Key Points
Mutation of the fibrinogen Aα chain in mice to selectively eliminate thrombin cleavage prevents fibrin polymer formation in vivo.
Fibrin polymer formation drives antimicrobial function and supports host survival following S aureus peritoneal infection.
Abstract
Fibrin(ogen) is central to hemostasis and thrombosis and also contributes to multiple physiologic and pathologic processes beyond coagulation. However, the precise contribution of soluble fibrinogen vs insoluble fibrin matrices to vascular integrity, tissue repair, inflammation, and disease has been undefined and unapproachable. To establish the means to distinguish fibrinogen- and fibrin-dependent processes in vivo, FibAEK mice were generated that carry normal levels of circulating fibrinogen but lack the capacity for fibrin polymer formation due to a germ-line mutation in the Aα chain thrombin cleavage site. Homozygous FibAEK mice developed to term and exhibited postnatal survival superior to that of fibrinogen-deficient mice. Unlike fibrinogen-deficient mice, platelet-rich plasma from FibAEK mice supported normal platelet aggregation in vitro, highlighting that fibrinogenAEK retains the functional capacity to support interactions with platelets. Thrombin failed to release fibrinopeptide-A from fibrinogenAEK and failed to induce polymer formation with FibAEK plasma or purified fibrinogenAEK in 37°C mixtures regardless of incubation time. FibAEK mice displayed both an absence of fibrin polymer formation following liver injury, as assessed by electron microscopy, and a failure to generate stable occlusive thrombi following FeCl3 injury of carotid arteries. FibAEK mice exhibited a profound impediment in Staphylococcus aureus clearance following intraperitoneal infection similar to fibrinogen-deficient mice, yet FibAEK mice displayed a significant infection dose-dependent survival advantage over fibrinogen-deficient mice following peritonitis challenge. Collectively, these findings establish for the first time that fibrin polymer is the molecular form critical for antimicrobial mechanisms while simultaneously highlighting biologically meaningful contributions and functions of the soluble molecule.
Introduction
Fibrin(ogen) is a key factor in the control of blood loss and the development of potentially fatal venous or arterial thrombotic events (eg, deep vein thrombosis, pulmonary embolism, myocardial infarction, and stroke). Fibrin(ogen) is also instrumental in reparative and protective inflammatory processes, but exuberant or persistent fibrin(ogen) is associated with many diseases, including cancer, vessel wall disease, and inflammatory diseases.1-4 Polymer is often presumed to be the key structural form of the molecule coupled to fibrinogen-dependent physiologic and pathologic processes in vivo, but resolving the precise contributions of soluble fibrinogen and fibrin in vivo has been formally problematic. The uncertainty is underscored by the known potential for soluble fibrinogen to support important functions, including the capacity of the soluble, circulating molecule to support integrin αIIbβ3-mediated platelet aggregation/thrombus formation. Similarly, leukocyte engagement of immobilized fibrinogen in vitro through integrin5-7 and nonintegrin8,9 receptors is thought to support cell adhesion, migration, phagocytosis, nuclear factor-κB–mediated transcription, chemokine and cytokine elaboration, degranulation, and other processes.9-13 Both fibrinogen and fibrin may have distinct and specialized properties that direct thrombotic and/or inflammatory events in vivo, but the precise form of the molecule driving fibrin(ogen)-associated events has not been established.
Host fibrin(ogen) is a known determinant of infection outcome for many bacterial pathogens (eg, Staphylococcus aureus, Yersinia pestis, and Streptococcus pyogenes). Depending on the context, fibrin(ogen) appears to support either microbial virulence or host antimicrobial defense and potentially both via different mechanisms. For example, the elimination of host fibrin(ogen) significantly reduced the virulence of S aureus in the context of an intravenous infection challenge.14 In contrast, in studies of S aureus peritonitis, fibrin(ogen) deficiency favored the virulence of the pathogen by impeding the rapid clearance of bacteria in the peritoneal cavity.15,16 Similar studies using mice with a genetically imposed reduction in circulating prothrombin or pharmacologic inhibition of thrombin activity also resulted in significantly compromised S aureus clearance from the peritoneal cavity.17,18 Such findings are consistent with, but do not prove, fibrin polymer as a critical molecular feature of the host antimicrobial response following S aureus peritoneal infection. The benefits and/or liabilities to the host and pathogen of the 2 molecular forms of host fibrin(ogen) remain an open question.
To establish an experimental system that provides the means to formally resolve the biologic contributions of fibrin and fibrinogen in any physiologic and pathologic process in vivo, we generated knock-in mice (termed FibAEK mice) in which the Aα chain of fibrinogen was selectively mutated to eliminate thrombin-mediated removal of fibrinopeptide A (FpA). Here, we report the phenotypic consequences for mice carrying normal levels of fibrinogen that is “locked” in the soluble, monomeric form with respect to development, reproductive success, hemostatic capacity, and clotting function both in vitro and in vivo. Furthermore, the power of FibAEK mice to resolve whether fibrinogen or fibrin is the molecular form of host fibrin(ogen) central to biologic outcome is exemplified through studies of fibrin(ogen)-mediated host defense in S aureus peritonitis.
Materials and methods
Generation of FibAEK gene-targeted mice
Details of gene targeting in mouse embryonic stem cells and of the generation of FibAEK mice can be found in the supplemental Materials and Methods available on the Blood Web site.
Analysis of FibAEK expression and hematologic profiles
Analyses of gene expression and hematological profile were performed as described in the supplemental Materials and Methods.
Purification and analysis of fibrinogen protein
Fibrinogen was purified from citrate-plasma and analyzed as described in the supplemental Materials and Methods.
Fibrin polymerization analysis
Fibrin polymerization analyses were performed as described in the supplemental Materials and Methods.
Comparative real-time analyses of thrombus formation by intravital microscopy
FeCl3 injury and analysis of the left carotid artery of mice were performed as described in the supplemental Materials and Methods.
Liver puncture injury and scanning electron microscopy
Needle puncture injury to the right medial lobe of the liver was performed and analyzed as described in the supplemental Materials and Methods.
Flow cytometry analysis of resident peritoneal cells
Flow cytometry-based phenotyping analyses of cells isolated by peritoneal lavage were performed as described in the supplemental Materials and Methods.
ClfA-dependent S aureus aggregation and coagulase-induced plasma clots
The capacity of purified fibrinogenAEK to support bacterial aggregation in vitro with wild-type ClfA+S aureus and participate in coagulase-induced polymerization was established as described in the supplemental Materials and Methods.
S aureus-induced peritonitis
Staphylococcus aureus strain Newman (kindly provided by T. J. Foster from Trinity College) and strain USA300 (obtained from ATCC) were used in infection studies as described in the supplemental Materials and Methods.
Results
Site-directed mutagenesis of the endogenous fibrinogen Aα chain gene
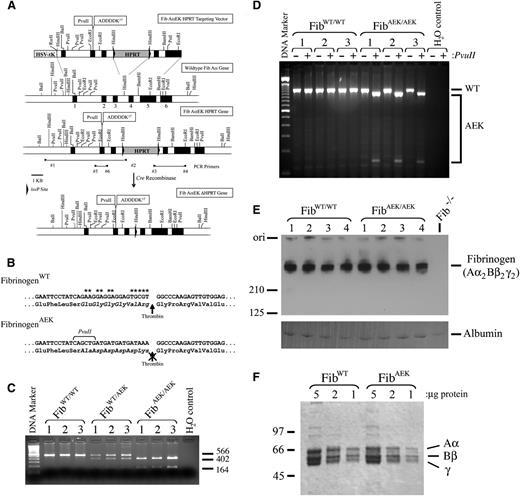
To generate mice carrying a mutant form of fibrinogen with no capacity for thrombin-mediated release of FpA, the Aα chain thrombin cleavage site residues EP6GGGVRP1 were converted to AP6DDDDKP1 (Figure 1A-B). The objective of this sequence change was to render the Aα chain completely insensitive to thrombin-mediated proteolysis, but allow for in vitro biochemical evaluation of enterokinase (EK)-dependent release of FpA.19 The choice of generating an EK cleavage site was based on the unusual sequence specificity of EK (P4-P1 residues DDDDK) with the distinct importance of the substrate residues upstream of the cleavage site but not the downstream residues (P1′-P3′; which must remain Gly-Pro-Arg to form a polymer in fibrin monomer), the complete lack of any DDDDK sequences in the 3 fibrinogen chains, and the fact that endogenous EK is a membrane-associated serine protease expressed only in the duodenum20 ; thus, barring traumatic injury to the duodenum, the enzyme would not be expected to encounter fibrinogen in vivo. Identification of homologous recombination for the Aα chain targeting vector, the AEK mutation, and Cre recombinase-mediated deletion of the HPRT selectable marker was confirmed by polymerase chain reaction (PCR) of genomic DNA (representative data in Figure 1C). Expression of the mutant allele was readily detected by reverse transcriptase (RT)-PCR analysis of hepatic mRNA isolated from adult mice (Figure 1D). Steady-state plasma fibrinogen levels (Figure 1E), as well as the size and integrity of individual chains of purified fibrinogen (Figure 1F), were similar in adult homozygous FibAEK and wild-type (WT) mice.
Generation of FibAEK mice that express normal levels of a mutant form of fibrinogen with a mutation in the fibrinopeptide sequence of the Aα chain immediately upstream of the thrombin cleavage site. (A) Schematic diagram of the gene targeting strategy for inserting 11 nucleic acid substitutions into the endogenous fibrinogen Aα-chain gene of mouse embryonic stem (ES) cells. Black boxes symbolize gene exons. Arrowheads indicate relative positions of nucleotide primers used for PCR-based genotyping. Note that ES cell clones were screened for incorporation of the Fib AαEK HPRT targeting vector by homologous recombination, and deletion of the HPRT minigene was accomplished by crossing mice carrying the targeted allele to transgenic mice expressing Cre recombinase under the control of the cytomegalovirus (CMV) promoter. (B) Summary of the nucleic acid substitutions, and resulting amino acid changes, for the mutated fibrinogen Aα-chain gene of FibAEK mice. Asterisks highlight positions of the nucleotide substitutions. (C) Representative PCR analyses to establish animal genotypes using DNA template from ear biopsies of WT, heterozygous, and homozygous mutant FibAEK mice. Primers 5 and 6 were used to amplify a 566-bp fragment, which was subsequently digested with PvuII to yield the diagnostic fragments of 402 and 164 bp. (D) RT-PCR analysis of total hepatic RNA isolated from each of 3 individual WT and FibAEK homozygous mice. Primers specific to sequences within exons 1 and 5 were used to generate an 1185-bp PCR product. The product generated from a WT transcript was insensitive to cleavage by PvuII, whereas the product generated from the mutant FibAEK transcript was cleaved into expected 1047- and 138-bp fragments. (E) Western blot analysis of plasma (nonreducing conditions) harvested from 4 WT mice, 4 FibAEK mice, and 1 Fib−/− mouse using fibrinogen-directed polyclonal antisera. (F) Coomassie blue-stained sodium dodecyl sulfate polyacrylamide gel (reducing conditions) showing affinity-purified fibrinogen preparations from WT and FibAEK mice. The positions of the Aα, Bβ, and γ chains are indicated.
Generation of FibAEK mice that express normal levels of a mutant form of fibrinogen with a mutation in the fibrinopeptide sequence of the Aα chain immediately upstream of the thrombin cleavage site. (A) Schematic diagram of the gene targeting strategy for inserting 11 nucleic acid substitutions into the endogenous fibrinogen Aα-chain gene of mouse embryonic stem (ES) cells. Black boxes symbolize gene exons. Arrowheads indicate relative positions of nucleotide primers used for PCR-based genotyping. Note that ES cell clones were screened for incorporation of the Fib AαEK HPRT targeting vector by homologous recombination, and deletion of the HPRT minigene was accomplished by crossing mice carrying the targeted allele to transgenic mice expressing Cre recombinase under the control of the cytomegalovirus (CMV) promoter. (B) Summary of the nucleic acid substitutions, and resulting amino acid changes, for the mutated fibrinogen Aα-chain gene of FibAEK mice. Asterisks highlight positions of the nucleotide substitutions. (C) Representative PCR analyses to establish animal genotypes using DNA template from ear biopsies of WT, heterozygous, and homozygous mutant FibAEK mice. Primers 5 and 6 were used to amplify a 566-bp fragment, which was subsequently digested with PvuII to yield the diagnostic fragments of 402 and 164 bp. (D) RT-PCR analysis of total hepatic RNA isolated from each of 3 individual WT and FibAEK homozygous mice. Primers specific to sequences within exons 1 and 5 were used to generate an 1185-bp PCR product. The product generated from a WT transcript was insensitive to cleavage by PvuII, whereas the product generated from the mutant FibAEK transcript was cleaved into expected 1047- and 138-bp fragments. (E) Western blot analysis of plasma (nonreducing conditions) harvested from 4 WT mice, 4 FibAEK mice, and 1 Fib−/− mouse using fibrinogen-directed polyclonal antisera. (F) Coomassie blue-stained sodium dodecyl sulfate polyacrylamide gel (reducing conditions) showing affinity-purified fibrinogen preparations from WT and FibAEK mice. The positions of the Aα, Bβ, and γ chains are indicated.
FibAEK blood fails to support thrombin-induced clot formation but can support platelet aggregation in vitro
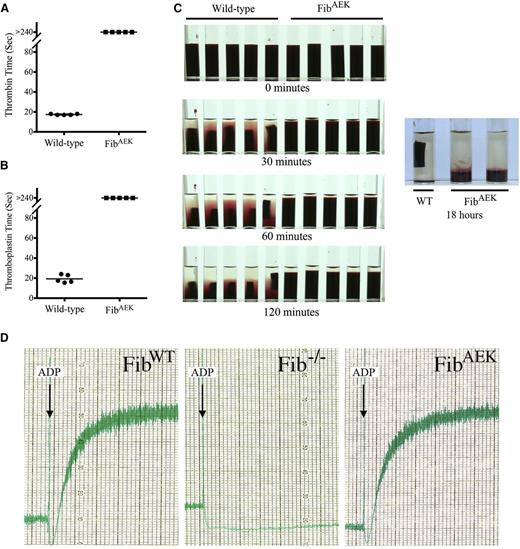
The capacity of thrombin to induce polymer formation for fibrinogenAEK was first evaluated in whole blood clotting assays. Coagulation of whole blood from WT mice was rapid in reactions initiated by the addition of either thrombin or thromboplastin (mean values of 17.4 and 19.3 seconds, respectively; Figures 2A-B). However, clot formation in whole blood from homozygous FibAEK mice was not detected following addition of either thrombin (Figure 2A) or thromboplastin (Figure 2B). Consistent with these findings, in all standard plasma coagulation tests where fibrin clot formation is the endpoint [i.e., prothrombin time (PT), activated partial thromboplastin time (aPTT), and thrombin time (TT)], samples from FibAEK mice failed to clot regardless of observation period at 37°C, whereas plasma from WT mice had average clotting times of 12.5, 29.3, and 14.9 seconds for the PT, aPTT, and TT (Table 1). Complementary whole blood studies were done over an 18-hour period at 37°C focusing on both clotting and platelet-mediated clot retraction. Under conditions where WT whole blood clots formed in just seconds and clot retraction became overtly evident within minutes, clot formation or retraction was not observed in any of the samples from FibAEK mice (Figure 2C). Even 18 hours after thromboplastin addition, neither clot formation nor retraction could be appreciated in FibAEK whole blood samples; the only observable event was red cell sedimentation. Nevertheless, the overall structural integrity of circulating fibrinogenAEK was strongly inferred by the capacity of FibAEK platelet-rich plasma, but not Fib−/− platelet-rich plasma, to support platelet aggregation following ADP stimulation (Figure 2D).
Whole blood isolated from FibAEK mice does not clot following the addition of thrombin or thromboplastin but supports platelet aggregation similar to WT fibrinogen. Clotting times for whole blood from each of 5 WT and FibAEK mice were determined following the addition of 2 U/mL (A) thrombin or (B) thromboplastin. The horizontal bars indicate average clotting times (seconds) for the groups. Note that clots were never detected for whole blood isolated from FibAEK mice following the addition of thrombin or thromboplastin. P < .001 by Fisher’s exact test for each analysis. (C) Qualitative analysis of whole blood clots and clot retraction following the addition of thromboplastin. Retracted clots were readily observed within 30 minutes for samples of whole blood isolated from WT mice; whereas even up to 18 hours following addition of thromboplastin, no clot of any form could be appreciated in whole blood samples isolated from FibAEK mice. (D) Platelet aggregation analysis using platelet-rich plasma isolated from WT, Fib−/−, and FibAEK mice. For each analysis, aggregation was initiated using the platelet agonist ADP.
Whole blood isolated from FibAEK mice does not clot following the addition of thrombin or thromboplastin but supports platelet aggregation similar to WT fibrinogen. Clotting times for whole blood from each of 5 WT and FibAEK mice were determined following the addition of 2 U/mL (A) thrombin or (B) thromboplastin. The horizontal bars indicate average clotting times (seconds) for the groups. Note that clots were never detected for whole blood isolated from FibAEK mice following the addition of thrombin or thromboplastin. P < .001 by Fisher’s exact test for each analysis. (C) Qualitative analysis of whole blood clots and clot retraction following the addition of thromboplastin. Retracted clots were readily observed within 30 minutes for samples of whole blood isolated from WT mice; whereas even up to 18 hours following addition of thromboplastin, no clot of any form could be appreciated in whole blood samples isolated from FibAEK mice. (D) Platelet aggregation analysis using platelet-rich plasma isolated from WT, Fib−/−, and FibAEK mice. For each analysis, aggregation was initiated using the platelet agonist ADP.
Hematologic profile of FibAEK mice
| . | FibWT (N = 6) . | FibAEK (N = 6) . |
|---|---|---|
| WBC (×109/L) | 4.95 ± 1.7 | 4.79 ± 1.8 |
| RBC (×1012/L) | 8.92 ± 0.5 | 9.02 ± 0.5 |
| Hemoglobin (g/dL) | 12.15 ± 0.7 | 12.23 ± 0.5 |
| Hematocrit (%) | 51.52 ± 3.7 | 50.80 ± 2.9 |
| Platelets (×109/L) | 1005 ± 157 | 892 ± 145 |
| PT (seconds) | 12.5 ± 0.2 | >180 |
| aPTT (seconds) | 29.3 ± 2.7 | >300 |
| Thrombin time (seconds) | 14.9 ± 1.1 | >90 |
| . | FibWT (N = 6) . | FibAEK (N = 6) . |
|---|---|---|
| WBC (×109/L) | 4.95 ± 1.7 | 4.79 ± 1.8 |
| RBC (×1012/L) | 8.92 ± 0.5 | 9.02 ± 0.5 |
| Hemoglobin (g/dL) | 12.15 ± 0.7 | 12.23 ± 0.5 |
| Hematocrit (%) | 51.52 ± 3.7 | 50.80 ± 2.9 |
| Platelets (×109/L) | 1005 ± 157 | 892 ± 145 |
| PT (seconds) | 12.5 ± 0.2 | >180 |
| aPTT (seconds) | 29.3 ± 2.7 | >300 |
| Thrombin time (seconds) | 14.9 ± 1.1 | >90 |
FibAEK mice exhibit a survival advantage over Fib−/− mice but cannot tolerate the challenge of pregnancy
Crosses between heterozygous FibAEK mice revealed that a significant fraction, but not all, of expected homozygous FibAEK mice survived to weaning (∼3 weeks of age). Of the first 193 pups generated from heterozygous breeding pairs, 51 (26%) were WT (WT/WT), 113 (58.6%) were heterozygous (WT/AEK), and only 29 (15%) were homozygous mutant (AEK/AEK). The partial loss of homozygous FibAEK offspring was typically associated with spontaneous perinatal abdominal hemorrhagic events and soft tissue bleeds similar to those previously reported in fibrinogen- and prothrombin-deficient neonates.21-23 However, homozygous FibAEK offspring surviving the perinatal period generally survived well into adulthood in the absence of other challenges. To formally compare the perinatal survival profile of homozygous FibAEK mice and homozygous fibrinogen-deficient (Fib−/−) mice within precisely the same genetic background and microenvironment, an analysis was performed using C57Bl/6-inbred breeding pairs made up of homozygous males and heterozygous females. Here, a significantly higher fraction of homozygous FibAEK offspring survived to weaning relative to Fib−/− offspring (Table 2). Heterozygous FibAEK female mice consistently carried litters to term without hemorrhagic consequences, but homozygous FibAEK female mice were unable to sustain pregnancies. Ten of 10 homozygous FibAEK mice impregnated by FibAEK homozygous males died or became moribund in midgestation (E9.5-E10.5), often with evidence of overt bleeding. Histologic analysis of uterine tissue from pregnant homozygous FibAEK mice revealed massive intrauterine hemorrhage characterized by free maternal blood cells (evidenced by enucleated red blood cells) within the uterus and placental tissue (supplemental Figure 1). Notably, the developing homozygous FibAEK embryos appeared developmentally sound, with no evidence of free embryonic (nucleated) red blood cells (supplemental Figure 1).
Analysis of postnatal survival comparing offspring with complete fibrinogen deficiency (Fib−/−) and mice homozygous for FibAEK
| . | ♀FibWT/Null × ♂FibNull/Null . | ♀FibWT/AEK × ♂FibAEK/AEK . | ||
|---|---|---|---|---|
| Offspring genotype | FibWT/− | Fib−/− | FibWT/AEK | FibAEK/AEK |
| Mendelian ratio | 1 | 1 | 1 | 1 |
| No. observed (at 3 weeks) | 170 | 85 | 130 | 93 |
| % of expected* | 100% | 50% | 100% | 72% |
| . | ♀FibWT/Null × ♂FibNull/Null . | ♀FibWT/AEK × ♂FibAEK/AEK . | ||
|---|---|---|---|---|
| Offspring genotype | FibWT/− | Fib−/− | FibWT/AEK | FibAEK/AEK |
| Mendelian ratio | 1 | 1 | 1 | 1 |
| No. observed (at 3 weeks) | 170 | 85 | 130 | 93 |
| % of expected* | 100% | 50% | 100% | 72% |
P < .05, χ2 analysis.
FibrinogenAEK fails to polymerize following incubation with thrombin but polymerization can be induced by enterokinase in vitro
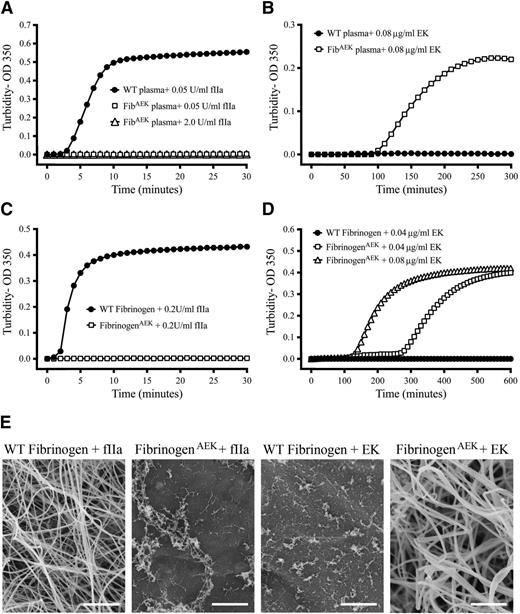
Using standard turbidity assays, plasma prepared from WT mice displayed a typical thrombin-induced fibrin assembly profile (Figure 3A). In contrast, no change in turbidity was detected following the addition of thrombin to plasma prepared from FibAEK mice, even at 40 times higher thrombin concentrations (Figure 3A). Consistent with the genetically imposed substitution of an enterokinase cleavage site in place of the thrombin cleavage site, plasma prepared from FibAEK, but not plasma from FibWT mice, exhibited a change in turbidity profile consistent with polymer formation following addition of enterokinase (Figure 3B). This turbidity change followed a typical lag phase but occurred over many hours (reflecting the relatively low enterokinase levels used). Reaction mixtures of purified fibrinogen preparations produced a similar pattern to that observed with plasma following the addition of thrombin or enterokinase (Figure 3C-D, respectively). Scanning electron microscopic analyses directly established that overall morphology of fibrin polymers generated with enterokinase-cleaved fibrinogenAEK was similar to those generated with thrombin-cleaved WT fibrinogen, with the exception that the fibers in the former seemed generally thicker (Figure 3E, far right and far left). Diffuse and thin macromolecular structures were observed in reactions of fibrinogenAEK and thrombin, perhaps representing thrombin-mediated fXIII (which copurifies with fibrinogen) activation and subsequent fibrinogen cross-linking (Figure 3E). Only comparatively small aggregates, potentially an artifact of processing for scanning electron microscopy, were observed in reactions of WT fibrinogen with EK enzyme.
FibrinogenAEK forms a polymer following incubation with enterokinase enzyme but not thrombin. Representative turbidity analyses using plasma isolated from WT (closed circles) and FibAEK (open symbols) mice following incubation with (A) thrombin or (B) enterokinase enzyme. Similar representative turbidity analyses using purified WT fibrinogen (closed circles) and fibrinogenAEK (open symbols) following incubation with (C) thrombin or (D) enterokinase enzyme. The purified fibrinogen concentration in each analysis was 0.04 mg/mL. To prevent spurious thrombin activity, reactions with enterokinase enzyme included the addition of the specific thrombin inhibitor lepirudin at 0.025 mg/mL. (E) Scanning electron micrographs of products formed from reaction mixtures of purified WT fibrinogen or fibrinogenAEK with thrombin or enterokinase. Scale bar, 2.5 μm.
FibrinogenAEK forms a polymer following incubation with enterokinase enzyme but not thrombin. Representative turbidity analyses using plasma isolated from WT (closed circles) and FibAEK (open symbols) mice following incubation with (A) thrombin or (B) enterokinase enzyme. Similar representative turbidity analyses using purified WT fibrinogen (closed circles) and fibrinogenAEK (open symbols) following incubation with (C) thrombin or (D) enterokinase enzyme. The purified fibrinogen concentration in each analysis was 0.04 mg/mL. To prevent spurious thrombin activity, reactions with enterokinase enzyme included the addition of the specific thrombin inhibitor lepirudin at 0.025 mg/mL. (E) Scanning electron micrographs of products formed from reaction mixtures of purified WT fibrinogen or fibrinogenAEK with thrombin or enterokinase. Scale bar, 2.5 μm.
Fibrinopeptide A of fibrinogenAEK cannot be released by thrombin
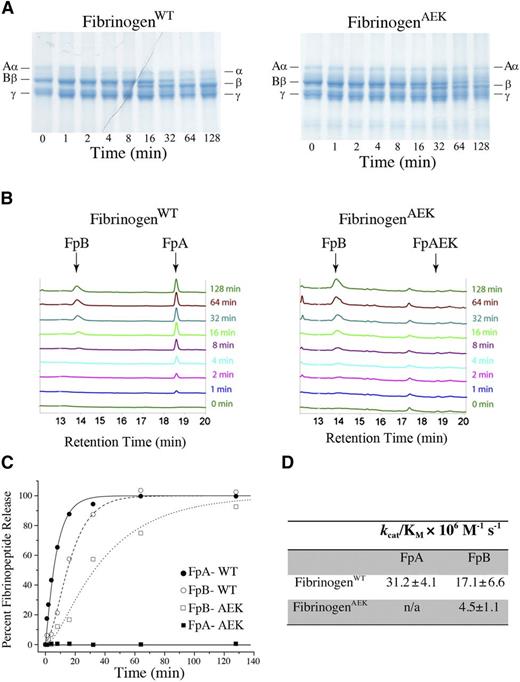
Thrombin-mediated fibrinopeptide release was compared using purified mutant and WT fibrinogen. A time-dependent molecular weight shift in the fibrinogen Aα chain (corresponding to the α chain without FpA) was observed with WT fibrinogen following thrombin addition, whereas only the intact Aα chain was observed for fibrinogenAEK reactions regardless of incubation time with thrombin (Figure 4A). Even fibrinogenAEK reactions with exceptionally high concentrations of thrombin (eg, 200 nM) over extensive incubation times (>30 minutes) revealed no proteolytic conversion of the Aα chain to the α chain (data not shown). The absence of FpA release was confirmed by high-performance liquid chromatography (HPLC) analysis of peptides generated in fibrinogen/thrombin incubation mixtures. Thrombin-released FpA from WT fibrinogen was detected on chromatograms within 1 minute (Figure 4B). However, thrombin-mediated FpA release was never detected with fibrinogenAEK (ie, FpA-AEK) regardless of the incubation time (Figure 4B).
Failure of thrombin-mediated proteolytic release of the mutant fibrinopeptide A of fibrinogen isolated from FibAEK mice. (A) Reaction mixtures of purified WT fibrinogen (left) and fibrinogenAEK (right) were incubated with thrombin and Ca2+ for various times, and the fibrinogen chains analyzed by reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The positions of the Aα, cleaved α, Bβ, cleaved β, and γ chains are indicated. Note that cleaved α chain was never observed in reaction mixtures containing fibrinogenAEK regardless of incubation time. (B) HPLC chromatograms of reaction mixtures containing either WT fibrinogen (left) or fibrinogenAEK (right) following incubation with thrombin and Ca2+ for various times. Indicated are the positions of peaks corresponding to fibrinopeptide A (FpA), fibrinopeptide B (FpB), and mutant fibrinopeptide A (FpAEK). The identification of peak positions was independently established by resolving synthetic peptides corresponding to the predicted fibrinopeptide sequence. Note that FpAEK is never observed in chromatograms derived from fibrinogenAEK reactions regardless of incubation time with thrombin and Ca2+. (C) Fibrinopeptide release curves were prepared by plotting the percent of fibrinopeptide released versus time. FpA data were fitted with a simple first-order equation, and the FpB data from normal fibrinogen were fitted to a standard equation describing 2 consecutive first-order processes. (D) Table of specificity constants (kcat/KM), which were determined by dividing first-order rate constants by the thrombin concentration.
Failure of thrombin-mediated proteolytic release of the mutant fibrinopeptide A of fibrinogen isolated from FibAEK mice. (A) Reaction mixtures of purified WT fibrinogen (left) and fibrinogenAEK (right) were incubated with thrombin and Ca2+ for various times, and the fibrinogen chains analyzed by reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The positions of the Aα, cleaved α, Bβ, cleaved β, and γ chains are indicated. Note that cleaved α chain was never observed in reaction mixtures containing fibrinogenAEK regardless of incubation time. (B) HPLC chromatograms of reaction mixtures containing either WT fibrinogen (left) or fibrinogenAEK (right) following incubation with thrombin and Ca2+ for various times. Indicated are the positions of peaks corresponding to fibrinopeptide A (FpA), fibrinopeptide B (FpB), and mutant fibrinopeptide A (FpAEK). The identification of peak positions was independently established by resolving synthetic peptides corresponding to the predicted fibrinopeptide sequence. Note that FpAEK is never observed in chromatograms derived from fibrinogenAEK reactions regardless of incubation time with thrombin and Ca2+. (C) Fibrinopeptide release curves were prepared by plotting the percent of fibrinopeptide released versus time. FpA data were fitted with a simple first-order equation, and the FpB data from normal fibrinogen were fitted to a standard equation describing 2 consecutive first-order processes. (D) Table of specificity constants (kcat/KM), which were determined by dividing first-order rate constants by the thrombin concentration.
A time-dependent release of FpB from the Bβ chain was maintained following thrombin addition with both fibrinogenWT and fibrinogenAEK (Figure 4A-B). However, consistent with findings with other fibrinogen variants,24-28 HPLC analyses suggested that FpB release from fibrinogenAEK was delayed relative to thrombin-mediated release of FpB from WT fibrinogen (Figure 4B-C). The calculated specificity constants (kcat/KM) confirmed a significant diminution in the efficiency of FpB release from fibrinogenAEK relative to WT fibrinogen (Figure 4D).
FibAEK mice exhibit compromised hemostasis and protection from occlusive thrombus formation following acute challenge
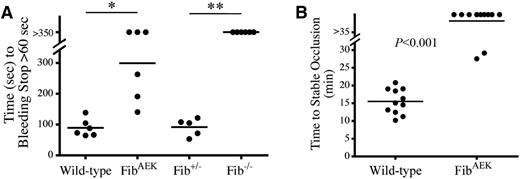
To evaluate the ability of FibAEK mice to control blood loss following acute vessel injury, we compared tail-bleeding times in cohorts of WT, FibAEK, Fib−/−, and Fib+/− mice. As shown in Figure 5A, WT and Fib+/− mice rapidly and uniformly stopped bleeding following tail tip amputation, whereas FibAEK and Fib−/− mice exhibited a major, albeit not identical, impediment in the control of blood loss. Unlike Fib−/− mice, which uniformly failed to stop blood loss over the entire observation period (>6 minutes), half of FibAEK mice analyzed ultimately stopped blood loss within this timeframe (Figure 5A). Thus, although hemostasis is compromised in FibAEK mice, these animals retain an advantage over mice with no fibrinogen in their capacity to control blood loss.
FibAEK mice display prolonged tail bleeding times and significant protection from occlusive thrombotic injury to the carotid artery. (A) Time to cessation of bleeding (>60 seconds) of WT, FibAEK, Fib+/−, and Fib−/− mice following 3-mm excision of the distal portion of the tail. Horizontal bars indicate mean times for each group. Note that 3 of 6 FibAEK mice and 6 of 6 Fib−/− mice did not stop bleeding during the evaluation period. *P < .01 by Student t test; **P < .01 by Fisher’s exact test. (B) Time to stable carotid vessel occlusion as indicated by detection of flow stop using a Doppler flowmeter following FeCl3 injury. A statistically significant difference of P < .001 was determined by Fisher’s exact test.
FibAEK mice display prolonged tail bleeding times and significant protection from occlusive thrombotic injury to the carotid artery. (A) Time to cessation of bleeding (>60 seconds) of WT, FibAEK, Fib+/−, and Fib−/− mice following 3-mm excision of the distal portion of the tail. Horizontal bars indicate mean times for each group. Note that 3 of 6 FibAEK mice and 6 of 6 Fib−/− mice did not stop bleeding during the evaluation period. *P < .01 by Student t test; **P < .01 by Fisher’s exact test. (B) Time to stable carotid vessel occlusion as indicated by detection of flow stop using a Doppler flowmeter following FeCl3 injury. A statistically significant difference of P < .001 was determined by Fisher’s exact test.
In complementary analyses, WT and FibAEK mice were challenged with FeCl3-induced carotid artery injury and time to occlusion was tracked using a Doppler flow probe (Figure 5B). WT mice developed a complete and sustained carotid vessel occlusion in an average time of 16 minutes. In contrast, the majority (9 of 11) of the FibAEK mice challenged never displayed complete vessel occlusion during the observation period (>35 minutes). The Doppler flow tracings for FibAEK mice suggested embolization events in all but 1 FibAEK animal (data not shown). Stable occlusion occurred in 2 FibAEK mice, but over a significantly longer timeframe than for any of the WT mice (ie, 28 and 29 minutes). Thus, the failure of thrombin-mediated fibrin polymer formation documented with fibrinogenAEK in vitro results in compromised hemostasis and resistance to occlusive thrombus formation in vivo.
FibAEK mice display a lack of extravascular fibrin deposition following liver injury
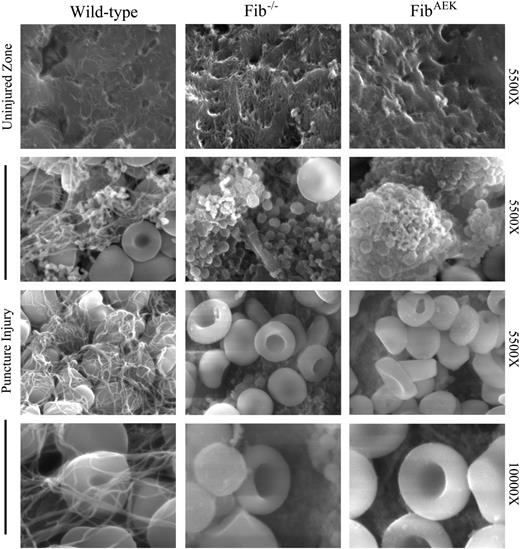
To directly interrogate the capacity of FibAEK mice to generate extravascular fibrin polymer in an in vivo setting, we challenged WT, Fib−/−, and FibAEK mice with a liver needle puncture injury and evaluated clot formation in the damaged zone using scanning electron microscopy. Fibrin polymer was readily visualized in the damaged zone of every WT mouse, with some fields having substantial numbers of platelets associated with the polymer and other areas revealing primarily polymers rich in red blood cells (representative views in Figure 6, left). Fib−/− mice challenged in parallel displayed a total absence of polymer within the injured zones. Here, fields within the injured zone of Fib−/− livers were rich in platelet clusters or free red blood cells (representative views in Figure 6, center). Importantly, studies of FibAEK mice established a distinct absence of any extravascular fibrin polymer following injury (representative views in Figure 6, right). Indeed, the injured hepatic zones in FibAEK mice were essentially indistinguishable from those observed in Fib−/− mice (Figure 6), despite the fact that FibAEK animals retain normal circulating levels of fibrinogen.
Failure of extravascular fibrin polymer formation in FibAEK mice following liver needle puncture injury. Representative scanning electron micrographs of right medial liver lobes from WT, Fib−/−, and FibAEK mice from uninjured areas (top row) or of the injured zone following needle puncture injury (bottom 3 rows). Note that the injured zone of WT mice was characterized by fibrin polymer structures associated with platelets and RBCs. In contrast, the injured zones of livers from both Fib−/− and FibAEK mice displayed platelet-rich clusters and RBCs, but a distinct absence of fibrin polymer structures.
Failure of extravascular fibrin polymer formation in FibAEK mice following liver needle puncture injury. Representative scanning electron micrographs of right medial liver lobes from WT, Fib−/−, and FibAEK mice from uninjured areas (top row) or of the injured zone following needle puncture injury (bottom 3 rows). Note that the injured zone of WT mice was characterized by fibrin polymer structures associated with platelets and RBCs. In contrast, the injured zones of livers from both Fib−/− and FibAEK mice displayed platelet-rich clusters and RBCs, but a distinct absence of fibrin polymer structures.
FibAEK mice fail to efficiently clear S aureus introduced into the peritoneal cavity, but retaining soluble fibrinogen offers a context-dependent host survival advantage in acute peritonitis
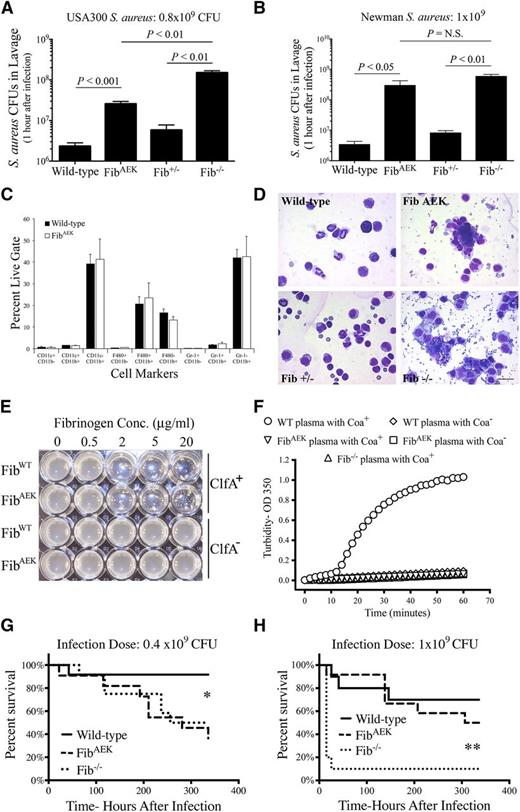
As a first illustration of the uniquely instructive nature of FibAEK mice, we sought to directly test the hypothesis that polymer formation is key to the implementation of the fibrinogen-dependent antimicrobial response in S aureus peritonitis challenge. Multiple prior reports have indicated that fibrin(ogen) is critical for a rapid and robust clearance of S aureus from the peritoneal cavity following an intraperitoneal infection.15,16 However, the precise mechanism, and whether this host antimicrobial response was fibrinogen- or fibrin-dependent, has remained unknown. Consistent with previous reports,16,18 WT mice challenged with an intraperitoneal injection of ∼109 colony-forming units (CFUs) of either the clinical isolate methicillin-resistant S aureus (MRSA) strain USA300 (Figure 7A) or strain Newman WT S aureus (Figure 7B) eliminated ∼99% of the bacteria within 1 hour based on analyses of peritoneal lavage fluid. In contrast, ∼15-fold higher USA300 CFUs were retrieved by peritoneal lavage of FibAEK mice and virtually the entire initial CFU inoculum of USA300 was retrieved from Fib−/− mice (Figure 7A). Similarly, the same numbers of strain Newman CFUs were retrieved by peritoneal lavage of FibAEK and Fib−/− mice as were present in the initial infection volume (Figure 7B). Collectively, the results indicate that the ability to form fibrin in WT and Fib+/− mice significantly contributes to rapid bacteria clearance in the peritoneal cavity.
FibrinogenAEK does not support rapid clearance of S aureus bacteria following acute peritoneal infection but retains some capacity to limit host lethality. The number of S aureus CFUs present in peritoneal lavage fluid collected 1 hour after intraperitoneal infection with (A) 0.8 × 109 CFUs strain USA300 S aureus and (B) 1 × 109 CFUs strain Newman S aureus from WT, FibAEK, Fib+/−, and Fib−/− mice (n = 6 per genotype). Data are presented as mean ± standard error of the mean with statistical comparisons made by Student t test. (C) Flow cytometric analysis of myeloid cell populations harvested from the peritoneal cavity of WT and FibAEK mice (n = 6 mice per genotype). Analyses are presented as the mean ± standard error of the mean. (D) Representative photomicrographs of cytospin preparations of peritoneal lavage fluid taken 1 hour after intraperitoneal infection with 1 × 109 CFUs S aureus. Note the cell-associated and free bacteria in samples from FibAEK and Fib−/− mice that are largely absent in samples from WT and Fib+/− mice. Scale bar, 20 μm. (E) Analysis of fibrinogen-dependent bacterial clumping with suspensions of strain Newman S aureus with and without expression of clumping factor A (ClfA). Notably, both WT and fibrinogenAEK support bacterial clumping at a concentration of 2 μg/mL and above. (F) Analyses of coagulase (Coa)-induced plasma clotting with citrate-plasma preparations from WT, homozygous FibAEK, and Fib−/− mice using supernatants prepared from stationary phase cultures of both strain Newman WT (Coa+) S aureus and Coa-negative (Coa−) strain Newman S aureus. Survival analyses of WT, FibAEK, and Fib−/− mice (n = 12 per genotype) following intraperitoneal infection with (G) 0.4 × 109 CFUs or (H) 1 × 109 CFUs strain Newman S aureus. *P < .01 for WT compared with Fib−/− or FibAEK; **P < .01 for FibAEK compared with Fib−/− using Kaplan-Meier log-rank analysis.
FibrinogenAEK does not support rapid clearance of S aureus bacteria following acute peritoneal infection but retains some capacity to limit host lethality. The number of S aureus CFUs present in peritoneal lavage fluid collected 1 hour after intraperitoneal infection with (A) 0.8 × 109 CFUs strain USA300 S aureus and (B) 1 × 109 CFUs strain Newman S aureus from WT, FibAEK, Fib+/−, and Fib−/− mice (n = 6 per genotype). Data are presented as mean ± standard error of the mean with statistical comparisons made by Student t test. (C) Flow cytometric analysis of myeloid cell populations harvested from the peritoneal cavity of WT and FibAEK mice (n = 6 mice per genotype). Analyses are presented as the mean ± standard error of the mean. (D) Representative photomicrographs of cytospin preparations of peritoneal lavage fluid taken 1 hour after intraperitoneal infection with 1 × 109 CFUs S aureus. Note the cell-associated and free bacteria in samples from FibAEK and Fib−/− mice that are largely absent in samples from WT and Fib+/− mice. Scale bar, 20 μm. (E) Analysis of fibrinogen-dependent bacterial clumping with suspensions of strain Newman S aureus with and without expression of clumping factor A (ClfA). Notably, both WT and fibrinogenAEK support bacterial clumping at a concentration of 2 μg/mL and above. (F) Analyses of coagulase (Coa)-induced plasma clotting with citrate-plasma preparations from WT, homozygous FibAEK, and Fib−/− mice using supernatants prepared from stationary phase cultures of both strain Newman WT (Coa+) S aureus and Coa-negative (Coa−) strain Newman S aureus. Survival analyses of WT, FibAEK, and Fib−/− mice (n = 12 per genotype) following intraperitoneal infection with (G) 0.4 × 109 CFUs or (H) 1 × 109 CFUs strain Newman S aureus. *P < .01 for WT compared with Fib−/− or FibAEK; **P < .01 for FibAEK compared with Fib−/− using Kaplan-Meier log-rank analysis.
To begin to determine the molecular or cellular basis for the impressive difference in peritoneal bacteria clearance between WT and FibAEK animals, analyses of resident peritoneal cells were performed. Both the number and distribution of myeloid (Figure 7C) and lymphoid (supplemental Figure 2) resident peritoneal cells found in unchallenged WT and FibAEK mice were indistinguishable. Cytospin preparations of lavage fluid from challenged FibAEK mice and Fib−/− mice confirmed a persistence of large numbers of free bacteria in the peritoneal cavity of these animals, whereas preparations from WT or Fib+/− mice were largely devoid of bacteria (Figure 7D). In vitro analyses indicated that both WT fibrinogen and fibrinogenAEK were capable of supporting S aureus bacterial clumping through the clumping factor A (ClfA) bacterial cell surface fibrinogen receptor (Figure 7E). S aureus produces coagulase (Coa), a virulence factor that is released into the extracellular milieu capable of binding prothrombin. Coa:prothrombin complexes mediate fibrin polymer formation independent of thrombin generation via the prothrombinase complex. Here, we found that culture supernatant obtained from Coa+S aureus, but not from Coa−S aureus, was capable of supporting fibrin polymer formation in plasma from WT mice (Figure 7F). In contrast, fibrin polymer formation was never detected in plasma from FibAEK following the addition of culture supernatant from either Coa+ or Coa−S aureus (Figure 7F). Consistent with a significant failure of bacterial clearance, both Fib−/− and FibAEK mice displayed significantly worse survival profiles following intraperitoneal infection with 0.4 × 109 CFU S aureus relative to similarly challenged WT mice (Figure 7G). Intriguingly, when challenged with a higher dose of 1 × 109 CFU S aureus, FibAEK mice exhibited a far superior survival profile relative to Fib−/− mice challenged in parallel. Here, ∼90% of Fib−/− mice succumbed to infection within 24 hours, whereas ∼50% of FibAEK mice survived for the 2-week observation period (Figure 7H). Of note, none of the infected animals (including Fib−/− mice) displayed evidence of significant hemorrhage. Collectively, these findings suggest that in specific contexts soluble fibrinogen has important functional significance even beyond a contribution to hemostasis.
Discussion
Fibrin(ogen) exists as either a soluble monomer or as an insoluble polymer. Each of these dramatically different structural forms appears to have distinct properties thought to contribute to the full spectrum of physiologic and pathologic activities attributable to fibrin(ogen) in vivo. However, formally resolving the biologic contributions of each molecular form to reproductive success, hemostasis/thrombosis, tissue repair, disease, and inflammatory processes has remained technically unapproachable using available genetic and pharmacologic tools. The inability of investigators to formally distinguish the contributions of fibrinogen and fibrin in vivo has led to a half-century-old practice of using parentheses when referring to “fibrin(ogen)”-dependent events. The genetic alteration imposed here was guided by prior studies suggesting that eliminating FpA release alone would be sufficient to prevent thrombin-mediated fibrin polymer formation. The human Aα chain variants fibrinogen Metz and fibrinogen Frankfurt XIII have a mutation in the P1 residue (ie, AαArg16Cys) that renders the Aα chain completely insensitive to thrombin cleavage and, comparable to our findings with fibrinogenAEK, impeded clotting function.29-32 Although fundamentally lacking the capacity to generate fibrin matrices in vivo, all other molecular/functional elements of the fibrinogenAEK molecule and FibAEK mice remain intact, including (1) support of platelet aggregation/thrombus formation through the platelet integrin receptor αIIbβ3, (2) the potential to engage all other integrin and nonintegrin receptors (eg, αMβ2, vascular cell adhesion molecule-1) as no receptor binding motifs were selectively mutated in fibrinogenAEK, (3) associations with circulating enzymes and proteins (eg, fXIII, fibronectin), (4) thrombin generation and thrombin action on all non-fibrinogen substrates (eg, fXIII, protein C and PARs), and (5) normal hemostatic factor levels. The availability of fibrinogen, either in a soluble form or immobilized form on other cell surface receptors or foreign bodies, may present biologic benefits never previously appreciated and impossible to recognize through comparative study of WT and fibrinogen-deficient mice. Thus, FibAEK mice provide a unique, clean experimental system for defining the biologic contribution(s) of fibrinogen monomer in any context in vivo that does not require other additional investigator-imposed manipulations such as fibrinogen- or other coagulation-targeted pharmacologic agents.
Hemostatic capacity in FibAEK mice was superior to that of Fib−/− mice, despite the lack of clotting function. Fib−/− mice consistently fail to stop blood flow following tail tip excision, whereas a significant fraction of FibAEK animals ultimately control blood loss following this hemostatic challenge. Additionally, FibAEK mice exhibit superior perinatal survival relative to Fib−/− mice, a time when both genotypes are at high risk of spontaneous bleeding events. Because fibrinogen-dependent platelet aggregation is maintained in FibAEK mice, we postulate the enhanced hemostasis in FibAEK mice is, in part, the result of retained fibrinogen-platelet interactions. The requirement of fibrin polymer formation for hemostasis is likely a function of the severity of the hemostatic challenge, where fibrin clotting is mandatory for control of blood loss following severe vessel injuries. More formal analyses on the role of fibrin formation in a range of hemostatic challenges will be the focus of future studies with FibAEK mice. The FibAEK mutant also provides a means to further define the contributions of soluble fibrinogen vs fibrin in thrombus formation and the functional properties of distinct domains recognized within arterial thrombi. Following vascular injury, thrombi form with distinct structural features, including a fibrin-rich extravascular/perivascular zone, an intravascular/lumenal inner core of densely packed platelets on a fibrin base, and an outer shell composed of loosely packed platelets.33,34 Our findings suggest soluble fibrinogen can contribute to thrombus formation, but fibrin polymer formation is required for the development of a fully occlusive thrombus following FeCl3 injury of the carotid artery. Fibrin(ogen) has been documented within defined domains of the thrombus, but the precise requirements of fibrin polymer compared with soluble fibrinogen for thrombus architecture, growth, capping, and resolution, as well as dynamic changes within the thrombus (eg, solute transport within and between domains), are open questions now approachable for the first time with FibAEK mice.35-37
The present studies provide strong direct evidence that FpA release is critical to, and sufficient for, fibrin polymer formation. Both turbidity measurements and scanning electron microscopy studies revealed robust polymerization of fibrinogenAEK following incubation with EK enzyme (supporting FpA, but not FpB, release), but a fundamental failure of polymerization following incubation with thrombin (supporting FpB, but not FpA, release). Our findings are compatible with prior biochemical analyses using snake venom proteases favoring FpA or FpB release,27,38-41 as well as previous reports indicating that polymerization is compromised in the fibrinogen variant γAsp364His, which alters the “a” binding pocket, whereas polymerization is similar to normal in the fibrinogen variant BβArg432Ala, which disrupts the “b” binding pocket.41-44 B:b interactions are reported to be exceptionally weak as characterized by high affinity constants and a low strength force to rupture the bonds.43,45 Prior studies suggest any assembly based on cleavage of FpB alone would be restricted to nonphysiologic conditions of low salt concentrations and low temperatures.29,39,46 FibAEK mice and fibrinogenAEK derived from these animals provide novel tools and reagents for more comprehensive studies exploring the consequences of FpA release, FpB release, or both to polymer formation and clot structure both in vitro and in vivo. Intriguingly, over very long incubation times and under nonphysiologic conditions, fXIII transglutaminase is known to covalently join intact soluble fibrinogen into macromolecular assemblies in vitro in the absence of any kind of fibrinopeptide release.47-50 Trace and disorganized macromolecular aggregates were occasionally encountered in scanning electron microscopy studies of reaction mixtures with fibrinogenAEK and thrombin, potentially a reflection of fXIII activity in vitro. Thus, although no appreciable fibrin polymer formation could be detected in FibAEK mice, it remains to be established whether fXIII-mediated cross-linking of fibrinogenAEK monomers occurs in vivo under any physiologic or pathologic conditions.
Fibrinogen is an important nexus of host-pathogen interaction as evidenced by the fact that many microbial pathogens have evolved and maintained bacterial-encoded fibrinogen binding proteins, direct plasminogen activators, and in the case of S aureus, 2 direct prothrombin activators: coagulase and vWbp.51-55 Interestingly, in the setting of intravenous S aureus infection (bacteremia), fibrinogen supports pathogen virulence and investigator-imposed fibrinogen deficiency improves host survival,14 whereas in the setting of S aureus peritonitis, fibrinogen diminishes pathogen virulence and investigator-imposed fibrinogen deficiency impedes bacterial clearance and reduces host survival.15 An impediment in the clearance of S aureus in the peritoneal cavity was also documented for mice carrying a mutant form of fibrinogen lacking the αMβ2 binding motif but maintaining full clotting function.16 As with FibAEK mice, elimination of the fibrinogen αMβ2-binding motif in Fibγ390-396A mice did not alter the composition or number of resident peritoneal immune cells. Retrieval of bacteria from the peritoneal cavity of Fibγ390-396A mice 1 hour after infection resulted in a 10-fold increase in CFUs relative to WT animals. These findings suggest both the importance of the fibrinogen αMβ2-binding motif in host defense and that fibrin formation is, in itself, insufficient to support the implementation of full antimicrobial function. Here, we show that fibrin polymer formation is vital to S aureus clearance and ultimately host survival in S aureus-induced peritonitis as we document an ∼200-fold increase in CFUs retrieved from FibAEK relative to WT mice 1 hour after peritoneal infection. This finding is also compatible with the view that soluble fibrinogen is a relatively poor ligand for αMβ2, whereas fibrin polymer (or immobilized fibrinogen) is a strong ligand for this leukocyte integrin receptor.7,56
The finding that FibAEK animals displayed a survival advantage over Fib−/− mice following high-dose intraperitoneal S aureus infection provided evidence that soluble fibrinogen is also biologically meaningful to the host regarding infection outcome. The precise mechanism(s) remain(s) to be fully explored, but 2 general hypotheses stand out. First, S aureus engagement of soluble fibrinogen in FibAEK mice by way of known microbial fibrinogen binding proteins (eg, ClfA) may change infection outcome at high intraperitoneal S aureus loads by promoting bacterial aggregation and impeding dissemination out of the peritoneal cavity to distant organs.53,57 Second, soluble fibrinogen interactions with host cells including platelets and inflammatory cells (ie, neutrophils and macrophages) may increase tolerance at high bacterial loads by supporting fibrinogen-mediated platelet interactions and platelet-linked bacterial killing mechanisms.58-60 Alternatively, fibrinogen either in the soluble form or adhered to peritoneal surfaces may support inflammatory cell survival and antimicrobial function including the formation of neutrophil extracellular traps.16,61-63 Whatever the precise mechanism(s) by which soluble fibrinogen limits host mortality relative to fibrinogen-deficient mice in this context, it does not appear to be coupled to overt hemorrhage. Furthermore, since fibrin(ogen) engagement of S aureus through ClfA is known to promote pathogen virulence and abscess formation following intravenous infection,51,64,65 the advantage to the host conferred by soluble fibrinogen in FibAEK mice over Fib−/− mice in high-load S aureus peritonitis could be reversed to a disadvantage for the host following intravenous-induced S aureus bacteremia. Insights gained through more comprehensive studies of FibAEK mice will not only provide a better understanding of biologic processes linked to fibrinogen but also may guide the development of novel intervention strategies for a wide spectrum of diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Carolina Cruz, Cheryl Rewerts, Leah Rosenfeldt, Maureen Shaw, and Kathryn McElhinney for excellent technical assistance and Dr James Luyendyk and Dr John Weisel for helpful technical suggestions in the preparation of this manuscript.
This work was supported by National Institutes of Health grants from the National Heart, Lung, and Blood Institute HL096126 (J.L.D.), from the National Institute of Arthritis and Musculoskeletal and Skin Diseases AR056990 (M.J.F.), and Cincinnati Rheumatic Diseases Center-Animal Models of Inflammatory Diseases core grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases P30 AR47363 (M.J.F.).
Authorship
Contribution: J.M.P., O.V.G., S.T., T.D., S.R.C., J.L.D., and M.J.F. designed the research, performed experiments, analyzed the data, and wrote the manuscript; Y.-P.K., M.H., E.S.M., and J.S.P. provided critical guidance on experimental procedures and helped write the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthew J. Flick, Cincinnati Children's Hospital Research Foundation, Division of Experimental Hematology and Cancer Biology, ML7015, 3333 Burnet Ave, Cincinnati, OH 45229-3039; e-mail: matthew.flick@cchmc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal