Key Points
PD-L1 expression is associated with poor prognosis in DLBCL.
The double-staining technique is a useful method for identifying and distinguishing PD-L1+ DLBCL.
Abstract
Programmed cell death ligand 1 (PD-L1) is expressed on both select diffuse large B-cell lymphoma (DLBCL) tumor cells and on tumor-infiltrating nonmalignant cells. The programmed cell death 1 (PD-1)/PD-L1 pathway inhibits host antitumor responses; however, little is known about how this pathway functions in the tumor microenvironment. The aim of this study was to determine the clinicopathological impact of PD-L1+ DLBCL. We performed PD-L1/PAX5 double immunostaining in 1253 DLBCL biopsy samples and established a new definition of PD-L1+ DLBCL. We also defined the criteria for microenvironmental PD-L1+ (mPD-L1+) DLBCL (ie, PD-L1– DLBCL in which PD-L1+ nonmalignant cells are abundant in the tumor microenvironment). Of the 273 patients whose clinical information was available, quantitative analysis of PD-1+ tumor-infiltrating lymphocytes (TILs) was performed. The prevalence rates of PD-L1+ and mPD-L1+ DLBCL were 11% and 15.3%, respectively. Both PD-L1+ and mPD-L1+ DLBCL were significantly associated with non–germinal center B-cell (GCB) type and Epstein-Barr virus positivity. The number of PD-1+ TILs was significantly higher in GCB-type tumors and lower in mPD-L1– and PD-L1+ DLBCL. Patients with PD-L1+ DLBCL had inferior overall survival (OS) compared with that in patients with PD-L1– DLBCL (P = .0009). In contrast, there was no significant difference in OS between mPD-L1+ and mPD-L1– DLBCL (P = .31). The expression of PD-L1 maintained prognostic value for OS in multivariate analysis (P = .0323). This is the first report describing the clinicopathological features and outcomes of PD-L1+ DLBCL. Immunotherapy targeting the PD-1/PD-L1 pathway should be considered in this distinct DLBCL subgroup.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is defined as diffuse proliferation of large neoplastic B lymphoid cells that efface the preexisting architecture. Although recent studies have subdivided DLBCL into some morphologically, biologically, or clinically distinct disease entities, a large number of cases remain heterogeneous.1 Anthracycline-based chemotherapies combined with rituximab, such as the R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) have long been the standard therapy for DLBCL.2,3 Although more than half of patients achieve long-term remission, these therapies are sometimes ineffective, particularly in patients with high-risk disease. Therefore, new treatment strategies based on underlying molecular oncogenic mechanisms are necessary.
Programmed cell death 1 (PD-1), a member of the B7 receptor family, is an inhibitory receptor expressed on the surface of T cells.4 Binding of PD-1 to its ligand PD-L1 expressed on antigen-presenting cells inhibits cytokine production and cell-cycle progression in T cells and functions as an important checkpoint in the regulation of immune responses.5 In a healthy host, PD-1/PD-L1 signaling regulates effector T-cell responses and protects bystander tissues from immune-mediated damage.
PD-L1 is upregulated in various types of solid tumors and inhibits local antitumor T-cell responses.6,7 Moreover, PD-L1 expression on tumor cells is associated with tumor progression and poor prognosis.8-17 Blockade of the PD-1/PD-L1 pathway by anti-PD-1 or anti-PD-L1 monoclonal antibodies may have significant potential for antitumor effects.18,19
PD-L1 is also expressed on DLBCL tumor cells and tumor-infiltrating nonmalignant cells, primarily macrophages.20,21 In contrast, PD-1 is expressed on tumor-infiltrating lymphocytes (TILs), and the presence of a large number of PD-1+ TILs is associated with favorable overall survival (OS) in patients with DLBCL.22,23 Furthermore, the presence of high levels of plasma-soluble PD-L1 is associated with poor OS and acts as a potent novel biomarker in DLBCL.24 These results suggest that the PD-1/PD-L1 pathway contributes to tumor cell survival and that manipulation of this pathway may be an applicable therapeutic modality to treat DLBCL. However, the detailed clinicopathological characteristics of PD-L1+ DLBCL are still unclear.
In this study, we retrospectively assessed the expression of PD-L1 in 1253 DLBCL samples by using a double-staining technique and analyzed the clinicopathological features of PD-L1+ DLBCL.
Patients and methods
Patients and samples
We reviewed 1557 consecutive biopsy samples from patients diagnosed with DLBCL and DLBCL subtypes that had been submitted to the Department of Pathology, Kurume University, Kurume, Japan, between 2008 and 2010. Relapsed cases, transformed cases, and inadequate/insufficient samples were excluded, and a total of 1253 newly diagnosed and untreated DLBCL cases with formalin-fixed paraffin-embedded (FFPE) samples were available for inclusion. These cases were reclassified according to the World Health Organization classification1 and Hans algorithm25 by experienced hematopathologists (D.N. and K.O.). Clinical information was available in 273 cases and was obtained by reviewing the patients’ medical charts. The observation period was from January 2008 to October 2013. The use of materials and clinical information was approved by the Research Ethics Committee of Kurume University and was in accordance with the Declaration of Helsinki.
Cell culture and formalin fixation
The Hodgkin lymphoma cell line HDLM2, which expresses PD-L1,21 was a kind gift from Dr M. Seto (Aichi Cancer Center Research Institute, Nagoya, Japan) and was cultured at 37°C in 5% CO2 in RPMI-1640 medium (Wako, Osaka, Japan) supplemented with 20% fetal bovine serum. The medium contained penicillin (100 U/mL) and streptomycin (100 μg/mL). The harvested cells were centrifuged, fixed in formalin, suspended in agar, and embedded in paraffin to produce a cell block.
Flow cytometry
Flow cytometry (FC) analysis was performed with a flow cytometer (FACS-Calive; Becton-Dickinson, Mountain View, CA), and data were analyzed using the Cell Quest software program (Becton-Dickinson) according to conventional methods described previously.26 For FC analysis, antibodies (clones) against CD5 (T1) (Beckman Coulter, CA) and CD10 (J5) (Beckman Coulter) were used.
In situ hybridization for Epstein-Barr virus–encoded RNA
Epstein-Barr virus (EBV) was detected by means of in situ hybridization with a fluorescein-conjugated EBV peptide nucleic acid probe kit (Dakocytomation, Glostrup, Denmark) following the manufacturer’s instructions. This probe was complementary to the 2 nuclear EBV -encoded RNAs encoded by the EBV.
Immunohistochemical staining
Tissue samples were fixed in formalin and embedded in paraffin by routine methods. The antibodies (clones) used for immunohistochemistry were anti-CD20 (L-26) (Dakocytomation), anti-bcl-6 (P1F6) (Leica Microsystems, Wetzler, Germany), anti-MUM-1 (MUM1p) (Dakocytomation), and anti-PD-1 (NAT105) (ab52587; Abcam, Cambridge, MA). When FC analysis could not be performed because of inadequate or insufficient samples, immunohistochemistry for detection of CD5 (4C7) (Leica Microsystems) and CD10 (56C6) (Leica Microsystems) was performed. When lymphoma cells were positive for CD5, cyclin D1 (SP4) (Thermo Scientific, Runcorn, UK) staining was used to exclude mantle cell lymphoma, an aggressive variant.
Classification of DLBCL by using PD-L1/PAX5 double staining
Immunohistochemical double staining of PD-L1 and PAX5 was carried out by using 2-μm-thick FFPE tissue sections for all cases. The slides were deparaffinized with xylene followed by ethanol. After rehydration with water, antigen retrieval was performed with EDTA buffer (pH 8.0) in a microwave oven at 95°C for 20 minutes. After cooling and rinsing with buffer, the slides were placed in a Dako autostainer (Dakocytomation, Kyoto, Japan). Endogenous peroxidase activity was blocked by incubating in 0.3% hydrogen for 5 minutes, and slides were incubated with anti-PAX5 mouse monoclonal antibodies (BC/24; 1:50 dilution; Biocare Medical, Concord, CA) for 2 hours. Slides were then washed with tris(hydroxymethyl)aminomethane buffer, taken off the autostainer, and manually treated with PolyView mouse-AP (#ADI-95-110; Enzo Life Sciences, Farmingdale, NY) for 20 minutes. After further washing, slides were incubated with HighDef red immunohistochemistry chromogen (AP, plus; #ADI-950-141; Enzo Life Sciences) for 15 minutes for visualization of PAX5 (red). Following these steps, slides were incubated with anti-PD-L1 rabbit monoclonal antibodies (EPR1161[2]; 1:200 dilution; ab174838; Abcam) at 4°C overnight. The slides were then placed in the autostainer again and incubated with an EnVision+ System horseradish peroxidase–labeled anti-rabbit polymer (#K4003; Dakocytomation) for 30 minutes. Visualization of PD-L1 was performed by using diaminobenzidine for 10 minutes (brown). Slides were counterstained with hematoxylin, dehydrated with ethanol, and mounted under coverslips. Sections from the HDLM2 cell block and human placenta (trophoblasts) were used as positive controls. For negative controls, tissue sections were incubated with antibody diluent (Dakocytomation) without primary antibody. All PD-L1/PAX5-immunostained slides were submitted for virtual microscope scanning under high-power magnification (×400) with a Hamamatsu Nanozoomer 2.0HT microscope (Hamamatsu Corp., Hamamatsu, Japan). The percentages of PD-L1+ and PD-L1– tumor cells and nonmalignant cells were estimated in 100 consecutive DLBCL not otherwise specified (NOS) samples and in all other DLBCL subtypes (n = 162) by using an ImageJ software cell counter plugin (National Institutes of Health, Bethesda, MD). The results were used to determine thresholds for evaluation of the tumor microenvironment (see the “Results” section).
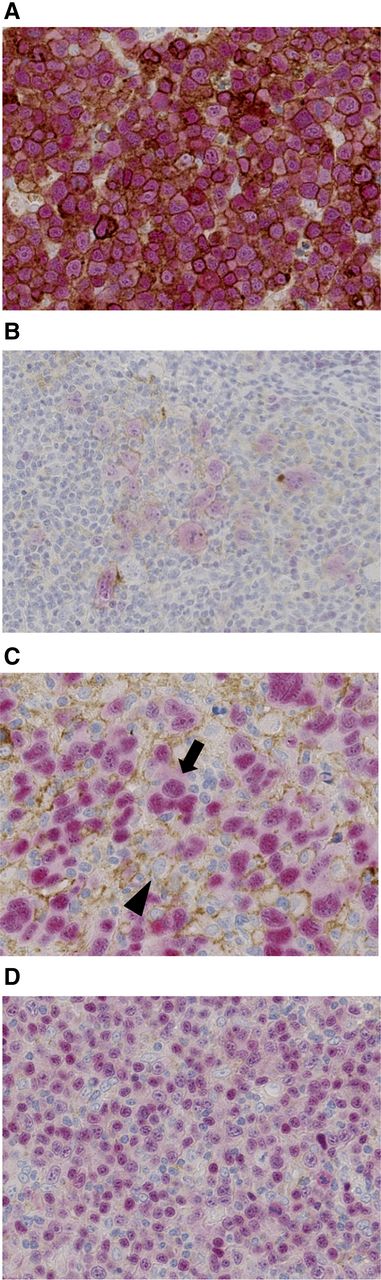
PD-L1+ DLBCL was defined as follows: 30% or more of the lymphoma cells showed distinct membranous and/or cytoplasmic staining of PD-L1 and nuclear staining of PAX5, regardless of the PD-L1 positivity of nonmalignant stromal cells. Among PD-L1– DLBCL cases in which PD-L1+ nonmalignant stromal cells represented 20% or more of the total tissue, cellularity was defined as microenvironmental PD-L1+ (mPD-L1+) DLBCL (Figure 1).
Representative immunohistochemical analysis of PD-L1 expression in DLBCL using a double-staining technique. (A) PD-L1+ DLBCL. Tumor cells were double positive for PD-L1 (brown) and PAX5 (red). Representative cases of (A) DLBCL NOS and (B) TCHRLBCL are presented. (C) mPD-L1+ DLBCL. Tumor cells (arrow) were positive for PAX5 and negative for PD-L1. PD-L1+ nonmalignant cells (arrowhead) represented 20% or more of the total tissue cellularity. (D) PD-L1– and mPD-L1– DLBCL. Tumor cells were positive for PAX5 and negative for PD-L1. Occasionally, PD-L1+ nonmalignant cells were identified; however, these represented less than 20% of the total tissue cellularity.
Representative immunohistochemical analysis of PD-L1 expression in DLBCL using a double-staining technique. (A) PD-L1+ DLBCL. Tumor cells were double positive for PD-L1 (brown) and PAX5 (red). Representative cases of (A) DLBCL NOS and (B) TCHRLBCL are presented. (C) mPD-L1+ DLBCL. Tumor cells (arrow) were positive for PAX5 and negative for PD-L1. PD-L1+ nonmalignant cells (arrowhead) represented 20% or more of the total tissue cellularity. (D) PD-L1– and mPD-L1– DLBCL. Tumor cells were positive for PAX5 and negative for PD-L1. Occasionally, PD-L1+ nonmalignant cells were identified; however, these represented less than 20% of the total tissue cellularity.
Chromosomal analysis
Chromosomal analysis using the standard G-band method was performed, and data were available in 1066 of 1253 cases. Of these, 420 cases had an abnormal karyotype, demonstrating clonality according to the International System for Human Cytogenetics Nomenclature guidelines (2013); these cases were included in the analysis. The presence of chromosomal gain or loss and the occurrence of structural abnormalities in each chromosome were compared between PD-L1+ and PD-L1– DLBCL and between mPD-L1+ and mPD-L1– DLBCL.
Quantitative analysis of PD-1+ TILs
Of the 273 patients for whom clinical information was available, we performed immunohistochemical staining for detection of PD-1 (clone NAT105; Abcam) and quantitative analysis of PD-1+ TILs in 236 cases based on tissue availability and sample size. All PD-1- and PD-L1/PAX5-immunostained slides from the same specimen were captured by using the same method as described earlier. For enumeration of PD-1+ TILs, representative fields of at least 3 mm2 were selected and captured from virtual microscopic images. The number of PD-1+ TILs was counted by using the ImageJ software cell counter plugin. For PD-L1 classification, corresponding areas of the same specimen were also captured from virtual microscopic images for histologic analysis. The PD-1+ cell count and PD-L1 classification results were recorded in ImageJ and were reviewed by one of the authors (H.M.). Any discrepancies were discussed until a consensus was reached. The reviewer was blinded to the patient’s clinical data.
Statistical analysis
Clinical, pathological, and chromosomal characteristics of the patients were compared by using χ2 tests or Fisher’s exact tests. Wilcoxon signed rank tests were performed to compare the median number of PD-1+ TIL counts in different groups. OS was defined as the time from the day of diagnosis to the day of death or the date of last follow-up. The Kaplan-Meier method was used to estimate the OS distributions, comparing the survival curves by using the log-rank test. Age- and sex-adjusted and multivariate-adjusted Cox proportional hazards regression models were used to evaluate the proposed prognostic factors. All reported P values are 2-sided, and those less than .05 were considered statistically significant. JMP version 11.0 and SAS version 9.2 (SAS Institute, Inc., Cary, NC) were used in all analyses.
Results
Histopathological, immunohistochemical, and chromosomal analysis
Table 1 summarizes the characteristics of the 1253 patients. Four hundred sixty-one patients (37%) were diagnosed with GCB-type DLBCL, whereas 792 patients (63%) were diagnosed with non-GCB type DLBCL. EBV in situ hybridization was positive in 114 cases (9%).
Pathological and chromosomal characteristics of 1253 DLBCL specimens
| Characteristic . | PD-L1+ (n = 132) . | PD-L1– (n = 1121) . | P* . | P† . | ||||
|---|---|---|---|---|---|---|---|---|
| mPD-L1+ (n = 172) . | mPD-L1– (n = 949) . | |||||||
| No. . | % . | No. . | % . | No. . | % . | |||
| Age (y) | ||||||||
| Median | 71.5 | 72 | 71 | |||||
| Range | 14-97 | 22-90 | 8-93 | |||||
| >60 | 102 | 77 | 138 | 80 | 712 | 75 | .71 | .14 |
| DLBCL subtypes | <.0001 | <.0001 | ||||||
| GCB | 22 | 17 | 31 | 18 | 408 | 43 | ||
| non-GCB | 110 | 83 | 141 | 82 | 541 | 57 | ||
| EBV-ISH positive | 22 | 17 | 37 | 22 | 55 | 6 | .0014 | <.0001 |
| Chromosomal abnormality | (n = 35) | (n = 47) | (n = 338) | |||||
| Gain of 9 | 8 | 23 | 1 | 2 | 16 | 5 | .0004‡ | .71‡ |
| 9p structural abnormalities | 8 | 23 | 7 | 15 | 39 | 12 | .11‡ | .51 |
| Characteristic . | PD-L1+ (n = 132) . | PD-L1– (n = 1121) . | P* . | P† . | ||||
|---|---|---|---|---|---|---|---|---|
| mPD-L1+ (n = 172) . | mPD-L1– (n = 949) . | |||||||
| No. . | % . | No. . | % . | No. . | % . | |||
| Age (y) | ||||||||
| Median | 71.5 | 72 | 71 | |||||
| Range | 14-97 | 22-90 | 8-93 | |||||
| >60 | 102 | 77 | 138 | 80 | 712 | 75 | .71 | .14 |
| DLBCL subtypes | <.0001 | <.0001 | ||||||
| GCB | 22 | 17 | 31 | 18 | 408 | 43 | ||
| non-GCB | 110 | 83 | 141 | 82 | 541 | 57 | ||
| EBV-ISH positive | 22 | 17 | 37 | 22 | 55 | 6 | .0014 | <.0001 |
| Chromosomal abnormality | (n = 35) | (n = 47) | (n = 338) | |||||
| Gain of 9 | 8 | 23 | 1 | 2 | 16 | 5 | .0004‡ | .71‡ |
| 9p structural abnormalities | 8 | 23 | 7 | 15 | 39 | 12 | .11‡ | .51 |
ISH, in situ hybridization.
PD-L1+ vs PD-L1–.
mPD-L1+ vs mPD-L1–.
Fisher’s exact test.
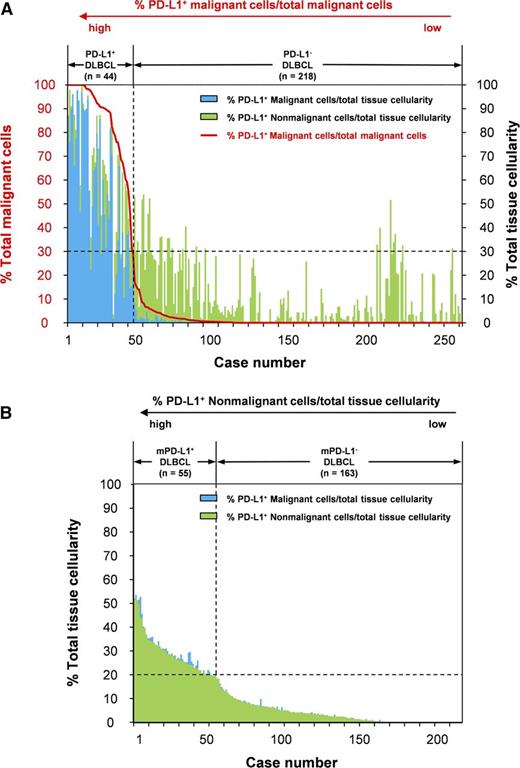
The percentages of PD-L1+ malignant cells and nonmalignant cells within the representative tumor area are summarized in Figure 2 and supplemental Figure 1, available on the Blood Web site. The percentage of PD-L1+ malignant cells among all malignant cells decreased dramatically to around 30%. However, the percentage of PD-L1+ nonmalignant cells among the total tissue cellularity varied regardless of the percentage of PD-L1+ malignant cells among all malignant cells (Figure 2A). Therefore, we set the threshold for PD-L1 positivity as 30% or more of the percentage of PD-L1+ malignant cells among all malignant cells. Among PD-L1– DLBCL, the percentage of PD-L1+ nonmalignant cells among the total tissue cellularity showed an almost linear decrease, with a median percentage of 26% (range, 0% to 52%; Figure 2B). We defined mPD-L1+ DLBCL as samples having 20% or more PD-L1+ nonmalignant cells among the total tissue cellularity in PD-L1– DLBCL. Using these thresholds, the prevalence rates of PD-L1+ DLBCL and mPD-L1+ DLBCL were 10.5% (132 of 1253) and 15.3% (172 of 1121), respectively.
The percentages of PD-L1+ malignant and nonmalignant cells within the representative tumor area in 262 DLBCL samples. (A) The percentages of PD-L1+ malignant cells (blue bars) and PD-L1+ nonmalignant cells (green bars) among the total tissue cellularity. The red line indicates the percentage of PD-L1+ malignant cells among all malignant cells. The cases were sorted according to the percentage of PD-L1+ malignant cells among the total tissue cellularity. We set the threshold for PD-L1 positivity as 30% or more of PD-L1+ malignant cells among the total tissue cellularity. (B) The percentages of PD-L1+ malignant cells (blue bars) and PD-L1+ nonmalignant cells (green bars) among the total tissue cellularity in PD-L1– DLBCL samples (n = 218). The cases were sorted according to the percentage of PD-L1+ nonmalignant cells among the total tissue cellularity. We defined mPD-L1+ DLBCL as samples containing 20% or more PD-L1+ nonmalignant cells among the total tissue cellularity in PD-L1– DLBCL.
The percentages of PD-L1+ malignant and nonmalignant cells within the representative tumor area in 262 DLBCL samples. (A) The percentages of PD-L1+ malignant cells (blue bars) and PD-L1+ nonmalignant cells (green bars) among the total tissue cellularity. The red line indicates the percentage of PD-L1+ malignant cells among all malignant cells. The cases were sorted according to the percentage of PD-L1+ malignant cells among the total tissue cellularity. We set the threshold for PD-L1 positivity as 30% or more of PD-L1+ malignant cells among the total tissue cellularity. (B) The percentages of PD-L1+ malignant cells (blue bars) and PD-L1+ nonmalignant cells (green bars) among the total tissue cellularity in PD-L1– DLBCL samples (n = 218). The cases were sorted according to the percentage of PD-L1+ nonmalignant cells among the total tissue cellularity. We defined mPD-L1+ DLBCL as samples containing 20% or more PD-L1+ nonmalignant cells among the total tissue cellularity in PD-L1– DLBCL.
PD-L1 expression was significantly associated with non-GCB subtype and EBV positivity (P < .0001 and P < .0014, respectively). Regarding World Health Organization classification, the prevalence rates of PD-L1+ DLBCL were 8.9% in patients with DLBCL NOS (97 of 1091), 15.6% in patients with EBV-positive DLBCL of the elderly (14 of 90), 30.8% in T-cell/histiocyte-rich large B-cell lymphoma (TCHRLBCL; 8 of 26), 5.6% in patients with other iatrogenic immunodeficiency-associated lymphoproliferative disorders (1 of 18), 45.5% in patients with intravascular large B-cell lymphoma (IVLBCL; 5 of 11), 42.9% in patients with primary mediastinal large B-cell lymphoma (PMLBCL; 3 of 7), 0% in patients with primary cutaneous DLBCL, leg type (0 of 4), 33% in patients with monomorphic posttransplant lymphoproliferative disorder (PTLD; 1 of 3), 100% in patients with DLBCL associated with chronic inflammation (2 of 2), and 100% in patients with DLBCL associated with HIV infection (1 of 1). With regard to mPD-L1, mPD-L1+ DLBCL was significantly associated with non-GCB subtype and EBV positivity (P < .0001 and P < .0001, respectively). The prevalence rates of mPD-L1+ DLBCL were 12.3% in patients with DLBCL NOS (124 of 994), 40.5% in patients with EBV-positive DLBCL of the elderly (30 of 76), 55.6% in patients with TCHRLBCL (10 of 18), 31.3% in patients with other iatrogenic immunodeficiency-associated lymphoproliferative disorders (6 of 17), 0% in patients with IVLBCL (0 of 6), 25.0% in patients with PMLBCL (1 of 4), 25.0% in patients with primary cutaneous DLBCL, leg type (1 of 4), and 0% in patients with monomorphic PTLD (0 of 2).
In chromosomal analysis, PD-L1+ DLBCL was significantly associated with gain of chromosome 9 (P = .0004), where the PD-L1 gene is located (9p24.1).5 In contrast, there was no association between PD-L1 expression and structural abnormalities in chromosome 9p (P = .11). Also in contrast, mPD-L1+ DLBCL was not associated with either gain of chromosome 9 or structural abnormalities in chromosome 9p (P = .71 and P = .51, respectively).
Quantitative analysis of PD-1+ TILs
Table 2 summarizes the results of the quantitative analysis of PD-1+ TILs. The number of PD-1+ TILs was significantly lower in patients with B symptoms (P = .024), extranodal sites (P = .042), and bulky mass (P = .041) compared with those without these clinical features. Pathologically, the number of PD-1+ TILs was significantly higher in GCB-type DLBCL (P = .034) and lower in mPD-L1+ DLBCL (P = .017) and PD-L1+ DLBCL (P < .0001). We defined the median number of TILs as the cutoff for the high and low PD-1+ TIL groups and performed survival analysis between the four groups according to the combined expression pattern of PD-L1 (positive or negative) and level of PD-1+ TILhigh or TILlow. In age- and sex-adjusted analyses, the PD-L1+/TILlow group (n = 25) was significantly associated with poor prognosis when the PD-L1–/TILlow group (n = 92) was used as a reference (P = .0086), whereas no prognostic impact was observed in the other two groups (PD-L1+/TILhigh group: n = 3 and PD-L1–/TILhigh group: n = 116). However, this difference disappeared in multivariate analysis (data not shown).
Quantitative analysis of PD-1+ TILs
| Clinical feature . | No. of patients . | Median TILs per mm2 (range) . | P* . |
|---|---|---|---|
| Age, years | |||
| >60 | 181 | 21 (0-1288) | NS |
| ≤60 | 55 | 21 (0-1041) | |
| ECOG PS | |||
| >1 | 74 | 30 (0-241) | NS |
| 0 or 1 | 162 | 19 (0-1288) | |
| B symptoms | |||
| Present | 57 | 12 (0-802) | .024 |
| Absent | 179 | 29 (0-441) | |
| Serum LDH | |||
| Elevated | 151 | 18 (0-1082) | NS |
| Normal | 85 | 37 (0-589) | |
| Serum sIL-2R | |||
| Elevated | 188 | 20 (0-326) | NS |
| Normal | 41 | 37 (0-1196) | |
| Extranodal sites | |||
| Present | 98 | 15 (0-1196) | .042 |
| Absent | 98 | 39 (0-1288) | |
| Bulky mass | |||
| Present | 36 | 10 (0-692) | .041 |
| Absent | 196 | 27 (0-1288) | |
| Ann Arbor stage | |||
| III or IV | 137 | 21 (0-1288) | NS |
| I or II | 99 | 21 (0-1196) | |
| DLBCL subtype | |||
| GCB | 97 | 40 (0-1196) | .034 |
| Non-GCB | 139 | 18 (0-1288) | |
| EBV-ISH | |||
| Positive | 24 | 58 (0-802) | NS |
| Negative | 212 | 19 (0-802) | |
| mPD-L1 | |||
| Positive | 47 | 10 (0-1196) | .017 |
| Negative | 161 | 29 (0-1288) | |
| PD-L1 | |||
| Positive | 28 | 3 (0-476) | <.0001 |
| Negative | 208 | 31 (0-1288) |
| Clinical feature . | No. of patients . | Median TILs per mm2 (range) . | P* . |
|---|---|---|---|
| Age, years | |||
| >60 | 181 | 21 (0-1288) | NS |
| ≤60 | 55 | 21 (0-1041) | |
| ECOG PS | |||
| >1 | 74 | 30 (0-241) | NS |
| 0 or 1 | 162 | 19 (0-1288) | |
| B symptoms | |||
| Present | 57 | 12 (0-802) | .024 |
| Absent | 179 | 29 (0-441) | |
| Serum LDH | |||
| Elevated | 151 | 18 (0-1082) | NS |
| Normal | 85 | 37 (0-589) | |
| Serum sIL-2R | |||
| Elevated | 188 | 20 (0-326) | NS |
| Normal | 41 | 37 (0-1196) | |
| Extranodal sites | |||
| Present | 98 | 15 (0-1196) | .042 |
| Absent | 98 | 39 (0-1288) | |
| Bulky mass | |||
| Present | 36 | 10 (0-692) | .041 |
| Absent | 196 | 27 (0-1288) | |
| Ann Arbor stage | |||
| III or IV | 137 | 21 (0-1288) | NS |
| I or II | 99 | 21 (0-1196) | |
| DLBCL subtype | |||
| GCB | 97 | 40 (0-1196) | .034 |
| Non-GCB | 139 | 18 (0-1288) | |
| EBV-ISH | |||
| Positive | 24 | 58 (0-802) | NS |
| Negative | 212 | 19 (0-802) | |
| mPD-L1 | |||
| Positive | 47 | 10 (0-1196) | .017 |
| Negative | 161 | 29 (0-1288) | |
| PD-L1 | |||
| Positive | 28 | 3 (0-476) | <.0001 |
| Negative | 208 | 31 (0-1288) |
ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; sIL-2R, soluble interleukin-2 receptor; NS, not significant.
Wilcoxon signed-rank test
Clinical features and outcomes in patients with PD-L1+ DLBCL
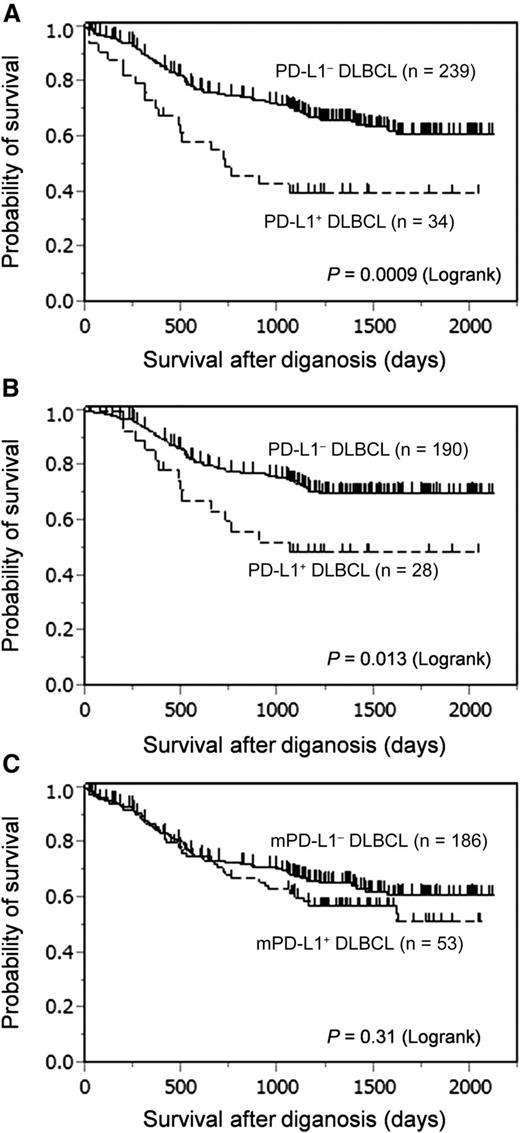
Table 3 summarizes the clinicopathological characteristics of the 273 patients whose clinical information was available. In this cohort, 218 (80%) received R-CHOP or R-CHOP-like chemotherapy. PD-L1+ DLBCL was significantly associated with the presence of B symptoms (P = .004), elevated serum soluble interleukin-2 receptor levels (P = .0004), International Prognostic Index (IPI) high-risk group (P = .04), and non-GCB subtype (P = .008), whereas mPD-L1+ DLBCL was associated with IPI high-risk group (P = .005), non-GCB subtype (P = .004), and EBV infection (P = .002). Death as a result of any reason occurred in 20 patients (59%) with PD-L1+ DLBCL compared with 76 patients (32%) with PD-L1– DLBCL and was mostly due to lymphoma progression. Patients with PD-L1+ DLBCL had inferior OS compared with that of patients with PD-L1– DLBCL in the entire cohort (P = .0009; Figure 3A). This difference was still significant when compared among patients who received an R-CHOP or R-CHOP-like regimen (P = .0133; Figure 3B). In contrast, there were no significant differences in OS between mPD-L1+ and mPD-L1– DLBCL (P = .31; Figure 3C).
Patient characteristics of PD-L1+ and PD-L1– DLBCL
| Characteristic . | PD-L1+ (n = 34) . | PD-L1– (n = 239) . | P* . | P† . | ||||
|---|---|---|---|---|---|---|---|---|
| No. . | % . | mPD-L1+ (n = 53) . | mPD-L1– (n = 186) . | |||||
| No. . | % . | No. . | % . | |||||
| Sex (male/female) | 23/11 | 68/32 | 24/29 | 45/55 | 95/91 | 51/49 | .07 | .42 |
| Age (y) | ||||||||
| Median | 74.5 | 73 | 70 | |||||
| Range | 30-92 | 29-87 | 21-93 | |||||
| >60 | 26 | 76 | 43 | 81 | 132 | 71 | .78 | .14 |
| ECOG PS >1 | 13 | 38 | 15 | 28 | 56 | 30 | .32 | .80 |
| Extranodal sites >1 | 14 | 41 | 15 | 28 | 49 | 26 | .09 | .78 |
| B symptoms | 16 | 47 | 17 | 32 | 37 | 20 | .004 | .06 |
| Elevated serum LDH | 26 | 76 | 35 | 66 | 114 | 61 | .10 | .53 |
| Elevated serum sIL-2R | 33 of 33 | 100 | 41 of 50 | 82 | 141 of 181 | 82 | .0005 | .97 |
| Ann Arbor stage >2 | 24 | 71 | 35 | 66 | 101 | 54 | .12 | .13 |
| IPI risk group | ||||||||
| Low (0–2) | 10 | 29 | 16 | 30 | 98 | 53 | .04 | .005 |
| High (3–5) | 24 | 71 | 37 | 70 | 88 | 47 | ||
| DLBCL subtypes | ||||||||
| GCB | 7 | 21 | 14 | 26 | 90 | 48 | .008 | .004 |
| non-GCB | 27 | 79 | 39 | 74 | 96 | 52 | ||
| EBV-ISH positive | 2 | 6 | 11 | 21 | 11 | 6 | .75‡ | .002‡ |
| Treatment | ||||||||
| R-CHOP/R-CHOP-like | 26 | 76 | 41 | 77 | 151 | 81 | ||
| First-line ASCT | 2 | 6 | 4 | 8 | 4 | 2 | ||
| Other chemotherapy | 5 | 15 | 6 | 11 | 21 | 11 | ||
| Radiotherapy (total) | 5 | 15 | 12 | 23 | 41 | 22 | ||
| Radiotherapy only | 1 | 3 | 2 | 4 | 8 | 4 | ||
| No therapy | 0 | 0 | 0 | 0 | 2 | 1 | ||
| (n = 33) | (n = 51) | (n = 176) | ||||||
| Treatment response | ||||||||
| CR/CRu | 21 | 64 | 41 | 80 | 127 | 72 | .21 | .28 |
| PR | 8 | 24 | 7 | 14 | 23 | 13 | ||
| SD or PD | 3 | 9 | 3 | 6 | 24 | 14 | ||
| Not evaluable | 1 | 3 | 0 | 0 | 2 | 1 | ||
| Characteristic . | PD-L1+ (n = 34) . | PD-L1– (n = 239) . | P* . | P† . | ||||
|---|---|---|---|---|---|---|---|---|
| No. . | % . | mPD-L1+ (n = 53) . | mPD-L1– (n = 186) . | |||||
| No. . | % . | No. . | % . | |||||
| Sex (male/female) | 23/11 | 68/32 | 24/29 | 45/55 | 95/91 | 51/49 | .07 | .42 |
| Age (y) | ||||||||
| Median | 74.5 | 73 | 70 | |||||
| Range | 30-92 | 29-87 | 21-93 | |||||
| >60 | 26 | 76 | 43 | 81 | 132 | 71 | .78 | .14 |
| ECOG PS >1 | 13 | 38 | 15 | 28 | 56 | 30 | .32 | .80 |
| Extranodal sites >1 | 14 | 41 | 15 | 28 | 49 | 26 | .09 | .78 |
| B symptoms | 16 | 47 | 17 | 32 | 37 | 20 | .004 | .06 |
| Elevated serum LDH | 26 | 76 | 35 | 66 | 114 | 61 | .10 | .53 |
| Elevated serum sIL-2R | 33 of 33 | 100 | 41 of 50 | 82 | 141 of 181 | 82 | .0005 | .97 |
| Ann Arbor stage >2 | 24 | 71 | 35 | 66 | 101 | 54 | .12 | .13 |
| IPI risk group | ||||||||
| Low (0–2) | 10 | 29 | 16 | 30 | 98 | 53 | .04 | .005 |
| High (3–5) | 24 | 71 | 37 | 70 | 88 | 47 | ||
| DLBCL subtypes | ||||||||
| GCB | 7 | 21 | 14 | 26 | 90 | 48 | .008 | .004 |
| non-GCB | 27 | 79 | 39 | 74 | 96 | 52 | ||
| EBV-ISH positive | 2 | 6 | 11 | 21 | 11 | 6 | .75‡ | .002‡ |
| Treatment | ||||||||
| R-CHOP/R-CHOP-like | 26 | 76 | 41 | 77 | 151 | 81 | ||
| First-line ASCT | 2 | 6 | 4 | 8 | 4 | 2 | ||
| Other chemotherapy | 5 | 15 | 6 | 11 | 21 | 11 | ||
| Radiotherapy (total) | 5 | 15 | 12 | 23 | 41 | 22 | ||
| Radiotherapy only | 1 | 3 | 2 | 4 | 8 | 4 | ||
| No therapy | 0 | 0 | 0 | 0 | 2 | 1 | ||
| (n = 33) | (n = 51) | (n = 176) | ||||||
| Treatment response | ||||||||
| CR/CRu | 21 | 64 | 41 | 80 | 127 | 72 | .21 | .28 |
| PR | 8 | 24 | 7 | 14 | 23 | 13 | ||
| SD or PD | 3 | 9 | 3 | 6 | 24 | 14 | ||
| Not evaluable | 1 | 3 | 0 | 0 | 2 | 1 | ||
ASCT, autologous stem cell transplantation; CR, complete response/remission; CRu, uncertain complete response/remission; IPI, international prognostic index; PD, progressive disease; PR, partial response/remission; SD, stable disease.
PD-L1+ vs PD-L1–.
mPD-L1+ vs mPD-L1–.
Fisher’s exact test.
PD-L1 expression on tumor cells was associated with poor OS in patients with DLBCL. (A) OS of the entire study cohort. (B) OS of patients who received rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or R-CHOP-like chemotherapy. (C) OS of patients according to mPD-L1 positivity.
PD-L1 expression on tumor cells was associated with poor OS in patients with DLBCL. (A) OS of the entire study cohort. (B) OS of patients who received rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or R-CHOP-like chemotherapy. (C) OS of patients according to mPD-L1 positivity.
Age- and sex-adjusted analyses identified the following variables as prognostic factors: PD-L1 expression, advanced clinical stage (III or IV), elevated serum lactate dehydrogenase level, presence of extranodal sites (more than 1), presence of B symptoms, and poor Eastern Cooperative Oncology Group performance status (more than 1). The variables included in multivariate analysis 1 were age, sex, clinical stage, lactate dehydrogenase, extranodal site, Eastern Cooperative Oncology Group performance status, B symptoms, Hans algorithm, and the expression of PD-L1 (all of the IPI risk factors were included). In this analysis, the expression of PD-L1+ DLBCL remained a significant prognostic factor (P = .0323; Table 4). When we performed multivariate analysis 2, in which B symptoms, Hans algorithm, IPI, and PD-L1 positivity were included as covariates, the impact of PD-L1 expression for OS was marginally significant (P = .0818; Table 4).
Prognostic factors affecting the OS of patients with DLBCL
| Characteristic . | Age- and sex-adjusted analysis . | Multivariate analysis 1* . | Multivariate analysis 2† . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| PD-L1+ vs PD-L1– | 2.206 (1.334-3.649) | .0021 | 1.809 (1.051-3.112) | .0323 | 1.599 (0.943-2.713) | .0818 |
| Stage III/IV vs I/II | 2.887 (1.791-4.654) | <.0001 | 1.704 (0.986-2.945) | .0561 | ||
| LDH elevated vs normal | 2.488 (1.499-4.129) | .0004 | 1.713 (1.008-2.912) | .0466 | ||
| Extranodal sites ≥2 vs ≤1 | 2.328 (1.549-3.500) | <.0001 | 1.618 (1.030-2.543) | .0368 | ||
| ECOG PS >1 vs ≤1 | 2.556 (1.706-3.830) | <.0001 | 1.976 (1.287-3.034) | .0019 | ||
| B symptoms present vs absent | 1.686 (1.103-2.575) | .0157 | 0.845 (0.526-1.358) | .4874 | 1.058 (0.673-1.664) | .8063 |
| Non-GCB vs GCB type | 1.445 (0.941-2.221) | .0928 | 1.256 (0.805-1.960) | .3159 | 1.287 (0.830-1.996) | .2593 |
| IPI high/high-intermediate vs low/low-intermediate | 3.773 (2.341-6.080)‡ | <.0001 | 3.447 (2.081-5.710) | <.0001 | ||
| Characteristic . | Age- and sex-adjusted analysis . | Multivariate analysis 1* . | Multivariate analysis 2† . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| PD-L1+ vs PD-L1– | 2.206 (1.334-3.649) | .0021 | 1.809 (1.051-3.112) | .0323 | 1.599 (0.943-2.713) | .0818 |
| Stage III/IV vs I/II | 2.887 (1.791-4.654) | <.0001 | 1.704 (0.986-2.945) | .0561 | ||
| LDH elevated vs normal | 2.488 (1.499-4.129) | .0004 | 1.713 (1.008-2.912) | .0466 | ||
| Extranodal sites ≥2 vs ≤1 | 2.328 (1.549-3.500) | <.0001 | 1.618 (1.030-2.543) | .0368 | ||
| ECOG PS >1 vs ≤1 | 2.556 (1.706-3.830) | <.0001 | 1.976 (1.287-3.034) | .0019 | ||
| B symptoms present vs absent | 1.686 (1.103-2.575) | .0157 | 0.845 (0.526-1.358) | .4874 | 1.058 (0.673-1.664) | .8063 |
| Non-GCB vs GCB type | 1.445 (0.941-2.221) | .0928 | 1.256 (0.805-1.960) | .3159 | 1.287 (0.830-1.996) | .2593 |
| IPI high/high-intermediate vs low/low-intermediate | 3.773 (2.341-6.080)‡ | <.0001 | 3.447 (2.081-5.710) | <.0001 | ||
CI, confidence interval; HR, hazard ratio.
The variables included in multivariate analysis 1 for OS were age, sex, stage, LDH, extranodal site, ECOG PS, B symptoms, Hans algorithm, and the expression of PD-L1.
The variables included in the second multivariate analysis for OS were sex, B symptoms, Hans algorithm, IPI, and the expression of PD-L1.
Sex-adjusted analysis.
Discussion
In this study, we demonstrated for the first time that PD-L1 expression on tumor cells was an independent prognostic factor for OS in patients with DLBCL by means of PD-L1/PAX5 double-staining using FFPE samples, which enabled us to identify PD-L1+ DLBCL. We also found that the number of PD-1+ TILs was significantly associated with PD-L1 positivity of lymphoma cells and tumor-infiltrating nonmalignant stromal cells. These results suggested that the PD-1/PD-L1 pathway contributed to the DLBCL tumor microenvironment and may be an effective therapeutic target.
Immunotherapy targeting of the PD-1/PD-L1 pathway has emerged as an effective strategy for certain types of malignancies.19 Regarding DLBCL, administration of pidilizumab, an anti-PD-1 monoclonal antibody, after autologous stem cell transplantation was reported as a promising therapeutic approach.18 Recently, nivolumab, a fully human immunoglobulin G4 monoclonal anti-PD-1 receptor antibody, was reported to have therapeutic activity in patients with lymphoid malignancies, including DLBCL.27,28 However, there is little information about the efficacy of blocking the PD-1/PD-L1 pathway using an anti-PD-L1 antibody in hematologic malignancies. The interaction of PD-1 with PD-L1 occurs between PD-1+ TILs and PD-L1+ nonmalignant cells and between PD-1+ TILs and PD-L1+ lymphoma cells. Distinguishing these interactions is critical for understanding the DLBCL tumor microenvironment.
We set the threshold for mPD-L1 positivity as 20% of the total tissue cellularity, which was the same as that used in a previous study21 ; the prevalence of mPD-L1+ DLBCL was 15.3%, which was also comparable with that observed in the previous study (14%). However, in this study, the threshold for PD-L1 positivity was 30%, considerably higher than those in previous studies. Several reports have described the expression of PD-L1 in DLBCL using FFPE samples. The percentages of PD-L1+ DLBCL in these studies were 11% (7 of 66),21 75% (55 of 73),24 and 20% (6 of 30).20 The threshold for PD-L1 positivity used in the former two studies was 5% of the tumor cell population, whereas the final study defined PD-L1 positivity as cases in which the majority of tumor cells were stained for PD-L1 (a threshold was not defined). This discrepancy suggests the difficulty in interpreting which cells are positive and which are negative, even for experienced hematopathologists. The prevalence of PD-L1+ DLBCL reported by Chen et al,21 in which a PD-L1/PAX5 double-staining technique was used in selected cases, was similar to that of our study, even when the threshold was raised from 5% to 30%; therefore, the double-staining technique may be the key for reducing discrepancies between studies.
We performed chromosomal analysis by using the G-band method; this experiment revealed a significant association between PD-L1 expression and gain of chromosome 9, where the PD-L1 gene is located. In contrast, this association was not evident in mPD-L1+ DLBCL. In a recent study that used an in-house break-apart fluorescence in situ hybridization assay, gain and amplification of the PDL locus, which contains the CD274 (PD-L1) and PDCD1LG2 (PD-L2) genes in DLBCL, were observed in 14% and 3% of samples, respectively.29 Although a direct comparison cannot be made, this result provides supporting evidence for the validity of the double-staining technique. This technique is a useful and cost-effective screening method for identifying PD-L1+ DLBCL and is generally available in research and clinical laboratories.
PD-L1-expressing cells have various mechanisms to evade T-cell immunity.7 One of the most important mechanisms is induction of apoptosis in PD-1+ tumor-associated antigen-specific T cells through the PD-1/PD-L1 pathway.6 With respect to the Hans algorithm, PD-L1+ DLBCL has been reported to be associated with non-GCB subtype.20,21 This association remained significant in our larger cohort. Andorsky et al20 described a PD-L1+ DLBCL cell line that exhibited characteristics consistent with a non-GCB phenotype (eg, inhibition of T-cell proliferation and interferon-γ [IFN-γ] secretion by tumor-associated T cells). These results correspond to our data, suggesting that PD-L1 expression on tumor cells plays a pivotal role in DLBCL tumor microenvironment and contributes to aggressive clinical outcomes. Blockade of the PD-1/PD-L1 pathway using monoclonal PD-L1 antibodies may be a novel and effective treatment approach for patients with this distinctive subgroup of DLBCL.
In our quantitative analysis of PD-1+ TILs, a low number of TILs was associated with PD-L1 and mPD-L1 positivity, and patients in the PD-L1+/TILlow group had poor prognoses. This is contrary to the results reported for melanoma, sarcoma, and various cancers in which the number of PD-1+ TILs is positively correlated with tumor PD-L1 expression.17,30,31 In contrast to these observations in solid tumors, the presence of a high number of PD-1+ TILs is a favorable prognostic factor in patients with follicular lymphoma, and patients with low numbers of PD-1+ TILs had a higher risk of histologic transformation.32,33 In normal tissue, PD-1 is expressed at high levels on germinal center follicular helper T-cells.34 Therefore, PD-1+ TILs are expected to be more abundant in GCB-type DLBCL. Our quantitative analysis confirmed that many PD-1+ TILs were associated with GCB-type DLBCL. This result suggested that the number of PD-1+ TILs reflected not only tumor-mediated T-cell exhaustion but also the lymphoma cell origin. In addition, although the mechanism remains unclear, the PD-1/PD-L1 pathway may have different roles in B-cell neoplasms. In clinical trials of nivolumab, the levels of PD-1 on TILs were less predictive of treatment response than was PD-L1 expression on tumor cells.27,30 Interestingly, nivolumab was shown to be efficacious in patients whose TILs did not express PD-1.30 Because nivolumab also had therapeutic activity in DLBCL,28 there is likely to be a tumor-mediated T-cell exhaustion mechanism in the DLBCL tumor microenvironment. Therefore, immunotherapy targeting the PD-1/PD-L1 pathway may have a therapeutic potential in patients with PD-L1+ DLBCL.
PD-L1+ DLBCL is also significantly associated with EBV infection. In cell lines, 9p24.1 amplification increases PD-L1 and JAK2 copy numbers and induces PD-L1 expression in classical Hodgkin lymphoma and PMLBCL.35 Furthermore, the same group demonstrated that EBV infection is an alternative mechanism for PD-L1 induction in Hodgkin lymphomas and EBV-positive PTLD as a result of latent membrane protein 1 (LMP1) –mediated effects on both the PD-L1 enhancer and promoter.36 The results of this study are consistent with previous studies, and patients with EBV-associated DLBCL may be appropriate candidates for anti-PD-1/PD-L1 immunotherapies.
Both malignant cells and tumor-associated antigen-presenting cells express PD-L1. Curiel et al37 reported that tumor-infiltrating PD-L1+ myeloid dendritic cells (MDCs) suppress T-cell IFN-γ induction and reduce IFN-γ-positive T cells, indicating that PD-L1+ MDCs induce T-cell immune suppression in the tumor microenvironment. The number of PD-1+ TILs was lower in mPD-L1+ DLBCL, which may be due to suppression of T-cell induction by mPD-L1+ MDCs. In addition, a relatively high incidence of mPD-L1 positivity was observed in TCHRLBCL (55.6%) and EBV-associated DLBCL (22%). These subtypes are pathologically characterized by infiltration of numerous inflammatory cells around tumor cells, suggesting the presence of a cytokine-rich tumor microenvironment. These cytokines may be responsible for upregulation of PD-L1 expression on tumor infiltrating nonmalignant cells, which may be another therapeutic target for blockade of the PD-1/PD-L1 pathway. Classification into the IPI high-risk group or with the non-GCB phenotype, both of which are well-known poor prognostic factors for DLBCL,38,39 was significantly associated with mPD-L1+ DLBCL. However, in contrast to PD-L1+ DLBCL, there was no survival disadvantage in patients with mPD-L1+ DLBCL. Although the reason for this difference is unclear, the function of PD-L1 may not be the same in PD-L1+ and mPD-L1+ DLBCL. Further studies are necessary to understand the function of PD-L1 in mPD-L1+ DLBCL.
Several limitations should be noted when interpreting the results of this work. First, the small sample size in the clinicopathological analysis may limit the power to detect differences between groups. Therefore, our findings should be verified in a larger population. Second, although the majority of patients were treated with R-CHOP or R-CHOP-like chemotherapy, which has long been the standard of care for DLBCL, and the treatment disparity between each center is relatively small, this was a retrospective study that lacked standardized treatment and follow-up protocols. A future prospective study is necessary for adequate evaluation. Finally, it still remains unclear whether expression of PD-L1 on tumor cells or on other nonmalignant cells is a key factor associated with clinical outcomes in response to PD-1/PD-L1 blockade in DLBCL. Future functional analyses are necessary to elucidate the complex roles of this pathway.
In conclusion, we have demonstrated that PD-L1 expression on tumor cells is correlated with poor prognosis and that the double-staining technique is a useful and cost-effective method for identifying and distinguishing PD-L1+ and mPD-L1+ DLBCL. Our findings support the hypothesis that immunotherapy targeting the PD-1/PD-L1 pathway may benefit patients with DLBCL, although therapeutic responses may differ according to PD-L1 expression profiles. Future investigations and clinical trials are needed to develop therapeutic strategies based on PD-L1-induced tumor immune evasion mechanisms and modulation of host immune responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The study was performed using tissue samples obtained from the many hospitals and/or institutes comprising the Kyushu Lymphoma Study Group, including the Department of Hematology, National Kyushu Cancer Center; Division of Hematology and Oncology, Department of Medicine, Kurume University School of Medicine; Department of Medicine and Bioregulatory Science, Graduate School of Medical Sciences, Kyushu University; Department of Medicine and Biosystemic Science, Kyushu University Faculty of Medicine; Department of Hematology, Iizuka Hospital; Department of Hematology, Kumamoto Social Insurance General Hospital; Department of Hematology, Kumamoto Shinto General Hospital; Department of Hematology and Oncology, Nakagami Hospital; Department of Hematology, Karatsu Red Cross Hospital; Department of Hematology, Nagasaki Prefecture Shimabara Hospital; Department of Hematology and Oncology, Fukuoka Higashi Medical Center; Department of Hematology, Sasebo City General Hospital; Department of Hematology, Imamura Hospital; Department of Hematology and Chemotherapy, Saiseikai Fukuoka General Hospital; Department of Internal Medicine, Chihaya Hospital; Department of Hematology and Infectious Diseases, National Hospital Organization Kokura Medical Center; Department of Internal Medicine, Heartlife Hospital; Department of Internal Medicine, Fukuoka Teishin Hospital; Department of Hematology, Hamanomachi Hospital; Department of Hematology, Atomic Bomb Disease and Hibakusha Medicine Unit, Atomic Bomb Disease Institute, Nagasaki University; Department of Hematology, Endocrinology and Metabolism, Niigata University Faculty of Medicine; Department of Hematology and Oncology, Kyushu Hospital; Department of Hematology and Oncology, Yame General Hospital; Department of Internal Medicine, Social Insurance Nakabaru Hospital; Department of Internal Medicine, Nakatsu Municipal Hospital; Department of Hematology, Yanagawa Hospital; and Department of Hematology and Oncology, Japanese Red Cross Musashino Hospital. The authors express their appreciation for the donated samples. They also thank Mayumi Miura, Kanoko Miyazaki, Konomi Takasu, Yuki Morotomi, Chie Kuroki, Kaoruko Nagatomo, and Kazutaka Nakashima for their technical assistance.
This research was supported by a Grant-in-Aid for Scientific Research by the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Authorship
Contribution: J.K. and K.O. were responsible for study conception and design; Y.Y., I.C., Y.A., N.U., K.N., T.O., K.A., R.T., and M.S. provided study materials or patients; J.K., H.M., F.A., and A.I. performed collection and assembly of data; J.K., H.M., A.H., A.I., D.N., Y.S., and K.O. performed data analysis and interpretation; J.K. wrote the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Koichi Ohshima, Department of Pathology, School of Medicine, Kurume University, Asahimachi 67, Kurume-city, Fukuoka 830-0011, Japan; e-mail: ohshima_kouichi@med.kurume-u.ac.jp.