To the editor:
We read with interest the letter by Burwick et al in Blood1 and were surprised by their conclusion that eculizumab failed to inhibit C5a generation in vivo. Eculizumab is a monoclonal antibody binding to human C5 preventing its cleavage to C5a and C5b. The authors investigated plasma concentrations of C5a and sC5b-9 in 1 patient with hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome treated with eculizumab.
They showed that, before the start of eculizumab, C5a concentration was low, whereas the sC5b-9 concentration was substantially elevated. This is consistent with what frequently is found when the terminal pathway is activated in vivo: sC5b-9 has a long half-life of ∼60 minutes2 compared with the very short half-life of ∼1 minute for C5a because of binding to the leukocyte C5a receptors.3 Thus, it is a typical pattern of human in vivo complement activation that is described for the HELLP patient at baseline. Immediately after the start of eculizumab, the sC5b-9 concentration fell abruptly, consistent with inhibiting C5 cleavage. Surprisingly, the C5a concentration apparently started to increase when eculizumab was initiated. Because this observation was unexpected and did not fit with any previous data, we aimed to reproduce these data and search for possible explanations for the findings.
Thus, we examined 3 patients with atypical hemolytic uremic syndrome (aHUS) that started with eculizumab and followed them over time for plasma C5a and sC5b-9. Because we suspected that the C5a enzyme-linked immunosorbent assay (ELISA) kit the authors had used (BD Bioscience, San Jose, CA) could have given false-positive results, we included this kit (BD HU C5a OPTEIA Kit II, Cat. No. 557965), as well as 2 other well-established C5a ELISA kits in our study (RND Duoset Human Complement Component C5a, Cat. No. DY2036; RND Systems, Minneapolis, MN; and Hycult C5a, Human ELISA kit, Cat. No. HK394-02; Hycult Biotech, Uden, The Netherlands).
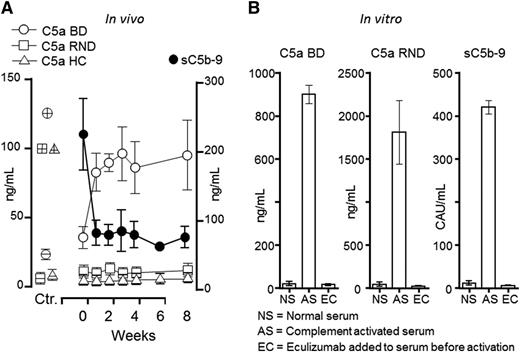
Notably, the BD C5a assay detected an abrupt increase in C5a in all 3 patients immediately after eculizumab treatment was started, whereas no increase was found using the RND or the Hycult (HC) kits (Figure 1A). Plasma sC5b-9 immediately decreased in the aHUS patients, as described for the patient with HELLP syndrome, using the same assay as Burwick et al, consistent with efficient blocking of C5. We then tested the effects of eculizumab in vitro by activating human serum and convincingly documented that eculizumab efficiently blocked C5a as measured by both the BD and the RND kits, as well as sC5b-9 measured by a singleplex assay developed in our laboratory (Figure 1B).
Effect of eculizumab on C5a and sC5b-9 generation in vivo and in vitro. (A) Plasma samples from 3 patients with aHUS were obtained at baseline (week 0) and during 8 weeks after the start of eculizumab treatment. C5a measured by the BD Biosience ELISA (BD) showed an abrupt increase in all 3 patients after the start of eculizumab, whereas no increase was seen in the C5a assays from RND Systems or HC. The controls (Ctr.) are shown to the left: negative controls (open circle, square and triangle symbols corresponding to those given for the kits, but with an inner horizontal line) represent 3 normal human EDTA plasma samples. Positive controls (similar open symbols as for the 3 kits, but with inner horizontal and vertical lines) represent C5a for the BD and RND kit and C5desarg for the Hycult kit. As expected, sC5b-9 (BD) decreased immediately after eculizumab treatment was started (right y-axis). (B) A pool of normal human serum (NS) was activated with heat aggregated IgG (AS), revealing an excessive increase in C5a both in the BD and RND kits, as well as in sC5b9. The latter was measured by a singleplex assay using an international complement activation standard given in complement activation units (CAU) per milliliter.8 Addition of eculizumab (EC) to the serum before activation completely abrogated C5a formation as detected in both the BD and the RND assay, as well sC5b-9 formation.
Effect of eculizumab on C5a and sC5b-9 generation in vivo and in vitro. (A) Plasma samples from 3 patients with aHUS were obtained at baseline (week 0) and during 8 weeks after the start of eculizumab treatment. C5a measured by the BD Biosience ELISA (BD) showed an abrupt increase in all 3 patients after the start of eculizumab, whereas no increase was seen in the C5a assays from RND Systems or HC. The controls (Ctr.) are shown to the left: negative controls (open circle, square and triangle symbols corresponding to those given for the kits, but with an inner horizontal line) represent 3 normal human EDTA plasma samples. Positive controls (similar open symbols as for the 3 kits, but with inner horizontal and vertical lines) represent C5a for the BD and RND kit and C5desarg for the Hycult kit. As expected, sC5b-9 (BD) decreased immediately after eculizumab treatment was started (right y-axis). (B) A pool of normal human serum (NS) was activated with heat aggregated IgG (AS), revealing an excessive increase in C5a both in the BD and RND kits, as well as in sC5b9. The latter was measured by a singleplex assay using an international complement activation standard given in complement activation units (CAU) per milliliter.8 Addition of eculizumab (EC) to the serum before activation completely abrogated C5a formation as detected in both the BD and the RND assay, as well sC5b-9 formation.
C5 might be cleaved directly, without a traditional C5 convertase (ie, in the absence of C3).4 However, it has never been shown that C5a is released without simultaneous C5b-9 formation. In fact, Krisinger et al5 showed that an even more effective C5b-9 complex was formed by direct cleavage of C5 by thrombin. Furthermore, C5-9 formation has been documented by a conformational change of C5, making a “C5b-like” molecule generating a C5-9 complex without release of C5a.6 Generation of C5a, without formation of C5b-9, has never been documented, and the data by Burwick et al do not document this, but rather reveal a false-positive reaction in their assay.
The authors described a patient with the HELLP syndrome. One possible explanation for their findings would be that this patient was exposed to an antigen related to this particular disease, detected in the BD C5a assay. This was definitely not the case, because the 3 patients we describe had aHUS and showed the same pattern. Thus, this reactivity seems to be directly related to eculizumab treatment. Interestingly, we found a correlation between the BD C5a assay and the eculizumab-C5 complexes, in an assay described by us recently.7
These data underscore the importance of confirming unexpected and surprising data by using alternative and different assays, instead of relying on a single commercial kit. This example illustrates how false conclusions were drawn based on results from an assay that was not satisfactorily validated for the purpose for which it was used. Thus, our data document that eculizumab efficiently inhibited C5a generation both in vitro and in vivo, in contrast to the wrong conclusion drawn in the paper by Burwick et al.1
This study was approved by the regional ethical committees and was performed in accordance with the appropriate version of the Declaration of Helsinki. Informed consent of the patients was obtained before analysis.
Authorship
Contribution: T.E.M. provided the conceptual design; all authors were involved in acquisition, analysis, and/or interpretation of data; T.E.M. drafted the manuscript; all authors critically revised the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: N.C.A.J.v.d.K. is a member of the international advisory board of Alexion Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: T. E. Mollnes, Reserach Laboratory, Nordland Hospital, 8092 NO-Bodø, Norway; e-mail: t.e.mollnes@medisin.uio.no.