In this issue of Blood, Offner et al report the results of LYM-2034, a phase 2 multinational trial in which 164 patients with nongerminal center B-cell–like diffuse large B-cell lymphoma (non-GCB DLBCL) were randomized to receive rituximab, cyclophosphamide, adriamycin, prednisone, and either vincristine (R-CHOP) or bortezomib (VR-CAP).1 DLBCL, previously recognized as a single disease entity, represents a heterogeneous group of diseases.
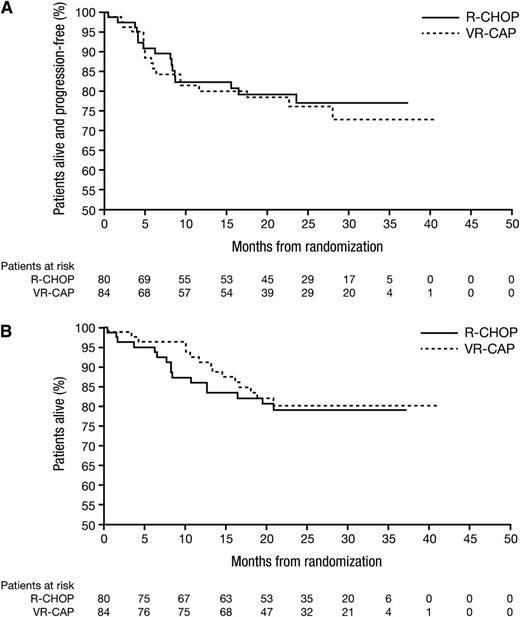
Kaplan–Meier analysis of PFS (A) and OS (B) for each treatment arm, R-CHOP and VR-CAP. There was no statistically significant difference between the arms. See Figure 2 in the article by Offner et al that begins on page 1893.
Kaplan–Meier analysis of PFS (A) and OS (B) for each treatment arm, R-CHOP and VR-CAP. There was no statistically significant difference between the arms. See Figure 2 in the article by Offner et al that begins on page 1893.
The cell-of-origin (COO), as defined by molecular and immunophenotypic differences, delineates 2 major subtypes of DLBCL, which bear prognostic significance for the patient. Gene expression profiling (GEP) originally identified these subtypes as activated B-cell (ABC) subtype, germinal center (GC) subtype, and a minority of unclassified DLBCL.2 Non-GCB subtypes encompass the ABC and unclassified types. Immunohistochemical means of COO identification remains the standard practice in the clinical setting, and has variable concordance with GEP, as reported in the literature.3 Despite being the gold standard for COO determination, currently GEP is neither widely available nor feasible for COO determination outside of research, even at large centers. In the era of targeted therapies, the molecular differences among the subtypes of DLBCL are particularly relevant, with efforts underway to tailor advantageous therapeutic strategies.
Since early published reports on the efficacy of the combination of rituximab with CHOP chemotherapy in 2001, R-CHOP has been established as the mainstay of therapy, as the chemoimmunotherapeutic offensive against DLBCL.4 The ABC (or non-GCB subtype) has been demonstrated as having an inferior outcome after treatment with CHOP-type chemoimmunotherapy.5 One key molecular distinction between the two subtypes is the understanding that nuclear factor (NF)-κB, a prosurvival and antiapoptotic molecule, is constitutively expressed and may be a key contributor to chemotherapy resistance in the ABC subgroup.6 Agents that negatively influence NF-κB therefore provide a possible strategy in impacting the biology of those lymphomas that rely on NF-κB for survival. Trial design with novel agents added to various R-CHOP–like regimens is underway. The goal therein is improved outcomes in the molecularly inferior ABC-subset and includes incorporation of agents such as lenalidomide, ibrutinib, and bortezomib, each with mechanistically different means of impacting NF-κB.
Bortezomib, a proteasome inhibitor, blocks proteosomal degradation of the NF-κB inhibitor, phosphorylated IκBα, among other therapeutic effects.7 Although Offner et al present results for the VR-CAP combination, botezomib was combined with R-CHOP in an earlier study. A phase 1 evaluation of the combination of bortezomib with R-CHOP was conducted in 16 previously untreated patients with DLBCL and 4 patients with mantle cell lymphoma.8 The maximum tolerated dose (MTD) was not reached, and bortezomib 1.3 mg/m2 given on days 1 and 4 was chosen as a safe and effective dose for phase 2 studies among the other doses evaluated with R-CHOP. Overall survival (OS) at 4 years was 75% and at a median follow-up time of 56 months, progression-free survival (PFS) was 58%. The COO was however not defined for the patients in the study. Another phase 1 study of bortezomib (MTD at 1.5 mg/m2) given on days 1 and 4, combined with dose-adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, in both ABC and GCB subtypes was evaluated in 12 and 15 cases, respectively.9 The overall response rate (ORR) was 83% in the ABC subtype and only 13% in the GC subtype, with median OS of 10.8 vs 3.4 months, indicating a significant impact with the addition of bortezomib to the chemotherapy backbone. With concerns of overlapping neurotoxicity between bortezomib and vincristine, Offner et al conducted a comparison between therapeutic arms containing either bortezomib or vincristine with R-CHOP, based on previous reports suggesting higher neurotoxicities with bortezomib–R-CHOP.10
In this report, Offner et al present their findings for 164 randomized, non-GCB subtype patients, including 84 patients on the VR-CAP arm and 80 patients treated with R-CHOP, after a median of ∼2 years follow-up time from randomization. Their evaluation revealed no difference between the VR-CAP or R-CHOP with respect to the complete response rate (64.5% vs 66.2%; P = .8) or ORR (93.4% vs 98.6%; P = .11) (see figure1 ). Importantly, bortezomib was given at 1.3 mg/m2 on days 1, 4, 8, and 11. More chemotherapy dose reductions were required with bortezomib (37%) as compared with vincristine (5%) in the R-CHOP arm, and the lack of dose intensity in the bortezomib arm may have played a role in the equivalent outcomes. Prior evaluations that combined bortezomib with R-CHOP or REPOCH incorporated bortezomib twice per cycle of combination therapy, as previously outlined, instead of 4 times per cycle. Even with the combination of vincristine with bortezomib in those prior evaluations, chemotherapy dose intensity was maintained more consistently. The majority of patients in each arm in the study presented by Offner et al completed 6 cycles of intended therapy. There was a high concordance between molecular and immunohistochemistry methods for the subtype classification.
Further evaluations with bortezomib in combination with chemoimmunotherapy may be warranted and not precluded by the results of Offner et al, given the possibility that dose intensity was not optimized. This study design assumes a difference in the efficacy of bortezomib as compared with vincristine. Perhaps revisiting the R-CHOP combination with and without bortezomib would allow for a more direct evaluation of the potential benefit of bortezomib in the treatment of the non-GCB subtype. Newer generation proteasome inhibitor combinations with chemotherapy provide therapeutic possibilities that may favorably impact the biology of the ABC subtype as well. Consideration of mechanisms of chemoresistance in the ABC subtype, in addition to the role of NF-κB, may further delineate patients who would preferentially respond to targeted therapy combinations, since NF-κB, although thought to be critical, is not the sole driver of aggressive disease biology in this subtype. Looking to the future of DLBCL therapeutics, subtype-directed therapy is a tangible goal with the targeted therapies that are currently available and results of ongoing evaluations with Revlimid–R-CHOP, as well as ibrutinib–R-CHOP combinations, are highly anticipated. The standard of care in non-GCB DLBCL will be shaped by these findings and may yield paradigm-shifting options for patients with the ABC subtype of DLBCL.
Conflict-of-interest disclosure: The authors declare no competing financial interests.