Key Points
This trial evaluated frontline VR-CAP and R-CHOP therapy for patients with centrally confirmed non-GCB DLBCL.
There was no significant improvement in response rates or long-term outcomes with VR-CAP vs R-CHOP in previously untreated non-GCB DLBCL.
Abstract
This phase 2 study evaluated whether substituting bortezomib for vincristine in frontline rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) therapy could improve efficacy in non-germinal center B-cell-like diffuse large B-cell lymphoma (non-GCB DLBCL), centrally confirmed by immunohistochemistry (Hans method). In total, 164 patients were randomized 1:1 to receive six 21-day cycles of rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, and doxorubicin 50 mg/m2, all IV day 1, prednisone 100 mg/m2 orally days 1-5, plus either bortezomib 1.3 mg/m2 IV days 1, 4, 8, 11 (rituximab, cyclophosphamide, doxorubicin, and prednisone with bortezomib [VR-CAP]; n = 84) or vincristine 1.4 mg/m2 (maximum 2 mg) IV day 1 (R-CHOP; n = 80). There were no significant differences between VR-CAP and R-CHOP in complete response rate (64.5%, 66.2%; odds ratio [OR], 0.91; P = .80), overall response rate (93.4%, 98.6%; OR, 0.21; P = .11), progression-free survival (hazard ratio [HR], 1.12; P = .76), or overall survival (HR, 0.89; P = .75). Rates of grade ≥3 adverse events (AEs; 88%, 89%), serious AEs (38%, 34%), discontinuations due to AEs (7%, 3%), and deaths due to AEs (2%, 5%) were similar with VR-CAP and R-CHOP. Grade ≥3 peripheral neuropathy rates were 6% and 3%, respectively. VR-CAP did not improve efficacy vs R-CHOP in non-GCB DLBCL. This trial was registered at www.clinicaltrials.gov as #NCT01040871.
Introduction
Diffuse large B-cell lymphoma (DLBCL) accounts for 25% to 35% of all new non-Hodgkin lymphoma diagnoses globally each year.1,2 Gene expression profiling (GEP) has identified at least 3 molecularly distinct DLBCL subtypes based on differential expression of genes involved in B-cell development,3-10 including activated B-cell-like (ABC), germinal center B-cell-like (GCB), and unclassified subtypes. GCB and non-GCB DLBCL subtypes can also be distinguished using immunohistochemistry (IHC) algorithms based on expression of markers including CD10, BCL6, and MUM-1; these algorithms have demonstrated 71% to 93% concordance with GEP.11-13
Clinical outcomes differ considerably between GCB and non-GCB DLBCL,6,8,14-16 with overall survival (OS) significantly inferior in non-GCB patients (5-year OS rates: 16%-64% vs 59%-76% GCB).3,7,17 Standard frontline treatment of DLBCL is rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP)18,19 ; however, outcomes with R-CHOP are inferior in non-GCB vs GCB DLBCL.4,13,20-23 More efficacious therapies targeting the molecular basis of non-GCB DLBCL are required.
The nuclear factor-κB (NF-κB) pathway is constitutively activated in non-GCB DLBCL,3,4,24-27 and represents a target for therapeutic intervention. The proteasome inhibitor bortezomib is a potent inhibitor of the transcriptional activity and nuclear translocation of NF-ĸB28-32 ; as such, bortezomib may have specific utility in non-GCB DLBCL. Bortezomib plus chemotherapy has demonstrated substantial activity in patients with previously untreated and relapsed DLBCL,14,33,34 potentially overcoming the negative prognosis associated with non-GCB vs GCB disease.14,33 Bortezomib plus R-CHOP appears to produce clinical benefit in non-GCB DLBCL.33 However, due to overlapping toxicity between bortezomib and vincristine, a higher-than-expected rate of dose-limiting neurotoxicity has been observed with this combination.35 In newly diagnosed mantle cell lymphoma (MCL), substitution of vincristine by bortezomib in the rituximab, cyclophosphamide, doxorubicin, and prednisone with bortezomib (VR-CAP) regimen has resulted in superior efficacy vs R-CHOP, while avoiding excessive neurotoxicity,36 and VR-CAP has recently been approved for MCL by the US Food and Drug Administration (FDA).37
LYM-2034 was a multinational, randomized phase 2 study designed to evaluate VR-CAP vs R-CHOP in patients with previously untreated non-GCB DLBCL, as classified by central review using the Hans algorithm.12 Here, we report efficacy and safety results after 24.9 months’ median follow-up from randomization.
Methods
Patients
Adults with previously untreated, de novo CD20+ non-GCB DLBCL, histologically confirmed by IHC at a central laboratory, were eligible. Other inclusion criteria were: stage II-IV disease (American Joint Committee on Cancer NHL Staging System) or stage I primary mediastinal (thymic) DLBCL; Eastern Cooperative Oncology Group (ECOG) performance status ≤2; at least 1 measurable site of disease per Revised Response Criteria for Malignant Lymphoma38 ; absolute neutrophil count ≥1500 cells per μL; platelets ≥100 × 109 cells per L (or ≥50 × 109 cells per L in the case of thrombocytopenia due to bone marrow infiltration); alanine aminotransferase and aspartate aminotransferase levels ≤3 × upper limit of normal (ULN); total bilirubin <2 mg/dL; and serum creatinine <1.5 × ULN or creatinine clearance ≥50 mL per minute.
Key exclusion criteria were: diagnosis of transformed lymphoma (follicular, T-cell, or Hodgkin lymphoma) or central nervous system lymphoma; previous chemotherapy or extended radiotherapy for lymphoma; grade ≥2 peripheral neuropathy; and uncontrolled or severe cardiovascular disease.
Study design and treatment
This randomized, open-label, phase 2 study was conducted at 57 centers in 18 countries worldwide between January 2010 and December 2011. Independent ethics committees or institutional review boards in the participating centers approved the study, which was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Patients provided written informed consent.
During screening, patients were required to submit tumor biopsies for DLBCL subtyping. Formalin-fixed, paraffin-embedded (FFPE) tumor tissue blocks were shipped to a central laboratory (PhenoPath Laboratories, Seattle, WA) for subtyping by IHC using the Hans method.12 The average turnaround time for central review from shipment to diagnosis was 5 days. Postrandomization GEP confirmation of DLBCL subtype was performed in a subset of patients. Patients with centrally confirmed non-GCB DLBCL by IHC were randomized to receive up to six 21-day cycles of VR-CAP or R-CHOP. Based on the importance of International Prognostic Index (IPI) score for tumor response and outcomes in DLBCL,39 randomization was stratified by IPI score (low [0 or 1] vs low-intermediate [2] vs high-intermediate [3] vs high [4 or 5] risk). Treatment comprised rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, all IV on day 1, and prednisone 100 mg/m2 orally (PO) on days 1 to 5, plus either bortezomib 1.3 mg/m2 IV on days 1, 4, 8, 11 (VR-CAP) of each cycle or vincristine 1.4 mg/m2 (maximum 2 mg) IV on day 1 (R-CHOP).
Bortezomib dose reduction was permitted for: grade ≥3 neutropenia with fever; grade 4 neutropenia lasting >7 days; platelets <10 × 109 cells per L; or any grade ≥3 nonhematologic toxicity, excluding neuropathy, considered by the investigator to be bortezomib-related. Bortezomib dose reductions were required for grade 2/3 peripheral sensory neuropathy, and bortezomib was discontinued for grade 4 peripheral sensory neuropathy. Dose adjustments for rituximab, cyclophosphamide, doxorubicin, and vincristine were made per the relevant prescribing information and current clinical practice. Concomitant medications, including growth factors, for conditions other than DLBCL were permitted, as were all supportive therapies other than anticancer treatments. Short-course steroid treatment (prednisone or equivalent; maximum 10 days, not exceeding 100 mg per day) was permitted for advanced disease in patients who had entered screening and were awaiting randomization. Radiotherapy was prohibited.
Treatment was discontinued for progressive disease (PD) or relapse, unacceptable toxicity, >3-week delay in treatment due to insufficient recovery from toxicity or patient withdrawal. Short-term follow-up visits to assess disease progression were required if treatment was discontinued before PD; these visits were completed every 6 weeks for 18 weeks, and then every 8 weeks thereafter until PD. During short-term follow-up, patients were evaluated by physical examination and laboratory tests only. At any visit, if there was clinical evidence for, or suspicion of, PD, then a computed tomography (CT) and positron emission tomography (PET) scan were performed at the investigator’s discretion to document progression. All patients underwent long-term survival follow-up after documented PD or at the start of alternate antineoplastic therapy. Long-term follow-up visits were completed every 12 weeks until death.
Objectives
The primary objective was to determine the complete response (CR) rate with VR-CAP and R-CHOP. Secondary objectives were to determine: overall response rate (ORR; CR plus partial response [PR] rate), duration of response (DOR), time to next lymphoma therapy (TTNT), 1- and 2-year progression-free survival (PFS) and OS rates, safety of VR-CAP and R-CHOP, and concordance between IHC and GEP for non-GCB DLBCL subtyping.
GEP confirmation of DLBCL subtype
RNA samples extracted from FFPE tumor tissue provided to the central laboratory for IHC confirmation of non-GCB DLBCL subtype were also evaluated by GEP using quantitative reverse transcription–polymerase chain reaction (RT-PCR). Tumor RNA was evaluated using the SensationPlus FFPE reagent kit prior to GEP as described previously.40 GeneChip Human Genome U133 Plus 2.0 Arrays were used for RNA profiling.
Assessments
Response and PD were assessed by CT and whole body 18Ffluorodeoxyglucose (FDG)-PET scan at the end of cycles 3 and 6, and thereafter as clinically indicated, according to the Revised Response Criteria for Malignant Lymphoma.38 PET review relied on standard visual assessment.41 A positive scan was defined as focal or diffuse FDG uptake above background in a location incompatible with normal anatomy or physiology, without a specific standardized uptake value cutoff. PET-negative status was required for an overall response assessment of CR by combined CT + PET scan; patients with PET-non-negative status were assigned an overall response assessment of PR. All efficacy assessments were centrally reviewed by an Independent Radiology Review Committee (IRC). Adverse events (AEs) were graded per National Cancer Institute–Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0.42
Statistical analyses
Assuming CR rates of 70% and 60% for VR-CAP and R-CHOP, respectively, and using Simon randomized phase 2 design43 with 1 preplanned interim futility analysis, a sample size of 75 evaluable patients per arm provided ≥85% probability of observing a better or equal CR rate with VR-CAP than with R-CHOP. This sample size would achieve 78% power if the CR rate was 80% with VR-CAP vs 60% with R-CHOP, using a likelihood ratio test with a 2-sided alpha of 0.05. Assuming that 10% of patients would be response-inevaluable, a sample size of 164 patients (82 per arm) with IHC-confirmed non-GCB DLBCL was planned, requiring screening of ∼364 patients based on the assumption that the non-GCB subtype constitutes ∼45% of all DLBCL.
The cutoff date for the primary end point (CR rate) was June 6, 2012, and the study completion date was June 6, 2013. The intent-to-treat population included all randomized patients. The safety population included all patients who had received at least 1 dose of study drug. The response-evaluable population included all randomized patients who had received at least 1 dose of study drug, had at least 1 measurable lesion at baseline, and had at least 1 postbaseline response assessment. The stratified Cochran-Mantel-Haenszel test was used for between-arm comparisons of response rates in the response-evaluable population. Kaplan-Meier methodology was used for time-to-event analyses (1- and 2-year PFS, OS, and TTNT rates). TTNT was measured from randomization to the start of new treatment; death due to PD prior to subsequent treatment was considered an event. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated based on a Cox regression model, with stratified log-rank test used for between-arm comparisons.
Results
Patients
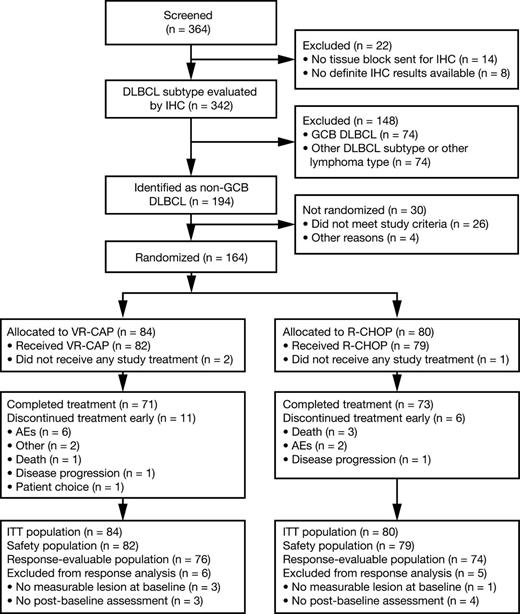
In total, 364 patients consented to DLBCL subtype screening and tumor samples from 342 patients were subtyped by IHC; 194 patients with non-GCB DLBCL were identified and of these, 26 did not meet the inclusion criteria and 4 were excluded for other reasons. Thus, 164 patients were randomized to VR-CAP (n = 84) or R-CHOP (n = 80). Figure 1 summarizes patient flow through the study.
Patient demographics and baseline disease characteristics were similar between the 2 arms, with a slight male preponderance in the R-CHOP group that was not statistically significant (Table 1). Median age was 59.0 years (range, 20-84), with 52 (32%) patients aged >65 years.
Patient demographics and baseline disease characteristics (intent-to-treat population)
| Parameter . | VR-CAP, n = 84 . | R-CHOP, n = 80 . | ||
|---|---|---|---|---|
| n . | % . | n . | % . | |
| Age, y | ||||
| Median | 59.5 | 58.5 | ||
| Range | 20-84 | 23-83 | ||
| Age >65 y | 26 | 31 | 26 | 33 |
| Gender | ||||
| Male | 41 | 49 | 47 | 59 |
| Race | ||||
| White | 64 | 76 | 52 | 65 |
| Asian | 15 | 18 | 14 | 18 |
| Other | 5 | 6 | 14 | 18 |
| ECOG performance status | ||||
| 0/1 | 73 | 87 | 64 | 80 |
| 2 | 11 | 13 | 16 | 20 |
| IPI* | ||||
| Low (0 or 1) | 21 | 25 | 20 | 25 |
| Low-intermediate (2) | 20 | 24 | 19 | 24 |
| High-intermediate (3) | 27 | 32 | 26 | 33 |
| High (4 or 5) | 16 | 19 | 15 | 19 |
| Disease stage at study entry | ||||
| I/II | 23 | 27 | 18 | 22 |
| III/IV | 61 | 73 | 62 | 78 |
| Symptomatic disease | 54 | 64 | 57 | 71 |
| Parameter . | VR-CAP, n = 84 . | R-CHOP, n = 80 . | ||
|---|---|---|---|---|
| n . | % . | n . | % . | |
| Age, y | ||||
| Median | 59.5 | 58.5 | ||
| Range | 20-84 | 23-83 | ||
| Age >65 y | 26 | 31 | 26 | 33 |
| Gender | ||||
| Male | 41 | 49 | 47 | 59 |
| Race | ||||
| White | 64 | 76 | 52 | 65 |
| Asian | 15 | 18 | 14 | 18 |
| Other | 5 | 6 | 14 | 18 |
| ECOG performance status | ||||
| 0/1 | 73 | 87 | 64 | 80 |
| 2 | 11 | 13 | 16 | 20 |
| IPI* | ||||
| Low (0 or 1) | 21 | 25 | 20 | 25 |
| Low-intermediate (2) | 20 | 24 | 19 | 24 |
| High-intermediate (3) | 27 | 32 | 26 | 33 |
| High (4 or 5) | 16 | 19 | 15 | 19 |
| Disease stage at study entry | ||||
| I/II | 23 | 27 | 18 | 22 |
| III/IV | 61 | 73 | 62 | 78 |
| Symptomatic disease | 54 | 64 | 57 | 71 |
ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic Index.
Percentages may not equal 100% due to rounding.
Treatment exposure
Of 164 randomized patients, 2 in the VR-CAP arm and 1 in the R-CHOP arm did not receive any study drug; 161 patients were therefore included in the safety population. Treatment exposure is summarized in Table 2. Seventy-one (87%) and 73 (92%) patients completed 6 cycles of treatment in the VR-CAP and R-CHOP arms, respectively. The remaining 11 (13%) and 6 (8%) patients, respectively, discontinued treatment before completing 6 cycles; reasons for discontinuation are noted in Figure 1. Mean relative dose intensity was similar between the 2 regimens for common drugs. However, the rate of dose reduction for bortezomib in the VR-CAP arm (37%) was greater than that for vincristine in the R-CHOP arm (5%).
Summary of treatment exposure (safety population)
| Parameter . | VR-CAP, n = 82 . | R-CHOP, n = 79 . |
|---|---|---|
| Completed 6 cycles of treatment, n (%) | 71 (87) | 73 (92) |
| Overall treatment duration in wk, median (range) | 16.7 (1-27) | 16.0 (1-21) |
| Median doses received, n | ||
| Rituximab | 6 | 6 |
| Doxorubicin | 6 | 6 |
| Cyclophosphamide | 6 | 6 |
| Prednisone | 30 | 30 |
| Bortezomib/vincristine | 22 | 6 |
| Patients receiving 6 cycles, n (%) | ||
| Rituximab | 72 (88) | 73 (92) |
| Doxorubicin | 70 (85) | 73 (92) |
| Cyclophosphamide | 70 (85) | 73 (92) |
| Prednisone | 72 (88) | 73 (92) |
| Bortezomib/vincristine | 67 (82) | 72 (91) |
| Relative dose intensity, mean (SD) | ||
| Rituximab | 1.00 (0.006) | 1.00 (0.009) |
| Doxorubicin | 0.96 (0.080) | 0.97 (0.082) |
| Cyclophosphamide | 0.96 (0.080) | 0.97 (0.056) |
| Prednisone | 0.99 (0.079) | 0.96 (0.101) |
| Bortezomib/vincristine* | 0.85 (0.140) | 0.79 (0.102) |
| Dose or schedule modified, n (%) | 70 (85) | 38 (48) |
| Any dose reduction | 46 (56) | 23 (29) |
| Dose bortezomib/vincristine withheld, n (%)† | 63 (77) | 3 (4) |
| Dose of bortezomib/vincristine reduced, n (%)† | 30 (37) | 4 (5) |
| Parameter . | VR-CAP, n = 82 . | R-CHOP, n = 79 . |
|---|---|---|
| Completed 6 cycles of treatment, n (%) | 71 (87) | 73 (92) |
| Overall treatment duration in wk, median (range) | 16.7 (1-27) | 16.0 (1-21) |
| Median doses received, n | ||
| Rituximab | 6 | 6 |
| Doxorubicin | 6 | 6 |
| Cyclophosphamide | 6 | 6 |
| Prednisone | 30 | 30 |
| Bortezomib/vincristine | 22 | 6 |
| Patients receiving 6 cycles, n (%) | ||
| Rituximab | 72 (88) | 73 (92) |
| Doxorubicin | 70 (85) | 73 (92) |
| Cyclophosphamide | 70 (85) | 73 (92) |
| Prednisone | 72 (88) | 73 (92) |
| Bortezomib/vincristine | 67 (82) | 72 (91) |
| Relative dose intensity, mean (SD) | ||
| Rituximab | 1.00 (0.006) | 1.00 (0.009) |
| Doxorubicin | 0.96 (0.080) | 0.97 (0.082) |
| Cyclophosphamide | 0.96 (0.080) | 0.97 (0.056) |
| Prednisone | 0.99 (0.079) | 0.96 (0.101) |
| Bortezomib/vincristine* | 0.85 (0.140) | 0.79 (0.102) |
| Dose or schedule modified, n (%) | 70 (85) | 38 (48) |
| Any dose reduction | 46 (56) | 23 (29) |
| Dose bortezomib/vincristine withheld, n (%)† | 63 (77) | 3 (4) |
| Dose of bortezomib/vincristine reduced, n (%)† | 30 (37) | 4 (5) |
SD, standard deviation.
The dose of vincristine was capped at 2 mg.
Proportion of patients with ≥1 dose of bortezomib (VR-CAP arm) or vincristine (R-CHOP arm) withheld or reduced.
Efficacy
A total of 150 patients were evaluable for response (VR-CAP, n = 76; R-CHOP, n = 74). Reasons for exclusion from the response-evaluable population are noted in Figure 1. Based on IRC assessment, there was no statistically significant difference between VR-CAP and R-CHOP for the primary end point, CR rate (64.5% vs 66.2%; odds ratio [OR], 0.91; P = .80), or for the secondary end point, ORR (93.4% vs 98.6%; OR, 0.21; P = .11) (Table 3). In both arms, median time to response was 2.1 months. There were no statistically significant differences between VR-CAP and R-CHOP with regard to durable (lasting ≥6 months) CR rate (61.8% vs 56.8%; OR, 1.25; P = .52) or durable CR/PR rate (75.0% vs 78.4%; OR, 0.84; P = .65) (Table 3). Fourteen (18.4%) and 15 (20.3%) patients had a DOR of <6 months in the VR-CAP and R-CHOP arms, respectively; of these patients, 7 and 11, respectively, were censored without PD. Subgroup analyses demonstrated no statistically significant differences between VR-CAP and R-CHOP in CR rate or ORR by baseline IPI score (supplemental Table 1, see supplemental Data available at the Blood Web site).
Best ORR and durable response rates per Independent Radiology Review Committee assessment (response-evaluable population)
| Response rate . | % (90% CI) . | Odds ratio* (95% CI) . | P† . | |
|---|---|---|---|---|
| VR-CAP, n = 76 . | R-CHOP, n = 74 . | |||
| CR | 64.5 (55.4-73.5) | 66.2 (57.1-75.3) | 0.91 (0.46-1.81) | .80 |
| PR | 28.9 | 32.4 | – | – |
| ORR (CR + PR) | 93.4 (88.7-98.1) | 98.6 (96.4-100.0) | 0.21 (0.03-1.76) | .11 |
| Durable CR‡ | 61.8 (52.6-71.0) | 56.8 (47.3-66.3) | 1.25 (0.64-2.42) | .52 |
| Durable CR/PR‡ | 75.0 (66.8-83.2) | 78.4 (70.5-86.3) | 0.84 (0.39-1.79) | .65 |
| Response rate . | % (90% CI) . | Odds ratio* (95% CI) . | P† . | |
|---|---|---|---|---|
| VR-CAP, n = 76 . | R-CHOP, n = 74 . | |||
| CR | 64.5 (55.4-73.5) | 66.2 (57.1-75.3) | 0.91 (0.46-1.81) | .80 |
| PR | 28.9 | 32.4 | – | – |
| ORR (CR + PR) | 93.4 (88.7-98.1) | 98.6 (96.4-100.0) | 0.21 (0.03-1.76) | .11 |
| Durable CR‡ | 61.8 (52.6-71.0) | 56.8 (47.3-66.3) | 1.25 (0.64-2.42) | .52 |
| Durable CR/PR‡ | 75.0 (66.8-83.2) | 78.4 (70.5-86.3) | 0.84 (0.39-1.79) | .65 |
R-CHOP vs VR-CAP; an odds ratio of >1 is in favor of VR-CAP.
Generalized Cochran-Mantel-Haenszel test for general association (stratified by International Prognostic Index score).
Durable response defined as CR or PR lasting ≥6 months.
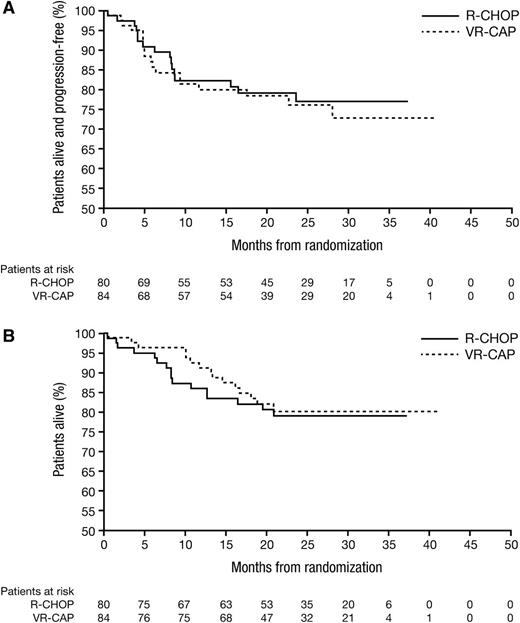
Median follow-up among all patients was 24.9 months (range, 0-40) (VR-CAP, 23.8 months [range, 0-40]; R-CHOP, 26.0 months [range, 0-37]). Median PFS, OS, and TTNT in the ITT population were not estimable due to insufficient events. For VR-CAP and R-CHOP, respectively, there were 18 (21%) and 16 (20%) PD/death events, 15 (18%) and 16 (20%) deaths, and 25 (30%) and 20 (25%) new treatments or deaths due to PD events. Kaplan-Meier analyses of PFS and OS are shown in Figure 2. For VR-CAP and R-CHOP, respectively, 2-year PFS rates were 76.2% and 77.1%, 2-year OS rates were 80.1% and 79.0%, and 2-year TTNT rates were 69.3% and 71.8% (Table 4). There were no significant between-arm differences in PFS (HR, 1.12; P = .76), OS (HR, 0.89; P = .75), or TTNT rates (HR, 1.25; P = .49). Two-year PFS and OS rates appeared more favorable in patients with lower vs higher baseline IPI score; however, there were no overt between-arm differences (supplemental Table 2). Two-year PFS and OS rates appeared higher in patients with PET-negative vs PET-non-negative status (overall CR vs PR by combined CT + PET assessment) (supplemental Table 3), an observation that was more notable in the VR-CAP arm; however, these results should be interpreted cautiously due to the low number of events.
Kaplan-Meier analysis of survival outcomes. (A) PFS and (B) OS by treatment arm.
Kaplan-Meier analysis of survival outcomes. (A) PFS and (B) OS by treatment arm.
One- and 2-year rates of PFS, OS, and patients remaining treatment-free (intent-to-treat population)
| Outcome . | % (95% CI) . | Hazard ratio* (95% CI) . | P† . | |
|---|---|---|---|---|
| VR-CAP, n = 84 . | R-CHOP, n = 80 . | |||
| PFS rate‡ | ||||
| 1-y | 80.0 (69.0-87.5) | 82.3 (71.4-89.3) | 1.12 (0.57-2.20) | .76 |
| 2-y | 76.2 (64.2-84.7) | 77.1 (65.1-85.4) | ||
| OS rate | ||||
| 1-y | 91.2 (82.3-95.7) | 85.9 (76.0-92.0) | 0.89 (0.44-1.81) | .75 |
| 2-y | 80.1 (69.0-87.5) | 79.0 (67.9-86.6) | ||
| Treatment-free rate§ | ||||
| 1-y | 70.6 (59.2-79.4) | 78.4 (67.2-86.2) | 1.25 (0.69-2.24) | .49 |
| 2-y | 69.3 (57.8-78.3) | 71.8 (59.6-80.9) | ||
| Outcome . | % (95% CI) . | Hazard ratio* (95% CI) . | P† . | |
|---|---|---|---|---|
| VR-CAP, n = 84 . | R-CHOP, n = 80 . | |||
| PFS rate‡ | ||||
| 1-y | 80.0 (69.0-87.5) | 82.3 (71.4-89.3) | 1.12 (0.57-2.20) | .76 |
| 2-y | 76.2 (64.2-84.7) | 77.1 (65.1-85.4) | ||
| OS rate | ||||
| 1-y | 91.2 (82.3-95.7) | 85.9 (76.0-92.0) | 0.89 (0.44-1.81) | .75 |
| 2-y | 80.1 (69.0-87.5) | 79.0 (67.9-86.6) | ||
| Treatment-free rate§ | ||||
| 1-y | 70.6 (59.2-79.4) | 78.4 (67.2-86.2) | 1.25 (0.69-2.24) | .49 |
| 2-y | 69.3 (57.8-78.3) | 71.8 (59.6-80.9) | ||
R-CHOP vs VR-CAP; a HR of <1 indicates an advantage for VR-CAP.
Based on the log-rank test (stratified by International Prognostic Index score).
PFS rate by Independent Radiology Review Committee assessment.
Patients who had not received subsequent lymphoma therapy or had not died due to progressive disease.
Twenty-three (27%) patients in the VR-CAP arm and 19 (24%) in the R-CHOP arm received subsequent treatment. The most common agents received as subsequent therapy (VR-CAP vs R-CHOP) were rituximab (17% vs 11%), cytarabine (17% vs 10%), etoposide (16% vs 10%), dexamethasone (13% vs 6%), and cisplatin (12% vs 6%). Nine patients in the VR-CAP arm and 4 in the R-CHOP arm went on to receive high-dose therapy and autologous stem cell transplantation (HDT-ASCT) during subsequent therapy. For VR-CAP, details on CD34+ stem cell collection were available for 7 patients. After mobilization with G-CSF (n = 4) or chemotherapy (n = 3), CD34+ stem cell collection was successful in all 7 patients with a median yield of 6 × 106 cells/kg.
Concordance between IHC and GEP for non-GCB subtype classification
GEP confirmation of DLBCL subtype was performed in 103 patients randomized after IHC subtyping (VR-CAP, n = 53; R-CHOP, n = 50); in this population, the CR rate was 62.3% for VR-CAP (33 of 53) and 62.0% for R-CHOP (31 of 50). Ninety-one patients (n = 45 and n = 46, respectively) were confirmed to be non-GCB by GEP (88.3% concordance); in this population, the CR rate was 60.0% for VR-CAP (27 of 45) and 60.9% for R-CHOP (28 of 46). There were no significant between-arm differences in PFS (HR, 0.91; P = .84) or OS (HR, 1.08; P = .87) in patients with GEP-confirmed non-GCB DLBCL (supplemental Table 4).
Safety
VR-CAP and R-CHOP had similar overall safety profiles (supplemental Table 5). Rates of all-grade AEs (99% vs 100%), grade ≥3 AEs (88% vs 89%), serious AEs (SAEs) (38% vs 34%), treatment discontinuation due to AEs (7% vs 3%), and on-study deaths due to AEs (2% vs 5%) were similar for VR-CAP and R-CHOP. Table 5 summarizes the most common AEs in both treatment arms.
Most common treatment-emergent AEs of any grade (≥15% of patients in either arm) and grade ≥3 (≥5% in either arm; safety population)
| Treatment-emergent AEs . | VR-CAP, n = 82 . | R-CHOP, n = 79 . | ||
|---|---|---|---|---|
| n . | % . | n . | % . | |
| Any grade | ||||
| Any AE | 81 | 99 | 79 | 100 |
| Neutropenia | 65 | 79 | 66 | 84 |
| Thrombocytopenia | 40 | 49 | 7 | 9 |
| Diarrhea | 26 | 32 | 11 | 14 |
| PN NEC | 26 | 32 | 17 | 22 |
| Vomiting | 25 | 31 | 11 | 14 |
| Constipation | 24 | 29 | 25 | 32 |
| Nausea | 22 | 27 | 20 | 25 |
| Pyrexia | 22 | 27 | 18 | 23 |
| Fatigue | 21 | 26 | 11 | 14 |
| Anemia | 19 | 23 | 17 | 22 |
| Leukopenia | 19 | 23 | 24 | 30 |
| Cough | 14 | 17 | 11 | 14 |
| Peripheral edema | 14 | 17 | 5 | 6 |
| Decreased appetite | 13 | 16 | 5 | 6 |
| Paresthesia | 12 | 15 | 10 | 13 |
| Asthenia | 10 | 12 | 14 | 18 |
| Febrile neutropenia | 7 | 9 | 16 | 20 |
| Grade ≥3 | ||||
| Any AE | 72 | 88 | 70 | 89 |
| Neutropenia | 64 | 78 | 64 | 81 |
| Thrombocytopenia | 30 | 37 | 2 | 3 |
| Leukopenia | 18 | 22 | 18 | 23 |
| Febrile neutropenia | 7 | 9 | 16 | 20 |
| Hyperglycemia | 5 | 6 | 0 | 0 |
| PN NEC | 5 | 6 | 2 | 3 |
| Anemia | 4 | 5 | 7 | 9 |
| Diarrhea | 4 | 5 | 1 | 1 |
| Pneumonia | 4 | 5 | 4 | 5 |
| Vomiting | 4 | 5 | 1 | 1 |
| Treatment-emergent AEs . | VR-CAP, n = 82 . | R-CHOP, n = 79 . | ||
|---|---|---|---|---|
| n . | % . | n . | % . | |
| Any grade | ||||
| Any AE | 81 | 99 | 79 | 100 |
| Neutropenia | 65 | 79 | 66 | 84 |
| Thrombocytopenia | 40 | 49 | 7 | 9 |
| Diarrhea | 26 | 32 | 11 | 14 |
| PN NEC | 26 | 32 | 17 | 22 |
| Vomiting | 25 | 31 | 11 | 14 |
| Constipation | 24 | 29 | 25 | 32 |
| Nausea | 22 | 27 | 20 | 25 |
| Pyrexia | 22 | 27 | 18 | 23 |
| Fatigue | 21 | 26 | 11 | 14 |
| Anemia | 19 | 23 | 17 | 22 |
| Leukopenia | 19 | 23 | 24 | 30 |
| Cough | 14 | 17 | 11 | 14 |
| Peripheral edema | 14 | 17 | 5 | 6 |
| Decreased appetite | 13 | 16 | 5 | 6 |
| Paresthesia | 12 | 15 | 10 | 13 |
| Asthenia | 10 | 12 | 14 | 18 |
| Febrile neutropenia | 7 | 9 | 16 | 20 |
| Grade ≥3 | ||||
| Any AE | 72 | 88 | 70 | 89 |
| Neutropenia | 64 | 78 | 64 | 81 |
| Thrombocytopenia | 30 | 37 | 2 | 3 |
| Leukopenia | 18 | 22 | 18 | 23 |
| Febrile neutropenia | 7 | 9 | 16 | 20 |
| Hyperglycemia | 5 | 6 | 0 | 0 |
| PN NEC | 5 | 6 | 2 | 3 |
| Anemia | 4 | 5 | 7 | 9 |
| Diarrhea | 4 | 5 | 1 | 1 |
| Pneumonia | 4 | 5 | 4 | 5 |
| Vomiting | 4 | 5 | 1 | 1 |
PN NEC, peripheral neuropathy not elsewhere classified, including peripheral sensory neuropathy, neuropathy peripheral, peripheral sensorimotor neuropathy, and peripheral motor neuropathy.
Focusing on the most common grade ≥3 AEs, VR-CAP was associated with neutropenia (78%), thrombocytopenia (37%), and leukopenia (22%), whereas with R-CHOP they were neutropenia (81%), leukopenia (23%), and febrile neutropenia (20%). Grade ≥3 AEs occurring with a ≥5% difference between VR-CAP and R-CHOP were thrombocytopenia (37% vs 3%), febrile neutropenia (9% vs 20%), and hyperglycemia (6% vs 0%) (Table 5). Grade ≥3 peripheral neuropathy not elsewhere classified (PN NEC) occurred in 5 (6%) and 2 (3%) patients in the VR-CAP and R-CHOP arms, respectively.
SAEs occurring in ≥5% of patients in either arm were febrile neutropenia (9% each), neutropenia (5% vs 6%), pyrexia (6% vs 0%), and pneumonia (5% vs 4%). The most common AEs (>2% of patients in either arm) leading to dose reduction were neutropenia (21%), thrombocytopenia (11%), peripheral sensory neuropathy (10%), neuralgia (9%), and febrile neutropenia (6%) with VR-CAP, and neutropenia (14%), febrile neutropenia (4%), and hypertension (3%) with R-CHOP. The only AEs leading to discontinuation in >1 patient were PN NEC and neutropenia (each n = 2, all VR-CAP).
Fifteen (18%) VR-CAP and 16 (20%) R-CHOP patients had died at data cutoff. There were 2 (2%) on-study deaths due to treatment-emergent AEs in the VR-CAP arm (cardiac arrest, upper gastrointestinal hemorrhage; each n = 1) and 3 (4%) in the R-CHOP arm (cardiac arrest, septic shock, and respiratory failure, each n = 1).
Discussion
In this phase 2 randomized study, substituting bortezomib for vincristine in the standard R-CHOP regimen did not lead to a significant improvement in CR rate (primary end point), ORR, or durable response rate in patients with previously untreated, IHC-confirmed non-GCB DLBCL. In addition, 2-year PFS, TTNT, and OS rates did not differ significantly between the arms. Subsequent therapies were generally similar between the 2 treatment groups. Few patients received HDT-ASCT following VR-CAP or R-CHOP, reflecting common treatment practice for previously untreated DLBCL.18,19 At the time of this final analysis, PFS and OS data had not reached full maturity; however, findings from a recent report44 indicate that 2-year PFS is a good surrogate marker for long-term outcomes in DLBCL, and therefore more extended follow-up is unlikely to reveal a survival benefit for VR-CAP. The lack of efficacy improvement with VR-CAP in non-GCB DLBCL is in contrast with recent observations from a phase 3 study in newly diagnosed MCL, in which VR-CAP produced superior PFS, CR rates, time to progression, and TTNT as compared with R-CHOP.36
The outcomes observed with R-CHOP administered as six 21-day cycles (per common treatment practice at the time of study design: now increasingly administered as eight 21-day cycles or six 14-day cycles)45-47 were largely consistent with previous studies of DLBCL.4,13,20,45 The median age of patients in the present study was 59.0 years, somewhat lower than that of newly diagnosed DLBCL patients typically observed in epidemiological studies,48,49 but consistent with data from previous clinical trials.11,45,50,51
To our knowledge, this is the first reported study to prospectively randomize patients with non-GCB DLBCL confirmed centrally prior to treatment. Only patients with non-GCB DLBCL classified by the Hans IHC method12 were randomized to VR-CAP or R-CHOP therapy. The confirmation of non-GCB diagnosis prior to randomization requires 2 considerations in the interpretation of the data. First, the concern for a delay in treatment caused by the central review procedure (only 5 days on average) may have excluded patients with the worst disease prognosis from being considered for participation in the trial, which may explain the relatively good outcomes in both arms of the study. Second, the IHC DLBCL subtype classification method was well accepted at the time of initiation of this study, but has subsequently become subject to discussion. For example, Gutiérrez-García et al demonstrated a correlation between the Hans and other algorithms and GEP, but found a higher percentage of misclassified GCB cases compared with non-GCB cases.52 The alternative IHC algorithms developed by Choi et al11 and Meyer et al (Tally method)13 have classified DLBCL subtype more reliably than other algorithms, including the Hans method.12 The Tally algorithm in particular, which tallies antibody results without order precedence, has demonstrated the best concordance with GEP (93%), while maintaining prognostic significance.13 For the present trial, development of the protocol preceded the Choi and Meyer publications, hence the rationale for using the globally available Hans IHC method for DLBCL classification. A recent metaanalysis of studies on GEP or IHC classification in DLBCL and impact on PFS and OS suggests superiority of GEP.53 However, in the present study, non-GCB subtype classified by IHC was subsequently confirmed by GEP in a representative subset of the total patient population. This demonstrated that the Hans IHC algorithm had 88.3% concordance with GEP in identifying the non-GCB subtype, and also showed that both methods were equally effective in predicting CR. It is therefore unlikely that DLBCL misclassification had a major impact on the results of this study.
Interestingly, some major clinical trials45,54,55 and registry studies56 evaluating R-CHOP treatment in patients with DLBCL have not confirmed the worse prognosis of the non-GCB phenotype when classified by the Hans IHC method. Differences in response have, however, been reported with the addition of etoposide to treatment.15,55,57,58
It is possible that the lack of difference in efficacy between VR-CAP and R-CHOP could be explained by limited differential efficacy with the addition of bortezomib or vincristine to the R-CHP base in frontline DLBCL. Genetic analyses have shown that DLBCL is characterized by a number of recurrent mutations along the B-cell receptor activation pathway (eg, in MyD88 or CARD11) leading to constitutive activation of NF-κB.59,60 Although inhibition of NF-κB signaling is among the mechanisms of action of bortezomib,28-32 not all compounds that inhibit the different steps of signal transduction pathways provide an equivalent clinical effect. Recent studies of ibrutinib, which inhibits upstream Bruton tyrosine kinase, have demonstrated proof-of-principle in a subset of patients with non-GCB DLBCL,61,62 whereas a phase 3 trial of enzastaurin, an inhibitor of the intermediate signal transducer PKC-β, failed to show clinical benefit in DLBCL, despite a strong association between high PKC-β expression and adverse prognosis.63-69 Thus, although bortezomib-mediated inhibition of key B-cell activation molecules underlying non-GCB DLBCL biology may have sound scientific basis, bortezomib may not be sufficiently active in the current dose, schedule, and combination for the treatment of non-GCB disease. Of note, recent in vitro data have demonstrated an unexpected role for bromodomain extraterminal domain proteins in regulating IκB kinase activity70 in DLBCL, suggesting that mere inhibition of NF-κB alone may be insufficient to kill lymphoma cells.71
An alternative explanation for bortezomib not being sufficiently active is that the NF-ĸB signature alone is not responsible for the adverse prognosis of non-GCB DLBCL, but instead enriches for a subpopulation that owes its adverse prognosis to another molecular mechanism. Besides the upregulated genes in non-GCB DLBCL, including BCL2, c-FLIP, and cyclin D2, thought to be driven by constitutive activation of NF-ĸB,24,72 other genes are dysregulated73,74 but their contribution at the protein level to the inferior prognosis of non-GCB patients is unclear. In recent years, attention has focused on identifying additional molecular markers that are predictive of outcomes in DLBCL. B-cell lymphoma patients with translocation of both MYC and BCL2 (“double-hit” lymphomas) have very poor prognosis.75-79 This dual translocation is observed in ∼5% of DLBCL cases.80 Overexpression of both MYC and BCL2 protein by IHC, with or without corresponding gene rearrangement, is also observed in approximately one-third of DLBCL cases,80,81 and is associated with poor prognosis.80-83 In a cohort of nearly 900 de novo DLBCL patients treated with R-CHOP, MYC/BCL2 coexpression was associated with an aggressive clinical course and poor prognosis, irrespective of DLBCL subclass, but occurred significantly more commonly in the ABC (non-GCB) than GCB subtype.81 MYC/BCL2 coexpression by IHC may, therefore, be a better prognostic factor in DLBCL than non-GCB subtype; however, other data argue against this.45 Evaluation of MYC/BCL2 coexpression in the present dataset may provide further insight into our results; however, such analyses were precluded by lack of sample availability.
VR-CAP and R-CHOP showed similar safety profiles, with similar rates of all-grade AEs, grade ≥3 AEs, SAEs, discontinuations due to AEs, and on-study deaths. However, grade ≥3 febrile neutropenia rates were lower, and grade ≥3 thrombocytopenia rates were higher, with VR-CAP, likely reflecting the different regimen components. Rates of dose reduction were numerically higher for bortezomib in VR-CAP than for vincristine in R-CHOP, an observation that may be influenced by asymmetry in potential dose administrations between the arms. Higher rates of grade ≥3 thrombocytopenia and dose modifications with VR-CAP were also observed in a recent phase 3 study in newly diagnosed MCL.36 Although any-grade PN NEC rates appeared higher with VR-CAP vs R-CHOP, rates of grade ≥3 PN NEC and discontinuations due to PN NEC were similar between arms. In this study, bortezomib replaced vincristine rather than being added to R-CHOP, due to concerns over potential overlapping neurotoxicity with the 2 agents.35 Since commencement of LYM-2034, other reports have suggested that bortezomib plus R-CHOP is tolerable (with acceptable and manageable neurotoxicity) in DLBCL patients, albeit using 2 doses of bortezomib 1.3 mg/m2 per cycle (days 1, 4) rather than the 4 used here.33,84 This modified schedule is being assessed in a randomized, US-based phase 2 study in previously untreated non-GCB DLBCL (NCT00931918).
In summary, VR-CAP does not improve CR rate, ORR, 2-year PFS rate, or 2-year OS rate in patients with IHC-confirmed non-GCB DLBCL when compared with the current standard of care, R-CHOP. This study highlights the need for better identification of markers of poor prognosis in DLBCL and of markers for patients who could achieve better outcomes with bortezomib-based therapy vs standard R-CHOP.
Presented in poster format at the 12th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 9-22, 2013.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all patients who participated and their families, as well as the investigators and staff at all LYM-2034 clinical sites for their valuable contribution to this study. They acknowledge Emma Landers of FireKite, an Ashfield company, part of UDG Healthcare plc, for writing support during the development of this manuscript, which was funded by Millennium Pharmaceuticals, Inc. and Janssen Global Services, LLC.
This work was supported by Janssen Research & Development, LLC.
Authorship
Contribution: F.O undertook provision of study materials and patients; F.O., O.S., E.O., H.-S.E., M.S.T., J.R., V.P., D.R., S.C., H.v.d.V., and B.F. were responsible for collection and assembly of data; F.O., D.R., S.C., E.Z., C.E., and B.F. were responsible for data analysis and interpretation; D.R., H.v.d.V., and A.R. were responsible for the conception and design of the study; and all authors were involved with the writing of the manuscript and the final approval of the manuscript to be published.
Conflict-of-interest disclosure: F.O., O.S., E.O., H.-S.E., V.P., and B.F. declare no competing financial interests; M.S.T. has received honoraria (Amgen), holds a consultant or advisory role (Amgen, Affymetrix, Janssen), and has received travel and/or accommodation expenses (Amgen, Roche); J.R. holds a consulting or advisory role (Roche); D.R. is under employment (Janssen), holds equity ownership (Johnson & Johnson) and patents, loyalties, or other intellectual property (Janssen), and has received travel and/or accommodation expenses (Janssen); S.C. and H.v.d.V. are under employment (Janssen), hold equity ownership (Johnson & Johnson), and have received research funding (Janssen) and travel and/or accommodation expenses (Janssen); E.Z., C.E., and A.R. are under employment (Janssen) and hold equity ownership (Johnson & Johnson).
The current affiliation for H.v.d.V. is Millennium Pharmaceuticals, Inc, Cambridge, MA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
Correspondence: Fritz Offner, Department of Hematology, University Hospital Ghent, De Pintelaan 185, 9000 Ghent, Belgium; e-mail: fritz.offner@ugent.be.