Burk plot used to generate kinetic parameters for FXIII-A2*, with data sets represented by different colors. The data point circled in pink is derived from the slope of the initial velocity in panel B (pink line). (B) The generation of FXIII-A2* cleavage products by plasmin (300 nM) at a representative FXIII-A2* concentration, determined using an ammonia release assay. (C) Standard curves of ammonia generation over time using various [FXIII-A2*]. (D) Ammonia generation over time, with varying times of FXIII-A2* digestion with plasmin. n = 3 for all experiments. Abs, absorbance.
The rate of inactivation of FXIII-A2* can occur on a physiologically relevant timescale. (A) Lineweaver-Burk plot used to generate kinetic parameters for FXIII-A2*, with data sets represented by different colors. The data point circled in pink is derived from the slope of the initial velocity in panel B (pink line). (B) The generation of FXIII-A2* cleavage products by plasmin (300 nM) at a representative FXIII-A2* concentration, determined using an ammonia release assay. (C) Standard curves of ammonia generation over time using various [FXIII-A2*]. (D) Ammonia generation over time, with varying times of FXIII-A2* digestion with plasmin. n = 3 for all experiments. Abs, absorbance.
Plasmin inactivates both plasma-derived and platelet-derived FXIII-A2*
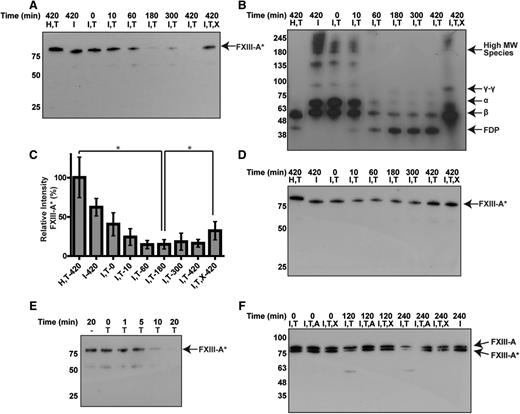
To test whether FXIII-A2* is sensitive to degradation by plasmin in plasma, plasmin was added to fibrin clots in platelet-poor plasma. FXIII-A2* was mostly degraded within 30 minutes by plasmin (3 µM, Figure 4A). Degradation of FXIII-A2* was inhibited when exogenous α2-antiplasmin or TXA was added (Figure 4B). These data show that plasma-derived FXIII-A2* can be degraded by plasmin in the presence of fibrin. To test whether cFXIII-A2 (from platelets) was sensitive to degradation by plasmin, platelets were initially activated by thrombin, followed by the addition of plasmin (1 nM to 1 µM). Platelet cFXIII-A2 was degraded, and transient degradation products were observed when concentrations of plasmin as low as 10 nM were added 16 hours after platelet activation, but not when plasmin was added 1 hour after platelet activation (Figure 4C-D). The degradation products were also observed in the presence of TXA, likely because reduced plasmin activity slowed their rate of cleavage. It is known that cFXIII-A2 translocates from the cytoplasm to the membrane upon platelet activation16 ; however, the results here suggest that cFXIII-A2 was not exposed to plasmin and was thus not degraded shortly after platelet activation. To test whether cFXIII-A2 was shielded from extracellular proteases, trypsin, which can cleave zymogen FXIII-A2B2,17 was added to platelets at 1 and 16 hours after activation. Platelet cFXIII-A2 was not degraded by trypsin at 1 hour, but was degraded by trypsin at 16 hours (supplemental Figure 4), indicating that exposure of cFXIII-A2 to extracellular proteases was delayed relative to platelet activation. Thus, platelet-derived FXIII-A2* can be degraded by plasmin following platelet activation.
Plasmin degrades plasma- and platelet-derived FXIII-A*. Western blots against the FXIII A subunit. (A) Endogenous FXIII-A2* from human plasma after adding plasmin (3 µM) for various times. (B) FXIII-A2* from plasma with plasmin (3 µM) and α2-antiplasmin (5 µM) or TXA (7.5 mM). (C-D) Endogenous FXIII-A2/FXIII-A2* from platelets (PLT), 1 hour (C) and 16 hours (D) after exposure to thrombin, and incubating with various concentrations of plasmin. Samples contain combinations of Innovin (I), plasmin (P), α2-antiplasmin (A), and TXA (X). n = 3 for all experiments.
Plasmin degrades plasma- and platelet-derived FXIII-A*. Western blots against the FXIII A subunit. (A) Endogenous FXIII-A2* from human plasma after adding plasmin (3 µM) for various times. (B) FXIII-A2* from plasma with plasmin (3 µM) and α2-antiplasmin (5 µM) or TXA (7.5 mM). (C-D) Endogenous FXIII-A2/FXIII-A2* from platelets (PLT), 1 hour (C) and 16 hours (D) after exposure to thrombin, and incubating with various concentrations of plasmin. Samples contain combinations of Innovin (I), plasmin (P), α2-antiplasmin (A), and TXA (X). n = 3 for all experiments.
Addition of tPA to plasma leads to degradation of pFXIII-A2* by endogenous plasmin
Addition of tPA (2 µM) to clotted normal plasma led to the degradation of FXIIIa and fibrin within 3 hours (Figure 5A-C). Degradation was inhibited by TXA. The degradation of pFXIII-A2* and fibrin occurred within a similar time frame, beginning within 10 minutes, and continuing to 180 minutes. To determine whether tPA degraded FXIIIa directly or by generating plasmin, plasminogen-deficient plasma was treated with tPA. Degradation of FXIIIa did not occur in plasminogen-deficient plasma, indicating that FXIIIa was degraded by tPA-mediated plasmin activity (Figure 5D). In fibrinogen-deficient plasma, FXIIIa was degraded more rapidly than in normal plasma when treated with tPA (comparing Figure 5E with Figure 5A), and was almost completely degraded within 10 to 20 minutes. In whole blood, FXIII-A2* was degraded when tPA was added, except in the presence of α2-antiplasmin or TXA (Figure 5F). Together, these data demonstrate that pFXIII-A2* degradation can occur by endogenous plasmin.
Endogenous plasminogen is activated by tPA to degrade endogenous FXIIIa. Plasma was analyzed by western blot against FXIII-A, after addition of tPA and TXA. (A-B) Time-dependent degradation of endogenous (A) FXIII-A2* and (B) fibrin(ogen) in normal plasma. (C) The relative amount of intact FXIII-A* from panel A using densitometry, calculated as a percentage of total signal in the lane. *P < .05. (D) Time course for plasminogen-deficient plasma. (E) Time-dependent degradation of FXIIIa in fibrinogen-deficient plasma after adding tPA. (F) Degradation of endogenous FXIII-A2*, but not FXIII-A2, in whole-blood clots with tPA. Samples contain combinations of hirudin (H), Innovin (I), tPA (2 µM) (T), TXA (7.5 mM) (X), and α2-antiplasmin (4 µM) (A). n = 3 for all experiments. FDP, fibrin degradation products; MW, molecular weight.
Endogenous plasminogen is activated by tPA to degrade endogenous FXIIIa. Plasma was analyzed by western blot against FXIII-A, after addition of tPA and TXA. (A-B) Time-dependent degradation of endogenous (A) FXIII-A2* and (B) fibrin(ogen) in normal plasma. (C) The relative amount of intact FXIII-A* from panel A using densitometry, calculated as a percentage of total signal in the lane. *P < .05. (D) Time course for plasminogen-deficient plasma. (E) Time-dependent degradation of FXIIIa in fibrinogen-deficient plasma after adding tPA. (F) Degradation of endogenous FXIII-A2*, but not FXIII-A2, in whole-blood clots with tPA. Samples contain combinations of hirudin (H), Innovin (I), tPA (2 µM) (T), TXA (7.5 mM) (X), and α2-antiplasmin (4 µM) (A). n = 3 for all experiments. FDP, fibrin degradation products; MW, molecular weight.
Plasmin-mediated FXIIIa inactivation occurs following fibrinolysis
To further characterize the pFXIII-A2* degradation by plasmin, we examined whether degradation occurred during or after clot formation, and if there were downstream effects on fibrin cross-linking. We first used western blotting to monitor pFXIII-A2* and fibrin during clot formation. tPA (200 pM) was added to plasma, and clotting was immediately initiated. Only a portion of pFXIII-A2* was degraded during fibrin formation and cross-linking, indicating that pFXIII-A2* remained active during clot formation in normal plasma (Figure 6A-B). We then evaluated pFXIII-A2* activity and its downstream effects using TEG because the mechanical strength (shear elastic modulus, G) of fibrin is closely tied to the activity of pFXIII-A2*. For these experiments, we used plasminogen-deficient plasma (reduced plasmin activity), normal plasma, and α2-antiplasmin–deficient plasma (increased plasmin activity), all containing tPA (200 pM). No differences in the moduli were observed between clots from the 3 types of plasma prior to fibrinolysis (Figure 6C). The moduli were all approximately threefold higher compared with samples containing an inhibitor of FXIIIa (T101), which does not inhibit the plasmin-mediated degradation of FXIII-A2* (supplemental Figure 5). These data indicate that pFXIII-A2* was not inactivated prior to fibrinolysis.
Plasmin-mediated inactivation of FXIII-A2* does not occur during normal clot formation, but does occur during fibrinolysis and thrombolytic conditions. (A-B) Clot formation in normal plasma with tPA (200 pM) and, in some cases, TXA (7.5 mM). Western blots against (A) FXIII-A and (B) fibrin(ogen) (n = 3). (C-E) TEG analyses of clot formation and cross-linking of exogenous (purified) fibrin in plasma. Schematics on the left show timelines of the procedures and characteristic shear elastic moduli (G, dashed lines), with shaded areas indicating time periods analyzed with TEG. Charts on the right show measured moduli of fibrin clots, a direct indicator of FXIII-A2* activity and fibrin structure. Control samples contain exogenous FXIIIa or T101. (C) Moduli of clots from plasminogen-deficient, normal, and α2-antiplasmin–deficient plasma formed in the presence of tPA (200 pM). (D) Moduli of exogenous fibrin (indicator of residual FXIIIa activity), added following clot lysis. Exogenous fibrinogen (1.4 mg/mL) and TXA were added 1 or 3 hours after clot lysis by tPA (800 nM). (E) Moduli of exogenous fibrin that was added during clot formation under thrombolytic conditions. TXA and then fibrinogen were added 4 minutes after clotting was initiated in the presence of tPA (50 nM or for α2-antiplasmin–deficient plasma, 5 nM). Samples contain combinations of Innovin (I), tPA (T), and TXA (X). **P < .01, ***P < .001. n = 3 for all experiments. TF, tissue factor.
Plasmin-mediated inactivation of FXIII-A2* does not occur during normal clot formation, but does occur during fibrinolysis and thrombolytic conditions. (A-B) Clot formation in normal plasma with tPA (200 pM) and, in some cases, TXA (7.5 mM). Western blots against (A) FXIII-A and (B) fibrin(ogen) (n = 3). (C-E) TEG analyses of clot formation and cross-linking of exogenous (purified) fibrin in plasma. Schematics on the left show timelines of the procedures and characteristic shear elastic moduli (G, dashed lines), with shaded areas indicating time periods analyzed with TEG. Charts on the right show measured moduli of fibrin clots, a direct indicator of FXIII-A2* activity and fibrin structure. Control samples contain exogenous FXIIIa or T101. (C) Moduli of clots from plasminogen-deficient, normal, and α2-antiplasmin–deficient plasma formed in the presence of tPA (200 pM). (D) Moduli of exogenous fibrin (indicator of residual FXIIIa activity), added following clot lysis. Exogenous fibrinogen (1.4 mg/mL) and TXA were added 1 or 3 hours after clot lysis by tPA (800 nM). (E) Moduli of exogenous fibrin that was added during clot formation under thrombolytic conditions. TXA and then fibrinogen were added 4 minutes after clotting was initiated in the presence of tPA (50 nM or for α2-antiplasmin–deficient plasma, 5 nM). Samples contain combinations of Innovin (I), tPA (T), and TXA (X). **P < .01, ***P < .001. n = 3 for all experiments. TF, tissue factor.
To determine whether inactivation occurred during fibrinolysis, normal plasma clots were fully formed and tPA (800 nM) was added to facilitate rapid lysis. The fibrinolytic system was inhibited by TXA at either 1 or 3 hours after addition of tPA and the contribution of residual pFXIII-A2* to the modulus of exogenous (purified) fibrin was determined. There was 42% and 60% decrease in the moduli at 1 hour and 3 hours, respectively, compared with control samples where fibrin was fully cross-linked with exogenous pFXIII-A2* (Figure 6D). These decreases in moduli indicate that FXIIIa became inactivated during fibrinolysis, and the downstream effect of this inactivation was reduced cross-linking of fibrin. This result is consistent with the western blots of degradation of pFXIII-A2* and fibrin shown in Figure 5A-C. Furthermore, in fibrinogen-deficient plasma, at 1 hour into lysis, the modulus of exogenous fibrin was 90% less than when fibrin was fully cross-linked, consistent with Figure 5E. The moduli of normal and fibrinogen-deficient plasma were similar when exogenous pFXIII-A2* was added; this indicates the moduli were dependent primarily on exogenous fibrin rather than residual endogenous fibrin.
Inactivation of pFXIII-A2* occurs during clot formation under thrombolytic conditions in plasma
Finally, we probed pFXIII-A2* activity and its downstream effects on fibrin during clot formation under thrombolytic conditions. Prior to clot initiation, thrombolytic levels of tPA were added to plasma with varying plasmin activities. The fibrinolytic system was inhibited 4 minutes into clot formation by adding TXA. Exogenous fibrinogen was then added and moduli were measured and compared with samples also containing exogenous pFXIII-A2* or T101. The resulting elastic modulus was 56% less in normal plasma than in plasminogen-deficient plasma (Figure 6E). Exogenous pFXIII-A2* rescued the modulus of normal plasma (bringing it to a similar value as plasminogen-deficient plasma) whereas it had little effect on plasminogen-deficient plasma. The loss of FXIIIa activity at 4 minutes was exacerbated in α2-antiplasmin–deficient plasma, where the modulus was 43% lower than normal plasma and 75% lower than plasminogen-deficient plasma, but could again be rescued by adding exogenous pFXIII-A2*. Thus, high fibrinolytic activity rapidly prevented pFXIII-A2* from cross-linking fibrin.
Discussion
The results show that FXIIIa can be inactivated by plasmin, and that plasmin is selective for FXIII-A2*, the active enzyme, over FXIII-A2B2, the zymogen. The specificity of plasmin for pFXIII-A2* over pFXIII-A2B2 may have confounded earlier reports, which only analyzed pFXIII-A2B2 and cFXIII-A2* to conclude that FXIII was resistant to degradation.7 Importantly, degradation of FXIII-A2* and resistance of FXIII-A2B2 were observed in both purified systems and in plasma. Thus, these findings reveal a newly recognized mechanism that may regulate cross-linking in physiologically relevant circumstances.
Degradation of FXIII-A2* in clots occurred within the same time period as degradation of fibrin. However, the rate of cleavage of FXIII-A2* in plasma was slower than the estimated half-life of FXIII-A2*, likely due to the presence of competing substrates such as fibrin18 and inhibitors of plasmin such as α2-antiplasmin.19 Notably, however, during normal clot formation, FXIII-A2* appeared to reach its full potential in cross-linking fibrin before it was inactivated, but fibrin was not cross-linked when added to reactions following FXIII-A2* inactivation. Thus, this mechanism may prevent FXIII-A2* from aberrantly cross-linking fibrin and other proteins in blood vessels.
Interestingly, degradation of platelet-derived cFXIII-A2* only occurred when plasmin was added 16 hours after platelet activation, but not 1 hour after activation. The slow availability of degradable cFXIII-A2* may be due to intracellular localization of cFXIII-A2 and thus inaccessibility by thrombin and plasmin. The initial resistance of cFXIII-A2 to degradation is consistent with the degradation of plasma FXIII-A2, which occurred primarily during fibrinolysis. Likewise, a subpopulation of FXIII-A2 was not readily activated in whole blood, as seen in the blot of Figure 5F; this initially spared a portion of the FXIII-A2, likely cFXIII-A2, from degradation.
Overall, these data support the notion that FXIII-A2* is inactivated during fibrinolysis, but not during clot formation. However, we note that these in vitro experiments do not rule out the possibility that plasmin may inactivate FXIIIa during clot formation in vivo. Moreover, plasmin may not be the sole inhibitor of FXIIIa in vivo. FXIIIa can also be cleaved and inhibited by thrombin and polymorphonuclear granulocyte proteases.4,5 In our experiments in plasma, FXIIIa was only degraded in the presence of plasmin, suggesting that cleavage of FXIIIa by thrombin may be secondary to that of plasmin. Questions remain regarding whether plasmin and polymorphonuclear granulocyte proteases work in concert or if they inactivate FXIIIa under distinct circumstances. Future studies may reveal additional points of FXIII-A2* inactivation in certain circumstances in vivo.
FXIII-A2* is at the interface between coagulation and fibrinolysis, and its ability to inhibit fibrinolysis is well established. The results presented here indicate that fibrinolytic enzymes can, in turn, downregulate FXIII-A2*. Thus, our findings reveal cross-talk between these pathways that may provide critical information for managing thrombosis and hemostasis.20,21 For example, the therapeutic use of plasminogen activators to treat embolic stroke22 and heart attack23 may be complicated by the novel discovery of plasmin-mediated inhibition of FXIII-A2*. Physiologically, tPA is present in the blood at ∼70 pM; however, therapeutic tPA is typically administered either IV, leading to systemic blood concentrations of ∼50 nM,24 or locally into clots from intravascular catheters at ∼400 nM.19 In our experiments, FXIII-A2* was degraded during clot formation under thrombolytic conditions, at 50 nM tPA. This mechanism may contribute to hemorrhaging associated with thrombolytic therapy.22 Notably, side effects of tPA administration resemble the phenotype of patients with FXIII deficiency, where in both cases there is a higher incidence of intracranial hemorrhage than would be expected when compared with other types of hemorrhage.20,22 Further research into the relevance of this mechanism in thrombolytic therapy is warranted.
Inhibition of FXIII-A2* by plasmin may also have implications in diseases where plasmin activity is abnormal, although this still needs to be verified in vivo. For example, patients with Quebec platelet disorder have elevated urokinase activity and thus are hyperfibrinolytic,25 suggesting that there may be less FXIII-A2* activity. In plasminogen deficiency, there may be higher FXIII-A2* activity, and increased aberrant cross-linking of proteins. In fibrinogen deficiency, degradation of FXIII-A2* could be enhanced because fibrin is a competing substrate for plasmin,18 or the degradation could be slower because fibrin is a cofactor for fibrinolytic enzymes.26 We observed the former in both western blots and TEG, and this rapid inactivation may have implications in congenital fibrinogen deficiency. Thus, these results provide insight into several physiological and pathophysiological scenarios that warrant further investigation.
In conclusion, the experiments show that plasmin preferentially inactivates FXIII-A2* over FXIII-A2B2, and that this mechanism occurs in a wide range of experimental conditions. The downstream effect of fibrinolytic inactivation of FXIII-A2* is that FXIII-A2* is no longer able to contribute to cross-linking and maintaining the mechanical strength of fibrin. However, to confirm that plasmin-mediated inactivation of FXIII-A2* plays a major role in thrombosis or hemostasis, additional data from human samples are necessary.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the blood donors for participation in the research, S. Novakowski and E. Pryzdial for helpful suggestions, S. Perry and J. Rogalski for help with spectrometry, and the UBC Centre for Blood Research for support with collecting blood.
This work was supported by the Canadian Institutes of Health Research (MOP-119426 and MSH-130166), the Canadian Foundation for Innovation (31928), and the BC Knowledge Development Fund.
Authorship
Contribution: W.S.H., N.M., X.J.D.L., and C.J.K. designed the study; W.S.H., N.M., X.J.D.L., and H.M.B. performed experiments; and W.S.H., N.M., X.J.D.L, J.R.B., A.S.W., and C.J.K. analyzed data and wrote the manuscript
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christian J. Kastrup, Michael Smith Laboratories, University of British Columbia, 2185 East Mall, Vancouver, BC, Canada; e-mail: ckastrup@msl.ubc.ca.

![Figure 3. The rate of inactivation of FXIII-A2* can occur on a physiologically relevant timescale. (A) Lineweaver-Burk plot used to generate kinetic parameters for FXIII-A2*, with data sets represented by different colors. The data point circled in pink is derived from the slope of the initial velocity in panel B (pink line). (B) The generation of FXIII-A2* cleavage products by plasmin (300 nM) at a representative FXIII-A2* concentration, determined using an ammonia release assay. (C) Standard curves of ammonia generation over time using various [FXIII-A2*]. (D) Ammonia generation over time, with varying times of FXIII-A2* digestion with plasmin. n = 3 for all experiments. Abs, absorbance.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/20/10.1182_blood-2015-07-650713/4/m_2329f3.jpeg?Expires=1765923532&Signature=P-lRS170qTwaW~gYe-XDT06v2bIaxpGXNfHSzYSK4TPTytlETkqpb4bAdG~BXy3e2UiBC0GJPqkMThqaK0M-jrYdxKutfy0XFo7yvpVDaetQpRSMW7zt1mXv1-I2k6p1jXXmYJmfCW3KirCT3hwAChM5d80LO~nQQwMXGKJAE41MK43OAjD66EZCpbQDcuYlT2Kl1f3ooUPsj6l8pOS6FPCC4TIkkTgQRrg1-kf4puxSKSxyXN3E~Bn4zsVlpB6PcChF79gNhmYvZ6lNqqHN8A25cKfE-hHK5-EwR1GgNqHU7G7BHbNTsnuWpFfTyXe7uLuapv4l99VWu~EO2-xgcg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal