Key Points
Biomarkers of complement activation can confirm the diagnosis of aHUS.
Complement biomarkers may be useful to differentiate aHUS from TTP.
Abstract
Atypical hemolytic uremic syndrome (aHUS) is characterized by dysregulated complement activity, the development of a thrombotic microangiopathy (TMA), and widespread end organ injury. aHUS remains a clinical diagnosis without an objective laboratory test to confirm the diagnosis. We performed a retrospective analysis of 103 patients enrolled in the Ohio State University TTP/aHUS Registry presenting with an acute TMA. Nineteen patients were clinically categorized as aHUS based on the following criteria: (1) platelet count <100 × 109/L, (2) serum creatinine >2.25 mg/dL, and (3) a disintegrin and metalloprotease with thrombospondin type 1 motif, 13 (ADAMTS13) activity >10%. Sixteen of 19 patients were treated with plasma exchange (PEX) therapy, with 6/16 (38%) responding to PEX. Nine patients were treated with eculizumab with 7/9 (78%) responding to therapy. In contrast to thrombotic thrombocytopenic purpura (TTP) patients, no aHUS patients demonstrated ultralarge von Willebrand factor multimers at presentation. Median markers of generalized complement activation (C3a), alternative pathway (Bb), classical/lectin pathway (C4d), and terminal complement activation (C5a and C5b-9) were increased in the plasma of these 19 patients. Compared with a cohort of ADAMTS13-deficient TTP patients (n = 38), C5a and C5-9 were significantly higher in the 19 patients clinically characterized as aHUS, suggesting that pretreatment measurements of complement biomarkers C5a and C5b-9 may confirm the diagnosis of aHUS and differentiate it from TTP.
Introduction
Significant advances in the treatment of atypical hemolytic uremic syndrome (aHUS) have placed an increased emphasis on the rapid and accurate differentiation of aHUS from acquired thrombotic thrombocytopenic purpura (TTP).1 Although both disorders share the common end point of widespread microvascular disease and end organ injury, their distinct underlying mechanisms of microvascular injury likely explain their differing responses to plasma exchange (PEX) and the response of patients with aHUS to complement inhibition therapy. Despite advances in our understanding of both aHUS and acquired TTP in recent years, both diseases remain clinical diagnoses.
Although severely deficient a disintegrin and metalloprotease with thrombospondin type 1 motif, 13 (ADAMTS13) activity can confirm the clinical diagnosis of acquired TTP, no such objective single test is available to confirm the clinical diagnosis of aHUS. This is especially problematic because aHUS is a condition that often requires prolonged complement inhibition therapy.1,2 The availability of an objective biomarker to confirm the clinical diagnosis of aHUS would be invaluable to clinicians who must diagnose and treat these challenging patients. In addition, a surrogate biomarker that could predict a response to complement inhibition early in the course of therapy would be a significant advance over our present method of using biomarkers of hemolysis and recovery of end organ function, a process that may take several weeks before any objective response to therapy is appreciated clinically.1,3
Patients and methods
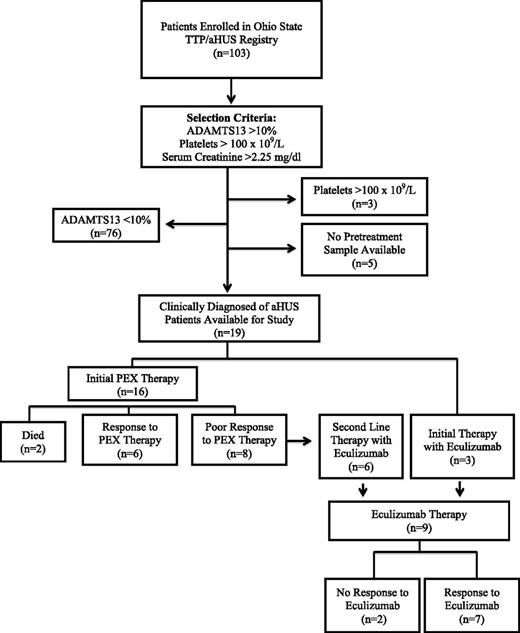
We performed a retrospective study of all patients enrolled into the Ohio State University TTP/aHUS Registry since its inception in 2003. This registry is composed of patients that present or are referred to our institution with an acute thrombotic microangiopathy (TMA). All patient data reported in this manuscript are from patients that have been enrolled in this Institutional Review Board-approved registry. This study was conducted in accordance with the Declaration of Helsinki. Beginning with a total of 103 enrolled patients, 76 patients were excluded from this analysis because of ADAMTS13 activity <10%, 3 patients were excluded because of their subacute presentation (platelet count >100 × 109/L), with an additional 5 patients excluded because a pretreatment plasma sample was not available (Figure 1). From this cohort of acute TMA patients, 19 patients with a platelet count <100 × 109/L, serum creatinine >2.25 mg/dL, and measurable ADAMTS13 activity (>10%) were selected for study. Although there is no objective diagnostic test that defines the diagnosis of aHUS, it has been hypothesized that these criteria identify a cohort of patients that is likely to be enriched for the diagnosis of aHUS.4,5 The clinical and demographic data for all 19 patients are shown in Table 1.6 This was the initial presentation for 17/19 (89%) patients, with 16/19 patients undergoing PEX therapy at the time of presentation.
Criteria used to select patients with aHUS for analysis and their response to PEX therapy and eculizumab.
Criteria used to select patients with aHUS for analysis and their response to PEX therapy and eculizumab.
Demographic and clinical features of the 19 subjects clinically characterized as aHUS compared with a cohort of 38 patients with TTP with severely deficient ADAMTS13 activity
| . | Median demographic and clinical data at presentation . | |||||||
|---|---|---|---|---|---|---|---|---|
| Age (range) . | Sex (M/F) . | Race (AA/W) . | Dialysis . | Platelets (150-400 × 109) . | Creatinine (<1.1 mg/dL) . | LDH (<190 U/L) . | C3 (970-1576 mg/L) . | |
| aHUS patients (n = 19) | 47 (20-69) | 3/16 | 3/16 | 14/19 (74%) | 46 (8-93) | 4.39 (2.38-12.61) | 1094 (140-3548) | 915 (561-1550) |
| TTP patients (n = 38) | 42.5 (18-76) | 12/26 | 8/30 | 0/38 | 12 (2-45) | 1.15 (0.65-4.11) | 966 (243-2449) | ― |
| . | Median demographic and clinical data at presentation . | |||||||
|---|---|---|---|---|---|---|---|---|
| Age (range) . | Sex (M/F) . | Race (AA/W) . | Dialysis . | Platelets (150-400 × 109) . | Creatinine (<1.1 mg/dL) . | LDH (<190 U/L) . | C3 (970-1576 mg/L) . | |
| aHUS patients (n = 19) | 47 (20-69) | 3/16 | 3/16 | 14/19 (74%) | 46 (8-93) | 4.39 (2.38-12.61) | 1094 (140-3548) | 915 (561-1550) |
| TTP patients (n = 38) | 42.5 (18-76) | 12/26 | 8/30 | 0/38 | 12 (2-45) | 1.15 (0.65-4.11) | 966 (243-2449) | ― |
AA, African American; F, female; LDH, lactate dehydrogenase M, male; W, white.
The criteria to define a response to therapy in this study included a normal platelet count and a stable or improved serum creatinine (at 6 months of follow-up) independent of PEX therapy or with continued therapy with eculizumab (Soliris). In patients presenting after September 2011 when eculizumab received regulatory approval from the US Food and Drug Administration, eculizumab was available for patients with a clinical diagnosis of aHUS. The criteria used by our group7 and others2 to define the failure of PEX therapy and to initiate therapy with eculizumab for a presumed diagnosis of aHUS include the following: (1) the failure to achieve a hematologic response (improvement in platelet count and decrease in the LDH) over the first 4-5 days; (2) progressive end organ injury (renal and/or neurologic) over the first 4-5 days of PEX therapy; and (3) nondeficient (>10%) ADAMTS13 activity. These criteria were used as evidence for the failure of PEX and the presumptive diagnosis of aHUS; in these cases, PEX was discontinued and therapy with eculizumab begun where it was available to patients. Follow-up for all TMA patients is standardized at our institution, with all patients being seen weekly for the first 4 weeks after discharge from the hospital, then monthly for the next 5 months, and then every 3 months longitudinally.
Complement biomarker assay methodology
Complement biomarker studies were performed on banked plasma samples at presentation, prior to the initiation of either PEX or eculizumab. Three patients underwent dialysis prior to samples being obtained, but there was no significant difference between these samples obtained after dialysis and those obtained from the remaining patients with samples obtained before undergoing hemodialysis (supplemental Table 4, available on the Blood Web site). In some patients, additional samples both during PEX and during long-term follow-up were available for study, but not at regular, predefined time points. Uniform banking and processing procedures for all banked samples have been in place since the initiation of our tissue bank in 2003, minimizing the potential impact of handling and storage on the results obtained. Complement proteins were chosen to study the pathway of complement activation and included the following: C4d (classical and lectin), factor Bb (alternative), generalized complement activation (C3a), and terminal complement activation (C5a and C5b-9).
Complement studies were performed using commercial enzyme-linked immunosorbent assay kits as reported previously.6 The assay kits for C4d, factor Bb, C3a, and C5b-9 were from Quidel (San Diego, CA). The assay kit for C5a was from BD (Franklin Lakes, NJ). The reference range for each of the complement assays was established using 40 local healthy donors.
Assay validation studies indicated that complement factor Bb, C5a, and C5b-9 are very stable analytes. The assay results were not significantly affected by sample storage at room temperature for 2 hours or overnight in a refrigerator prior to processing, nor were they affected by 1 cycle of sample freeze/thaw. C4d was also a relatively stable analyte, but the results were 30% higher when the blood was stored overnight in a 4°C refrigerator before processing. C3a was the least stable analyte with extended storage and freeze/thaw cycles giving rise to a result that was >50% higher than the result from a freshly banked sample without experiencing a freeze/thaw cycle. Given that all samples in this study have been banked and processed in a uniform manner, these potential sources of error/variability are less relevant to these data. Nonetheless, these issues must be considered when comparing the results of C4d and C3a studies from different laboratories, and our data support the use of Bb, C5a, and C5b-9 as more reliable clinical assays of complement activation.
ADAMTS13 assay methodology
ADAMTS13 activity was measured using a surface-enhanced laser desorption/ionization time-of-flight mass spectrometer as described previously.8 ADAMST13 inhibitor titer was determined using the same technology and calculated as a dilution of patient plasma that neutralizes 50% ADAMTS13 activity in pooled normal plasma. ADAMTS13 immunoglobulin G levels were determined using a commercial kit from American Diagnostica as reported previously.8
von Willebrand factor (VWF) multimer analysis
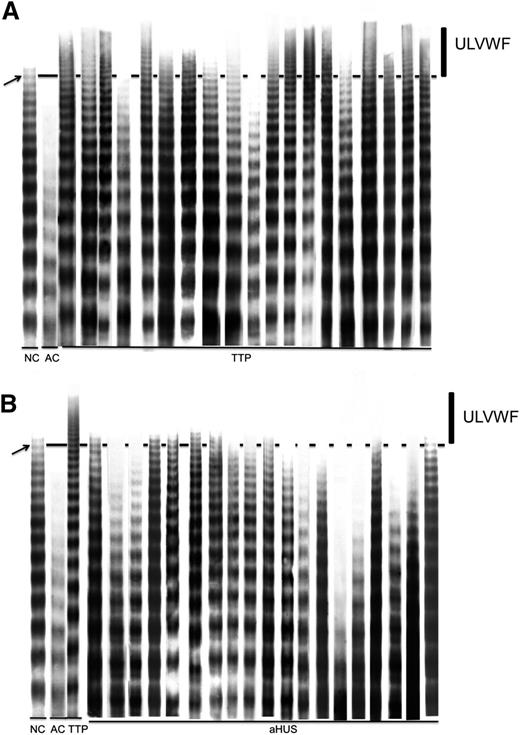
Citrated plasma from each patient was diluted 1:40 in 70 mM tris(hydroxymethyl)aminomethane-HCl (pH: 8.8) containing 4 mM EDTA, 2.4% sodium dodecyl sulfate, 0.67 M urea, 0.1% bromophenol blue, and 5% glycerol and incubated in a 60°C water bath for 20 minutes. A 5-μL sample was then electrophoresed on a 1.6%, vertical, discontinuous agarose gel (11 × 15 cm) at a constant current of 4 mA for 18 hours. After the run, proteins were transferred to a polyvinylidene fluoride membrane at a constant current of 500 mA for 4 hours. After blocking the membrane with 5% dry milk solution, immobilized proteins were reacted with rabbit anti-human VWF polyclonal antibody (1:2500 dilution; Dako) for 2 hours, followed by application of the HyGLO Chemiluminescent HRP Antibody Detection Kit (Denville Scientific). The immunoreactive bands of VWF multimers on the film were scanned by densitometry to get images for analysis. In most of the experiments, normal control plasma showed 15-17 distinct immunoreactive bands. Similar numbers of immunoreactive bands were seen when 40 normal, healthy donors were evaluated. In order to more objectively define the fraction best representing ultralarge VWF (ULVWF), we grouped the large VWF multimers together, with the area higher than band #15 indicated by an arrow, and collectively referred to as ULVWF (Figure 2).
VWF multimeric analysis of pretreatment samples for TTP and aHUS patients. In each run shown, a normal control plasma (NC) and an abnormal control plasma (AC, obtained from a patient with type 2 von Willebrand disease) were included to verify the performance and resolution of the analysis. The area above the solid line in the figure represents the larger multimers with molecular weight higher than band #15 (indicated by an arrow) counted from the bottom of the gel. This area, indicated by an vertical line in the figure, is referred to as ULVWF. (A) Multimeric analysis from 19 acquired TTP patients. (B) Multimeric analysis from 19 aHUS patients. A sample from a TTP patient containing ULVWF is included in panel B for comparison. In a few aHUS patients, faint bands above the dotted line can be seen that represent normal variation rather than pathologically increased ULVWF multimers.
VWF multimeric analysis of pretreatment samples for TTP and aHUS patients. In each run shown, a normal control plasma (NC) and an abnormal control plasma (AC, obtained from a patient with type 2 von Willebrand disease) were included to verify the performance and resolution of the analysis. The area above the solid line in the figure represents the larger multimers with molecular weight higher than band #15 (indicated by an arrow) counted from the bottom of the gel. This area, indicated by an vertical line in the figure, is referred to as ULVWF. (A) Multimeric analysis from 19 acquired TTP patients. (B) Multimeric analysis from 19 aHUS patients. A sample from a TTP patient containing ULVWF is included in panel B for comparison. In a few aHUS patients, faint bands above the dotted line can be seen that represent normal variation rather than pathologically increased ULVWF multimers.
Complement protein mutation analysis
The reported complement protein mutation studies were performed by a reference laboratory at the University of Iowa.
Statistical methods
A general linear model was used for comparison of the complement biomarkers in terms of the presence of mutations, response to PEX, and the comparison of the aHUS cohort with the cohort of acquired TTP. Race, gender, and age were used as covariates. Where necessary, data sets were log transformed to fit a normal distribution. If after log transformation the data set still did not fit a normal distribution, an independent Mann-Whitney U test was used for comparison using age, sex, and race as covariables. The SPSS program was used for all analyses. All calculated P values were 2-sided with the level of significance set at P < .05.
Results
A total of 19 patients were included in this analysis. The demographic features and the clinical laboratory data at presentation are shown in Table 1. Three of the 19 (16%) patients presented immediately postpartum, and 4/19 (21%) had previously undergone kidney transplantation. The finding that 7/19 aHUS patients presented postpartum and post–kidney transplantation is not surprising given that these 2 scenarios are common clinical presentations of aHUS. PEX was started in 16/19 (84%) of patients at the time of their initial presentation. Three patients were not treated with PEX therapy based on their subacute presentations, which were judged to be atypical for an acute TTP episode by the treating physician, and were treated initially with eculizumab. Renal failure requiring dialysis was present in 13/16 (81%) of patients treated with PEX. In the 3 patients that were not treated with PEX, acute renal injury was present in all 3 patients (serum creatinine >3 mg/dL), but only one-third of these patients required dialysis at presentation.
Response to PEX
Six of 16 patients (38%) met the criteria for a response to PEX therapy, including independence from dialysis in the 4 patients that required dialysis at presentation. The median number of PEX procedures to achieve a normal platelet count in these 6 patients was 11.5 (range, 5-32). Ten patients did not respond to PEX after a median of 6 PEX procedures (range, 4-13). Two nonresponding patients died 3 weeks and 3 months after their initial presentations secondary to acute respiratory failure and a massive hemispheric infarct, respectively. In the 8 remaining patients that failed PEX, 6 discontinued PEX therapy and immediately started therapy with eculizumab. The 2 remaining patients had PEX discontinued and were discharged with a persistent thrombocytopenia and end-stage renal disease requiring hemodialysis. Eculizumab was not available for these 2 patients because their presentation occurred prior to the regulatory approval of eculizumab for the treatment of aHUS.
Response to eculizumab
A total of 9 patients were treated with eculizumab, including the 3 patients who received eculizumab without previously being treated with PEX. Hematologic responses (normalization of the platelet count) occurred in 7/9 (78%); 8/9 (89%) patients had improvements in renal function after therapy with eculizumab. Six of the 9 eculizumab-treated patients were dialysis dependent at the start of therapy, with all 6 becoming independent of the need for dialysis. Two patients did not normalize their platelet count after 8 and 10 months of therapy, respectively. The first patient did, however, recover renal function and became independent of dialysis despite the lack of a hematologic response. The second patient never showed any significant improvement in renal function on therapy with eculizumab and started hemodialysis 10 months after starting therapy with eculizumab. This patient was found to have heterozygous mutations of both complement factor (CF)I and CF H-related genes 3 and 1 (CFHR3-CFHR1), but testing for CFH autoantibodies was not performed. Significant renal injury was present in this patient at the time of presentation (serum creatinine >3 mg/dL, estimated glomerular filtration rate <15 mL/minutes per m2) that had been present for at least 3 months prior to her presentation at our institution. No other extrarenal manifestations attributable to aHUS were found in this patient.
Complement biomarker studies
The median complement biomarker data for all 19 subjects prior to the initiation of therapy are shown for all 19 patients in Table 2. Activation of complement as defined by an increase in both C3a and C5a was seen in 18/19 (95%) of patients. More pronounced activation of the alternative pathway (factor Bb) and the terminal complement pathway (C5b-9) was seen in this cohort of 19 patients. Sixteen of 19 (84%) patients had increased levels of factor Bb at presentation, and increased levels of C5b-9 were seen in all 19 patients. The complement biomarker measuring activation of the classic complement pathway (C4d) was increased (15/19 [79%]), but to a lesser degree than to what was seen in the alternative and terminal pathways. All patients that were treated with either PEX or eculizumab therapy had increased pretreatment biomarkers of terminal complement activation (C5a and C5b-9).
Complement biomarker studies at presentation for all subjects and in the context of mutation status and response to PEX
| . | Median complement biomarkers (ng/mL) (normal range) . | ||||
|---|---|---|---|---|---|
| Factor Bb (244.3-960.8) . | C4d (278.5-1845.9) . | C5b-9 (33.9-238.2) . | C5a (18.6-47.9) . | C3a (6.9-242.3) . | |
| All patients (n = 19) | 7386 (603-30 614) | 2914 (1394-15 210) | 1098 (422-4840) | 115 (55-280) | 1237 (79-13 726) |
| Mutation present* (n = 6) | 9299 (1050-30 614) | 2571 (1640-3831) | 1480 (422-4840) | 125 (56-269) | 1353 (79-3490) |
| No mutation (n = 6) | 1756 (910-15 096) | 3338 (1394-4981) | 954 (621-1317) | 97 (55-280) | 759 (566-1391) |
| P value | .322 | .401 | .206 | .795 | .189 |
| PEX response (n = 6) | 14 945 (7386-20 443) | 3495 (1640-4680) | 1394 (1024-1920) | 141 (105-214) | 1266 (590-13 726) |
| PEX nonresponder (n = 10) | 3103 (603-30 614) | 3066 (1394-4981) | 1063 (422-4840) | 108 (55-269) | 1158 (79-3834) |
| P value | .185 | .818 | .593 | .675 | .389 |
| . | Median complement biomarkers (ng/mL) (normal range) . | ||||
|---|---|---|---|---|---|
| Factor Bb (244.3-960.8) . | C4d (278.5-1845.9) . | C5b-9 (33.9-238.2) . | C5a (18.6-47.9) . | C3a (6.9-242.3) . | |
| All patients (n = 19) | 7386 (603-30 614) | 2914 (1394-15 210) | 1098 (422-4840) | 115 (55-280) | 1237 (79-13 726) |
| Mutation present* (n = 6) | 9299 (1050-30 614) | 2571 (1640-3831) | 1480 (422-4840) | 125 (56-269) | 1353 (79-3490) |
| No mutation (n = 6) | 1756 (910-15 096) | 3338 (1394-4981) | 954 (621-1317) | 97 (55-280) | 759 (566-1391) |
| P value | .322 | .401 | .206 | .795 | .189 |
| PEX response (n = 6) | 14 945 (7386-20 443) | 3495 (1640-4680) | 1394 (1024-1920) | 141 (105-214) | 1266 (590-13 726) |
| PEX nonresponder (n = 10) | 3103 (603-30 614) | 3066 (1394-4981) | 1063 (422-4840) | 108 (55-269) | 1158 (79-3834) |
| P value | .185 | .818 | .593 | .675 | .389 |
Twelve of the 19 subjects had mutation studies performed.
Mutations studied included the following: factor H, factor I, factor B, C3, membrane cofactor protein (MCP), thrombomodulin, and CFHR3-CFHR1.
We compared the pretreatment complement biomarkers from this cohort of 19 patients clinical diagnosed with aHUS with a previously published cohort of 38 TTP patients enrolled in the Ohio State TTP/aHUS Registry but characterized by severely deficient ADAMTS13 activity.6 In comparing these 38 acquired TTP patients to this cohort of 19 patients, pretreatment levels of C5a, C3a, and C5b-9 were significantly higher in the cohort of 19 patients clinically diagnosed with aHUS compared with published data from the 38 patients with TTP characterized by severely deficient ADAMTS13 activity (Table 3). Although the median and mean complement biomarkers were significantly different between these groups, there was still overlap in these markers between the diagnosis groups. A clear cutoff to differentiate one group vs the other based on these markers was not readily apparent in these data, even when evaluating the log-transformed levels of the markers (supplemental Figure 3A-E).
Comparison of pretreatment complement biomarkers obtained from 19 patients with clinically diagnosed aHUS vs a cohort of 38 patients with ADAMTS13-deficient TTP
| Clinical diagnosis . | Complement biomarkers (ng/mL) . | ||||
|---|---|---|---|---|---|
| Factor Bb (244.3-960.8) . | C4d (278.5-1845.9) . | C5b-9 (33.9-238.2) . | C5a (18.6-47.9) . | C3a (6.9-242.3) . | |
| Acquired TTP*6 (n = 38) | 2153 (343-5448) | 3534 (458-7450) | 585 (210-1924) | 75 (29-210) | 777 (128-4782) |
| aHUS* (n = 19) | 7386 (603-30 610) | 2914 (1394-15 210) | 1098 (422-4840) | 115 (55-280) | 1237 (79-13 730) |
| P value† | .063 | .706 | < .0001 | .004 | .031 |
| Clinical diagnosis . | Complement biomarkers (ng/mL) . | ||||
|---|---|---|---|---|---|
| Factor Bb (244.3-960.8) . | C4d (278.5-1845.9) . | C5b-9 (33.9-238.2) . | C5a (18.6-47.9) . | C3a (6.9-242.3) . | |
| Acquired TTP*6 (n = 38) | 2153 (343-5448) | 3534 (458-7450) | 585 (210-1924) | 75 (29-210) | 777 (128-4782) |
| aHUS* (n = 19) | 7386 (603-30 610) | 2914 (1394-15 210) | 1098 (422-4840) | 115 (55-280) | 1237 (79-13 730) |
| P value† | .063 | .706 | < .0001 | .004 | .031 |
Compared with the acquired TTP patients, patients clinically diagnosed aHUS tended to have significantly higher C5b-9 and C5a levels. P values reflect comparisons of complement biomarkers between diagnosis groups using Wilcoxon rank sum tests. Bolded text signifies the values that were statistically significant at the P < .05 level.
For the acquired TTP patients, the mean data for each complement biomarker are shown; whereas for the smaller aHUS cohort, the median biomarker data are presented.
Compared with the 19 clinically diagnosed aHUS patients. The Mann-Whitney independent U test was used for the Bb and C4d comparisons, whereas a generalized linear model was used to compare C5b-9, C5a, and C3a, using age, sex, and race as covariables.
Complement mutation studies
Twelve patients were studied for the presence of mutations (factor H, factor I, factor B, C3, MCP, thrombomodulin, and CFHR3-CFHR1) of complement proteins, with 6/12 having documented heterozygous mutations (CFH, 2; complement factor I, 2; C3, 1; MCP, 1; and CFHR3-CFHR1, 1) and 1 patient found to have 2 mutations. There was no significant difference in any of the complement biomarkers studied between those with and without documented mutations (Table 2). Similarly, there was no significant difference in these same pretreatment biomarkers in the 6 patients that responded to PEX compared with the 10 PEX nonresponders. Eight of the 9 eculizumab-treated patients had mutation studies performed, with 5/8 (63%) found to have one of the above-mentioned mutations. Six of the 7 eculizumab-responding patients had mutation studies performed, with 4/6 responders having a documented complement protein mutation.
VWF multimer analysis
In this study, the VWF multimers present at the time of acute presentation were compared between patients clinically characterized as aHUS and a cohort of ADAMTS13-deficient TTP patients. Samples studied included the pretreatment samples from the 19 patients clinically diagnosed with aHUS in this report and pretreatment samples from 19 TTP patients randomly selected from patients enrolled in our registry. As shown in Figure 2A, at least 10 of the 19 TTP patients demonstrated a significant accumulation of ULVWF at the time of acute clinical presentation. In contrast, as shown in Figure 2B, all 19 patients with a diagnosis of aHUS did not show evidence for any significant accumulation of ULVWF multimers.
Discussion
The reported efficacy and regulatory approval of eculizumab for the treatment of aHUS have emphasized the need for more objective diagnostic testing to confirm the diagnosis of aHUS and differentiate it from acquired TTP.1 Although the presence of severely deficient ADAMTS13 activity confirms the diagnosis of acquired TTP, no single such biomarker exists to confirm the diagnosis in patients with a suspected diagnosis of aHUS. Such a biomarker would be an important confirmatory test for the clinical diagnosis of aHUS given both the expense of therapy with eculizumab as well as the presumed need for long-term therapy.2 Complement biomarkers documenting activation of the alternative pathway and terminal pathway activation at the time of presentation would also eliminate the complete reliance on the response to complement inhibition therapy to confirm the clinical diagnosis of aHUS, which can take several weeks in the case of renal injury. In addition, in a patient clinically suspected to have aHUS, the finding of normal biomarkers of terminal complement activation (C5a and C5b-9) would argue against the role of dysregulated complement activity as the etiology of a patient’s TMA findings.
One limitation in the development of objective diagnostic testing for the diagnosis of aHUS is the dependency on clinical and laboratory criteria to define the diagnosis for study. Although aHUS remains a clinical diagnosis, there is mounting data that the ADAMTS13 activity, the extent of renal injury at presentation, and the response to PEX can be used collectively to define a cohort of patients consistent with the diagnosis of aHUS.2,5,7 These criteria are presently used by our group to prospectively identify patients consistent with the diagnosis of aHUS and were used to identify patients for this study.7 The accuracy of our classification cannot be absolutely confirmed, but the finding of complement mutations in half of the studied patients consistent with previously published studies2,9,10 and the high rates of response to therapy in the eculizumab-treated patients support the hypothesis that this cohort of patients is accurately classified as aHUS. In addition, postpartum and post–kidney transplantation are common clinical scenarios associated with the diagnosis of aHUS. The finding of 7 of 19 patients presenting in the immediate postpartum state and postkidney transplantation (given the high rates of recurrence after kidney transplantation) would further support the clinical diagnosis of aHUS.10
Given the recent clinical availability of eculizumab, patients were more likely to be categorized as nonresponders to PEX after a fewer number of PEX procedures, resulting in more recently presenting patients being classified as nonresponders after a fewer number of PEX procedures compared with those presenting prior to the availability of eculizumab. Seven of 10 patients in the PEX nonresponding cohort had the option of going on to therapy with eculizumab after failing PEX, explaining in part the earlier clinical decision to discontinue PEX and initiate therapy with eculizumab. It is plausible that the PEX nonresponding patients could have recovered after a more prolonged course of PEX similar to the longer PEX treatments seen in the PEX-responding patients. The question could also be raised as to whether these 6 patients that responded to PEX therapy might also have responded to supportive care equally. One of the 6 PEX-responding patients had complement mutation studies performed that demonstrated a mutation of MCP, a mutation that predicts a more benign course and identifies patients that may also recover with supportive care alone. These issues limit the application of these data to accurately define the response rates to PEX in patients with clinically diagnosed aHUS in this study.
Although pathological activation of the alternative pathway and dysregulated complement activity are central to the pathophysiology of aHUS, complement activation has also been reported in patients with ADAMTS13-deficient TTP.6,11 Réti et al11 reported the finding of increased levels of C3a and C5b-9 in 23 patients with ADAMTS13-deficient TTP, indicative of generalized and terminal complement (membrane attack complex formation), respectively. Similar data have also been published by our group showing complement activation at presentation in 38 patients with ADAMTS13-deficient TTP.6 In this report, 33/38 (87%) patients had evidence for complement activation as defined by increases in both C3a and C5a. Although activation of complement is seen in both disorders, we hypothesize that terminal complement activation as measured by C5a and C5b-9 may be useful to objectively confirm the clinical diagnosis of aHUS and differentiate it from acquired TTP. At presentation, more significant elevations of C5a and C5b-9 and nonseverely deficient ADAMTS13 activity in the context of a patient responding poorly to PEX may collectively be useful to differentiate aHUS from acquired TTP. Additionally, measurement of terminal complement activation in TTP patients as measured by C5a and C5b-9 might also be useful to identify patients at greater risk of death during that acute TMA episode as reported previously.6
It could also be hypothesized that these same complement biomarkers might find utility as a biomarker to predict the response therapy with eculizumab. Support for the potential utility of these complement biomarkers as markers of response to therapy can also be found in the additional plasma samples that were available from 5 of 9 eculizumab-treated patients during follow-up at varied time points (1.5 weeks to 16 months) during continuous eculizumab therapy. In these 5 patients that were recovering clinically on eculizumab therapy, there was a trend showing decreased levels of C5b-9 in patients clinically responding to therapy with eculizumab with variability in C5a levels during ongoing therapy with eculizumab (data not shown). Although it would be predicted that both C5a and C5b-9 should both decline after therapy with eculizumab, the follow-up samples were obtained on the day that they were due for their next dose of eculizumab, at the nadir of the anti-C5 antibody levels, providing one possible explanation for lack of a clear decline in posttreatment C5a levels. Although the limited number of samples obtained at varying time points during their course of therapy precludes any definite conclusions from being drawn, these data should serve as hypothesis-generating data for future prospective studies.
It is important to mention the potential confounding effects that can be seen with differing methods of handling and processing of clinical samples for complement biomarker studies.12 As described previously, we found C3a to be the least stable analyte of the complement biomarkers studied, with differing storage times and freeze-thaw cycles markedly affecting the results obtained. In contrast, C5a and C5b-9 assays performed well across many conditions, providing reliable and reproducible results that make them the most attractive complement biomarkers for routine clinical study.
The data from VWF multimer analysis confirm the work from previous publications that the formation of ULVWF is associated with clinical development of acute TTP.13,14 Additionally, our data confirm previous work that ULVWF multimers are not abnormally accumulated in all cases of acquired TTP at the time of acute presentation.13 Conceivably, an early presentation of disease may exhibit the accumulation of ULVWF in the blood, whereas a fulminant TTP case may not demonstrate the presence of ULVWF multimers because of consumption related to the development of extensive platelet thromboses. Future studies using a larger TTP cohort with longitudinal sampling may allow for a more detailed study of the relationship between severity of ULVWF accumulation, laboratory data, and clinical outcome, in the context of the measured ADAMTS13 activity. However, importantly for this study, we demonstrate for the first time that the abnormal accumulation of ULVWF is not present in a cohort of clinically diagnosed aHUS patients at the time of acute presentation. This novel observation strongly suggests that in contrast to TTP, VWF-mediated platelet thrombosis is not a primary mechanism for development of TMA in patients with aHUS.
In summary, these data suggest that the use of these biomarkers of complement activation, specifically C5a and C5b-9, may be useful clinically to confirm the clinical diagnosis of aHUS and differentiate it from acquired TTP. Although limited by the retrospective nature of the data, they should form the basis for future studies to evaluate their role in the confirmation of the diagnosis as well as a tool to objectively monitor the response to complement inhibition therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported in part by a US Food and Drug Administration government grant (R01 FD003932).
Authorship
Contribution: S.R.C. designed the research, analyzed the data, and wrote the manuscript; V.M.H. analyzed the data and edited the manuscript; S.G. performed the statistical analysis of the data; S.Y. performed the experiments, analyzed the data, and edited the manuscript; and H.M.W. designed the research, analyzed the data, and wrote/edited the manuscript.
Conflict-of-interest disclosure: S.R.C. and H.M.W. are consultants to and received research funding from Alexion. V.M.H is a consultant to Alexion. The remaining authors declare no competing financial interests.
Correspondence: Spero R. Cataland, Department of Internal Medicine, Ohio State University, A361 Starling Loving Hall, 320 W 10th Ave Columbus, OH 43210; e-mail: spero.cataland@osumc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal