Key Points
Unusual sugars on the tips of sIg of FL cells interact with a tissue lectin to activate tumor-specific signaling.
Activating lectin does not allow endocytosis of sIg, leading to persistent, essential, and targetable antigen-independent stimulation.
Abstract
The vast majority of cases of follicular lymphoma (FL), but not normal B cells, acquire N-glycosylation sites in the immunoglobulin variable regions during somatic hypermutation. Glycans added to sites are unusual in terminating at high mannoses. We showed previously that the C-type lectins, dendritic cell–specific intercellular adhesion molecule-3 grabbing non-integrin (DC-SIGN) and mannose receptor, bound to FL surface immunoglobulin (sIg), generating an intracellular Ca2+ flux. We have now mapped further intracellular pathways activated by DC-SIGN in a range of primary FL cells with detection of phosphorylated ERK1/2, AKT, and PLCγ2. The SYK inhibitor (tamatinib) or the BTK inhibitor (ibrutinib) each blocked phosphorylation. Activation by DC-SIGN occurred in both IgM+ and IgG+ cases and led to upregulation of MYC expression, with detection in vivo observed in lymph nodes. Unlike cells of chronic lymphocytic leukemia, FL cells expressed relatively high levels of sIg, unchanged by long-term incubation in vitro, indicating no antigen-mediated downregulation in vivo. In contrast, expression of CXCR4 increased in vitro. Engagement of sIg in FL cells or normal B cells by anti-Ig led to endocytosis in vitro as expected, but DC-SIGN, even when cross-linked, did not lead to significant endocytosis of sIg. These findings indicate that lectin binding generates signals via sIg but does not mediate endocytosis, potentially maintaining a supportive antigen-independent signal in vivo. Location of DC-SIGN in FL tissue revealed high levels in sinusoidlike structures and in some colocalized mononuclear cells, suggesting a role for lectin-expressing cells at this site.
Introduction
It is known that the t(14;18) translocation found in the majority of cases of FL, which leads to upregulation of BCL2 expression, is necessary but not sufficient for tumor development.1 Although further genomic changes are being revealed, particularly in histone-modifying genes,2 the role of the microenvironment is also likely to be critical.3 Another important feature of FL is that, despite the loss of one Ig allele by the t(14;18) translocation, surface immunoglobulin (sIg) is retained. Initially this retention and the ongoing somatic hypermutation (SHM) led to speculation about a role for persistent antigen in FL, which was difficult to explain, given the high variability of IGV gene usage and the sequence changes resulting from SHM. A striking observation on the nature of sIg in FL has revealed a possible explanation both for retention of sIg and for an influence of microenvironmental factors. This involves a “universal” antigen-independent mechanism able to engage sIg of all cases of FL.4 Specifically, in FL there is a tumor-specific structural change of sIg variable regions by introduction of motifs for N-addition of unusual glycans.5,6 The placement of these glycans at the tip of the sIg on FL cells not only provides a common molecular feature for interaction with lectin, but, also allows a functional bridge between the sIg and the microenvironmental cells that express candidate lectins.4
The introduced glycosylation motifs are in the sIg variable regions of most, if not all, FL cases, but are rare in normal B cells.7 Motifs are generated during SHM and appear to be positively selected by FL cells. They are also maintained over time, even while SHM continues,8 indicating an important role in pathogenesis and maintenance in the germinal center (GC) site. There is additional evidence for acquisition of motifs in other GC-located B-cell malignancies such as endemic Burkitt lymphoma, and a proportion of diffuse large B-cell lymphoma, but not in multiple myeloma, mucosa-associated lymphoid tissue lymphomas, or mutated chronic lymphocytic leukemia (CLL).9
We showed previously that the glycan added to the sites is unusual in terminating at the high mannose stage.5 In contrast, available sites in the constant region are fully N-glycosylated,10 suggesting that the motifs in the IGHV region, although present at various sequence positions, and sometimes in IGLV, are sterically unavailable for glycosyl transferase activity in the Golgi. The outcome is expression in all cases of sIg, with mannosylated sites in the V regions and fully glycosylated sites in the constant region.4,5
Candidate lectins in lymphoid tissue able to interact with FL sIg include dendritic cell–specific intercellular adhesion molecule-3 grabbing non-integrin (DC-SIGN) and mannose receptor.11 These are both calcium-dependent (C-type) lectins with a major role in innate immunity, being able to bind to mannoses expressed by a range of pathogens and by self-ligands.12 Both recombinant proteins were also able to bind to the mannosylated sIg and induced a tumor-specific sIg-mediated intracellular [Ca+2] flux in primary FL cells, with no signal in normal B cells. A control protein, consisting of mannose receptor with the carbohydrate-recognition domain removed, was used to confirm the specificity for binding to glycan expressed by FL cells. A blocking experiment using mannosylated or nonmannosylated single-chain Fv indicated binding to mannosylated sIgM.4 We have now focused on DC-SIGN as a paradigm and have analyzed the intracellular pathways activated by binding of either the soluble lectin or after attachment to beads. Exposure of sIgM+ or sIgG+ FL cases to either form of DC-SIGN induced phosphorylation of ERK1/2, PLCγ, and AKT. Bead-bound DC-SIGN also increased levels of MYC, with MYC protein detected in FL lymph nodes. In contrast, normal B cells that express similar levels of sIg failed to respond to DC-SIGN. Another intriguing finding was that, unlike anti-Ig, soluble DC-SIGN, even when crosslinked, did not mediate significant endocytosis of sIg. These features may have been captured by opportunistic FL cells for long-term survival and proliferation in the hostile GC site.
Methods
Patient and healthy donor material
Primary cells from lymph nodes of 18 FL patients and peripheral blood mononuclear cells from the blood of 5 healthy donors were isolated from frozen stocks.7 Approval was obtained from the Southampton and South West Hampshire Research Ethics Committee and informed consent was obtained in accordance with the Declaration of Helsinki. Cells were rapidly thawed at 37°C before purification of viable cells using Lymphoprep. After washing in complete medium (RPMI 1640 medium supplemented with 10% [vol/vol] fetal calf serum, 2 mM glutamine, and 1% [wt/vol] sodium pyruvate) and counting using Trypan Blue exclusion, cells were allowed to rest for 30 minutes at 37°C/5% CO2.
Phenotype and IGHV sequences
Live FL cells were analyzed using anti-IgM-R-Phycoerythrin (PE), anti-IgD-fluorescein isothiocyanate (FITC), anti-IgG-FITC (all DAKO, Ely, United Kingdom [UK]), anti-human CD19 Pacific Blue or allophycocyanin (APC), and anti-human CD5 PerCP/Cy5.5 (BioLegend, Cambridge, UK). Cells were labeled with anti-IgM/IgD/CD19/CD5 or anti-IgM/IgG/CD19/CD5 or corresponding isotype controls. Surface Ig expression was measured in CD19+/CD5– population. Samples were acquired on a FACSCanto II (BD Biosciences, Oxford, UK).
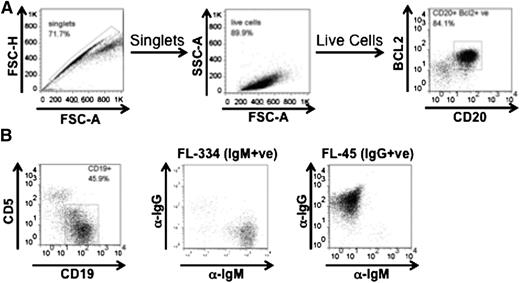
BCL2+ FL cells were identified for immunefluorescent analysis after fixation in paraformaldehyde (final concentration 1.6%) (VWR, Lutterworth, UK). Cells were incubated for 5 minutes at room temperature, washed and resuspended in ice-cold 90% (vol/vol) methanol, and incubated on ice for 30 minutes. Then cells were washed and reconstituted in Flow Buffer (1% bovine serum albumin, 4 mM ethylenediamine tetraacetic acid (EDTA), and 0.15 mM NaN3 in phosphate-buffered saline). Staining was done with directly labeled anti-CD20 PerCP-Cy 5.5 (clone H1/FB1), anti-BCL2-FITC (clone 6C8). Samples were acquired on a FACSCanto II. Gating was first for singlets (FSC-H/FSC- A), then lymphocytes (SSC-A/FSC-A) before gating based on CD20 and BCL2 double-positivity.
Tumor-derived IGV(D)J gene sequences were identified in FL samples as described.7
DC-SIGN, anti-Ig, and bead conjugation
Recombinant human DC-SIGN/CD209-Fc chimera (soluble DC-SIGN) (R&D Systems, Abingdon, UK) comprises the dimeric extracellular portion (aa64-404) covalently linked to human Fcγ, goat F(ab′)2 anti-μ, anti-γ, or goat F(ab′)2 isotype control (Southern Biotech, Cambridge Biosciences UK), which were used as soluble proteins or were each conjugated to Dynabeads M-280, 2.8-µm diameter, with epoxy surface groups (Invitrogen, Paisley, UK) according to the manufacturer’s instructions. Briefly, beads were washed in the provided C1 buffer, and proteins were added at 10 µg per mg of beads. The suspension was incubated on a rotator at 37°C overnight, then washed in the provided C2 buffer and resuspended in the provided SB storage buffer at a final bead concentration of 10 mg beads per mL so that 1.5 μL includes 106 beads. For long-term storage, 0.02% NaN3 was added.
Binding of DC-SIGN to surface Ig on primary FL
FL cells were divided into fluorescence-activated cell sorting (FACS) tubes and were placed on ice before treatment with soluble DC-SIGN. Tubes were incubated on ice for 45 minutes before 4% paraformaldehyde was applied to fix cells at room temperature for 15 minutes. Cells were washed twice in phosphate-buffered saline and centrifuged at 1500 rpm for 5 minutes and then resuspended in Flow Buffer. To determine the level of DC-SIGN binding, a monoclonal mouse anti-human-CD209 IgG2b antibody (BD Biosciences) was added and incubated at room temperature for 45 minutes. As a control, some cells were not treated with antibody. Cells were washed and resuspended using Flow Buffer before the addition of Alexa Fluor488-conjugated goat anti-mouse-IgG Fcγ2b (Jackson ImmunoResearch, Newmarket, UK) to detect bound anti-CD209 or Dylight549-conjugated goat anti-human IgM (Jackson ImmunoResearch) to detect sIgM. Tubes were incubated at room temperature in darkness for 30 minutes then washed and resuspended in Flow Buffer before analysis with a BD FACSCanto. Data were analyzed using FlowJo (Tree Star, Ashland, OR). Singlet live cells were gated on to detect sIgM expression, and cells positive for this were gated on to determine DC-SIGN binding.
To ensure that binding was unaffected by the Fc portion of the DC-SIGN molecule potentially attaching to FcγRIIb, we masked this receptor by 2 strategies. First, using the AT10 antibody,13 and second, using an FcR-blocking reagent (Miltenyi Biotec, Surrey, UK). Cells were treated on ice for 10 minutes with either 100 μg/mL AT10 or FcR-blocking reagent, per the manufacturer’s instructions. After washing in complete medium, the binding protocol was carried out as described.
Immunoblot analysis
Immunoblotting was performed as described14 using: anti-phospho-ERK1/2 (9101), anti-ERK1/2 (9102), anti-phospho-AKT (S473) (4060), anti-AKT (9272) (all from Cell Signaling Technology, Hitchin, UK), anti-MYC (clone 9E10) (Merck Millipore, Beeston, UK), and anti-β-actin (2066; Sigma-Aldrich, Poole, UK). Secondary horseradish peroxidase-conjugated antibodies were obtained from GE Healthcare (Little Chalfont, UK). Images were collected using a Fluor-S MultiImager (BioRad, Hemel Hempstead, UK) and quantified using Fluor-S software Quantity One Version 4.6.3 (BioRad). All values were normalized to the relevant loading control, and relative fold change was calculated with the isotype control antibody-treated cells taken as a 1.0 fold of expression.
PhosFlow analysis
For the PhosFlow assay,15 FL cells were left in complete medium for 60 minutes to recover at 37°C. 0.5 to 1 × 106 cells in 0.1 mL of medium were left untreated or treated with anti-Ig mix (10 μg/mL goat F[ab′]2 anti-human IgM and anti-human Fcγ) or DC-SIGN (10 μg/mL) crosslinked with anti-human IgG (Fc-specific), F(ab′)2 antibody (Sigma-Aldrich) (DC-SIGN:anti-IgG [3:1 molar ratio] to avoid nonspecific binding from anti-IgG in the experiment, 30 min preincubation) for indicated time points. H2O2 was added immediately before stimulation at a final concentration of 3.3 mM,15 except for one unstimulated control. Stimulation was stopped by the addition of paraformaldehyde (final concentration 1.6%) (wt/vol), and cells were incubated for 5 minutes at room temperature and then washed and resuspended in ice-cold 90% (vol/vol) methanol and incubated on ice for 30 minutes. Then cells were washed and reconstituted in Flow Buffer and stained with directly labeled anti-CD20 PerCP-Cy™ 5.5 (clone H1/FB1), anti-BCL2-FITC (clone 6C8), anti-ERK1/2 (pT202/pY204)-PE or PLC-γ2 (pY759) Alexa Fluor 647, or appropriate isotype controls antibodies (all from BD Biosciences) for 30 minutes on ice and then washed and reconstituted in the Flow Buffer. Samples were acquired on a FACSCanto II. Change in phosphorylation was determined on the gated CD20+/Bcl2+ cells, and geometric mean fluorescence intensity (GeoMFI) was calculated for PE (pERK) or Alexa Fluor 647 (pPLC-γ2) at the different time points. The results were analyzed by the online analytical platform Cytobank (Cytobank, Inc., Mountain View, CA). The resulting GeoMFI were normalized to controls samples (H2O2 treatment only) for each time point and fold changes in phosphorylation were calculated.
Immunohistochemistry
4-μ-thick formalin-fixed, paraffin-embedded sections obtained from reactive tonsils and follicular lymphomas (encompassing grades 1, 2, and 3a) were stained with the following antibodies: CD20 (clone L26, Dako, Glostrup, Denmark; dil. 1:100), CD21 (clone EP3/93, cell Marque, Rocklin, CA; dil. 1:50), c-MYC (clone Y69 Abcam, Cambridge, UK; dil. 1:200), and CD209 (clone EPR5588, Epitomics, Burlingame, CA; dil 1:100). For CD20, CD21, and c-MYC antibodies, antigen retrieval was obtained with Tris-EDTA at pH 9 for 30 minutes. CD209 and corresponding double stains were performed on frozen sections.
Kinase inhibitors
The clinical BTK and SYK inhibitors ibrutinib and tamatinib (the active form of fostamatinib) (Stratech Scientific Ltd, Suffolk, UK) were used at 10-μM final concentration. Cells were pretreated with the inhibitor for 30 minutes before stimulation.
Endocytosis of surface immunoglobulin
Internalization of sIgM was assessed by measuring the remaining anti-Ig or DC-SIGN on the cell surface of CD19+ve cells with secondary FITC-labeled antibodies. Briefly, washed and precooled cells were preincubated with 20 μg/mL goat F(ab′)2 anti-human Ig or DC-SIGN for 40 minutes on ice. Aliquots (1 × 106 cells) were left for the unstained control and the secondary antibody controls. Then the cells labeled with anti-Ig or DC-SIGN and were divided into aliquots (1 × 106 cells) for incubation on ice or at 37°C for 0, 10, 30, and 60 minutes. At each time point, internalization was stopped by the addition of ice-cold Flow Buffer and cells were left on ice. Next, cells were washed in ice-cold Flow Buffer and labeled with anti-CD19-APC and rabbit anti-goat F(ab′)2-FITC (Jackson ImmunoResearch,) for anti-Ig labeled cells or goat anti-human IgM secondary antibody, and FITC (Cambridge Bioscience, Cambridge, UK) for DC-SIGN–labeled cells. Samples were acquired on a FACSCanto II and GeoMFI for the green channel in CD19+ve gated cells were used to calculate percentage of internalization. The GeoMFI value at time 0 was used as 100% of sIg expression.
Results
Primary FL tissue analysis
The primary FL samples investigated were from lymph nodes of 18 cases of grades 1-3a (supplemental Table 1, available on the Blood Web site) and included 12 IgM+ and 6 IgG+ cases by phenotype. Analysis of the IGHVHDHJ-constant region by polymerase chain reaction analysis confirmed the isotype and revealed Asn-X-Ser/Thr motifs in 13 of 13 cases, with 6 of these having additional sites in IGLV. All cases of FL where clonal sequences of both IGHV and IGLV were analyzed had motifs. Samples were analyzed for tumor cells by CD20/BCL2 staining (Figure 1A) and expression of surface Ig with representative IgM+ or IgG+ cases shown in Figure 1B. The percentage of B cells in the mononuclear cell fraction was usually 40% to 50% and the proportion of normal (BCL2–) cells in the B-cell population was low (∼10%-20%).
Characterization of FL cells. Phenotyping of representative patients used in this study, including FACS plots detailing gating strategy of singlet CD20+ cells to determine BCL2 expression (A) and gating of singlet, live CD19+ FL cells expressing to determine IgM (FL334) or IgG (FL45) isotype (B).
Characterization of FL cells. Phenotyping of representative patients used in this study, including FACS plots detailing gating strategy of singlet CD20+ cells to determine BCL2 expression (A) and gating of singlet, live CD19+ FL cells expressing to determine IgM (FL334) or IgG (FL45) isotype (B).
DC-SIGN binds to primary FL cells and specifically activates signaling pathways
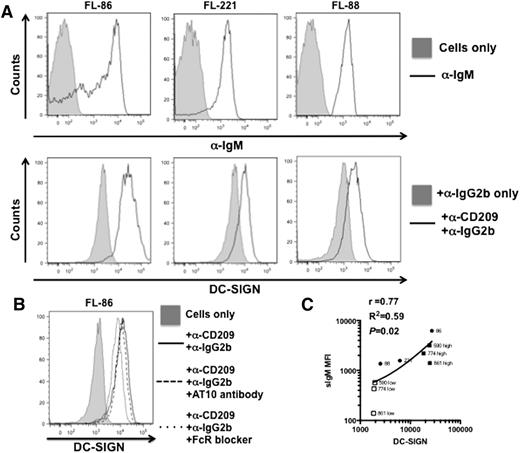
Primary FL cells express relatively high but variable levels of sIg. We compared the level of binding of DC-SIGN with the amount of expressed sIg in 6 cases of sIgM+ FL. Results on 3 cases are illustrated in Figure 2A and controls using either an Fcγ RIIb–blocking antibody (AT10)13 or the commercial FcR blocker showed that binding was specific for the DC-SIGN component of DC-SIGN-Fc (Figure 2B). Data from the 6 patients indicated correlation between sIgM levels and binding of DC-SIGN (Figure 2C). In 2 cases (774 and 861) (supplemental Figure 1), we detected 2 populations of BCL2+ cells expressing different (high and low) levels of sIgM (supplemental Figure 1A), and in a further case (590) there was a broad peak indicating a range of sIgM expression. Analysis of these samples for DC-SIGN binding showed double peaks (supplemental Figure 1B), and back-gating to determine the level of sIgM on the peaks revealed a correlation between DC-SIGN binding and the level of sIgM expression (supplemental Figure 1C). This supports the conclusion that DC-SIGN is binding to sIgM and that the ability to bind varies with the level of sIgM expression. However, in addition to sIgM levels, another variable is the number of glycan addition sites per molecule. Some correlation with binding is apparent, with cases 86 and 590 having 5 and 3 sites, respectively (Figure 2C), but case 221 with 4 sites was an exception being only a moderate binder, perhaps indicating heterogeneity in glycan addition.
DC-SIGN binding to surface IgM+ primary FL cells. Representative FACS plots of 3 FL samples simultaneously stained within the same tube to detect sIgM expression and soluble DC-SIGN binding (n = 6). Analysis (FlowJo) of singlet live cells initially allowed examination of sIgM expression (A). Populations positive for sIgM were gated to determine DC-SIGN binding on these cells. (B) Representative FACS plot of a control experiment to show that recombinant DC-SIGN-Fc binding is not affected by AT10 (anti-CD32) antibody or by the FcR-blocking reagent. (C) Analysis of GeoMFI data (GraphPad Prism) found that expression of sIgM was significantly correlated to binding of DC-SIGN (Spearman rank test). This correlation was confirmed further within samples of FL cells showing double peaks of high and low sIgM expression.
DC-SIGN binding to surface IgM+ primary FL cells. Representative FACS plots of 3 FL samples simultaneously stained within the same tube to detect sIgM expression and soluble DC-SIGN binding (n = 6). Analysis (FlowJo) of singlet live cells initially allowed examination of sIgM expression (A). Populations positive for sIgM were gated to determine DC-SIGN binding on these cells. (B) Representative FACS plot of a control experiment to show that recombinant DC-SIGN-Fc binding is not affected by AT10 (anti-CD32) antibody or by the FcR-blocking reagent. (C) Analysis of GeoMFI data (GraphPad Prism) found that expression of sIgM was significantly correlated to binding of DC-SIGN (Spearman rank test). This correlation was confirmed further within samples of FL cells showing double peaks of high and low sIgM expression.
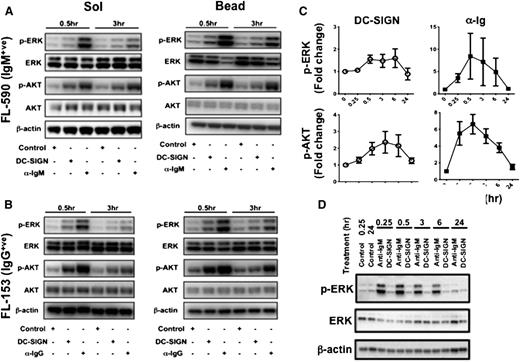
Exposure of primary FL cell samples to DC-SIGN led to phosphorylation of ERK1/2 and AKT. The level of constitutive phosphorylation before stimulation is difficult to estimate, especially because phosphorylation can be reversed by cellular phosphatases during the processing of tissue samples. In contrast, phosphorylation induced by DC-SIGN in vitro is clear and occurred in both IgM+ (Figure 3A) and IgG+ cases (Figure 3B). The level of response induced by bead-bound DC-SIGN was generally higher than the soluble form. Again, blocking of the FcR had no effect on responses, indicating specificity for DC-SIGN (supplemental Figure 2). Increasing the bead:cell ratio to 4:1 did generate higher responses against DC-SIGN, which was especially evident with p-AKT (supplemental Figure 2). Because DC-SIGN is a calcium-dependent lectin, we measured responses in 2 cases over a range of calcium concentrations and found that the level in medium plus FCS (1.5 mM) was adequate (supplemental Figure 3); therefore, this was used in all experiments.
Induction of FL-specific signaling pathways by DC-SIGN. Western blotting demonstrating ERK and AKT phosphorylation in primary FL cells taken from sIgM+ and sIgG+ cases. Representative examples of 16 samples: (A) sIgM+ and (B) sIgG+ cases treated with soluble or bead isotype control (IC), DC-SIGN, or anti-Ig for 0.5 and 3 hours. (C) Fold change analysis was used to measure the extent of ERK and AKT phosphorylation induced in the 16 samples by either DC-SIGN or anti-Ig over time. (D) Normal B cells were stimulated with either DC-SIGN or anti-IgM beads, and western blotting analysis was done for ERK and AKT phosphorylation.
Induction of FL-specific signaling pathways by DC-SIGN. Western blotting demonstrating ERK and AKT phosphorylation in primary FL cells taken from sIgM+ and sIgG+ cases. Representative examples of 16 samples: (A) sIgM+ and (B) sIgG+ cases treated with soluble or bead isotype control (IC), DC-SIGN, or anti-Ig for 0.5 and 3 hours. (C) Fold change analysis was used to measure the extent of ERK and AKT phosphorylation induced in the 16 samples by either DC-SIGN or anti-Ig over time. (D) Normal B cells were stimulated with either DC-SIGN or anti-IgM beads, and western blotting analysis was done for ERK and AKT phosphorylation.
Comparative data showing stimulation with DC-SIGN or with anti-Ig on 16 cases are illustrated in Figure 3C. Although responses to anti-Ig are higher, the responses to DC-SIGN appear to persist longer, with little reduction from 0.5 to 6 hours. In contrast to primary FL cells, normal B cells did not respond to bead-bound DC-SIGN, although the response to anti-Ig was similar to FL cells (Figure 3D). The gel shows one example of the same results obtained from 4 normal donors, using circulating B cells. Because of very small numbers,15 it was not possible to investigate the normal B cells remaining in the tissue sites.
Analysis of responses to DC-SIGN in single FL cells
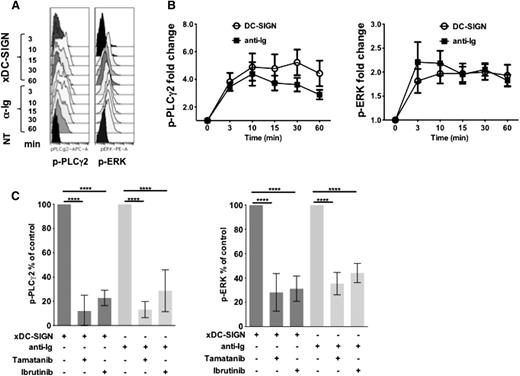
Responses of single cells were analyzed by flow cytometry using soluble DC-SIGN crosslinked via anti-Fcγ. Soluble anti-Ig was used as a control, and detection of responses was done by PhosFlow using FITC-labeled antibodies against either p-ERK or p-PLCγ. The basal phosphatase activity was inhibited by the addition of hydrogen peroxide.15 A typical result (Figure 4A) using an IgG+ sample (FL-33), gated on CD20 and BCL-2 double-positive expression, confirms the early phosphorylation of ERK seen by western blotting, with a similar pattern observed for PLCγ. No change in cell size was observed after either stimulus.
Downstream signaling induced by anti-Ig or DC-SIGN is ablated by kinase inhibitors. PhosFlow data analysis to examine phosphorylation of PLCγ2 and ERK in FL cells. Cells were pretreated with H2O2 to inhibit phosphatases before stimulation with either crosslinked DC-SIGN (xDC-SIGN) or soluble anti-Ig. (A) Cytobank software (representative heat map traces shown) was used to measure phosphorylation of PLCγ2 and ERK for each treatment at each time point. (B) Fold-change analysis was used to chart the level of phosphorylation over time in 13 samples tested. (C) PhosFlow was used to measure the impact of the SYK inhibitor tamatinib or BTK inhibitor ibrutinib on phosphorylation of PLCγ2 and ERK. Analysis of data for 9 samples (6 for SYK and 3 for BTK) was compiled (GraphPad Prism) (Student t test; ****P < .0001).
Downstream signaling induced by anti-Ig or DC-SIGN is ablated by kinase inhibitors. PhosFlow data analysis to examine phosphorylation of PLCγ2 and ERK in FL cells. Cells were pretreated with H2O2 to inhibit phosphatases before stimulation with either crosslinked DC-SIGN (xDC-SIGN) or soluble anti-Ig. (A) Cytobank software (representative heat map traces shown) was used to measure phosphorylation of PLCγ2 and ERK for each treatment at each time point. (B) Fold-change analysis was used to chart the level of phosphorylation over time in 13 samples tested. (C) PhosFlow was used to measure the impact of the SYK inhibitor tamatinib or BTK inhibitor ibrutinib on phosphorylation of PLCγ2 and ERK. Analysis of data for 9 samples (6 for SYK and 3 for BTK) was compiled (GraphPad Prism) (Student t test; ****P < .0001).
We constructed heat maps of scalable geometric means of responses for 13 FL cases. Compared with the unstimulated controls (NT), all cases responded, but at variable levels (supplemental Figure 4A). Higher responses to DC-SIGN were generally found in cells with higher responses to anti-Ig. The non–B-cell fraction, mainly T cells, gave only background (∼9% pPLCγ2 level and ∼6% p-ERK level) of maximal responses for FL cells when exposed to DC-SIGN (data not shown).
Compiled kinetic responses of FL cells to lectin are similar to those with anti-Ig, but again tended to be more persistent (Figure 4B). We could find no consistent significant differences in the level of phosphorylation between IgM+ or IgG+ cases (supplemental Figure 4B), although numbers are limited.
Inhibitors of B-cell receptor–associated proximal kinases decrease DC-SIGN–dependent signaling in FL
We then tested whether inhibitors of SYK (tamatinib) or BTK (ibrutinib) acted against pathways stimulated in FL cells via DC-SIGN. Analysis by single-cell flow cytometry for the effects of pre-exposure to inhibitor for 30 minutes on signaling showed strong inhibition of responses against either anti-Ig or DC-SIGN. Compiled data for 6 cases (SYK) and 3 cases (BTK) are shown in Figure 4C.
DC-SIGN stimulates MYC expression in primary FL cells
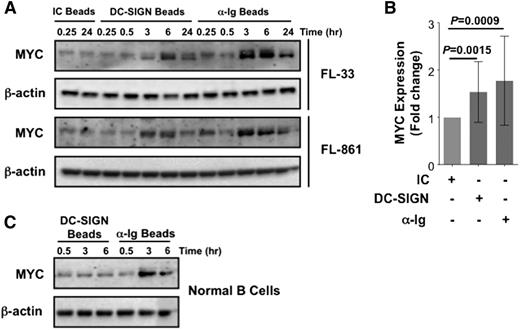
To investigate the downstream B-cell receptor–associated targets activated by DC-SIGN, we analyzed MYC expression in FL. Basal levels of MYC were mostly low, but were increased by exposure to bead-bound DC-SIGN (2 representative examples shown in Figure 5A). The response was again weaker than that induced by bead-bound anti-Ig but had a similar kinetic being maximal at the 6-hour time point. Data from 11 samples confirmed a significant increase in MYC levels after exposure to DC-SIGN (Figure 5B).
DC-SIGN treatment induces MYC expression in FL cells. Comparison of the ability of DC-SIGN to upregulate MYC in primary FL cells in relation to anti-Ig stimulation. (A) Western blotting was used to measure MYC protein expression in FL cells treated with isotype control, DC-SIGN, or anti-Ig beads over time (2 representative samples shown). (B) Fold-change analysis of band densitometry was used to compare MYC expression induced by each treatment of 11 FL samples tested (Student t test). (C) Western blotting was used to examine MYC expression in normal B cells treated with either DC-SIGN or anti-IgM beads over time.
DC-SIGN treatment induces MYC expression in FL cells. Comparison of the ability of DC-SIGN to upregulate MYC in primary FL cells in relation to anti-Ig stimulation. (A) Western blotting was used to measure MYC protein expression in FL cells treated with isotype control, DC-SIGN, or anti-Ig beads over time (2 representative samples shown). (B) Fold-change analysis of band densitometry was used to compare MYC expression induced by each treatment of 11 FL samples tested (Student t test). (C) Western blotting was used to examine MYC expression in normal B cells treated with either DC-SIGN or anti-IgM beads over time.
In contrast, normal B cells did not upregulate MYC after exposure to DC-SIGN, but did after bead-bound anti-Ig (Figure 5C). The potential relevance of this upregulation in vivo was investigated by staining of FL tissue for MYC protein. We analyzed 2 cases of classical FL for each of the grades 1, 2, and 3a, as well as 2 control reactive lymphoid tissues (data not shown). MYC protein was detectable in a small fraction of cells in all FL cases. Immunohistochemistry of a representative FL case (grade 2) revealed FL cells, with characteristic size range and heterogeneous neoplastic cell nuclei, embedded within the CD21+ follicular dendritic cell network, among which were detectable MYC+ FL cells (supplemental Figure 6). Although numbers of cells are small, MYC is a highly regulated protein, so expression would be expected to be limited.
Effects of incubation in vitro on expression of sIg or CXCR4
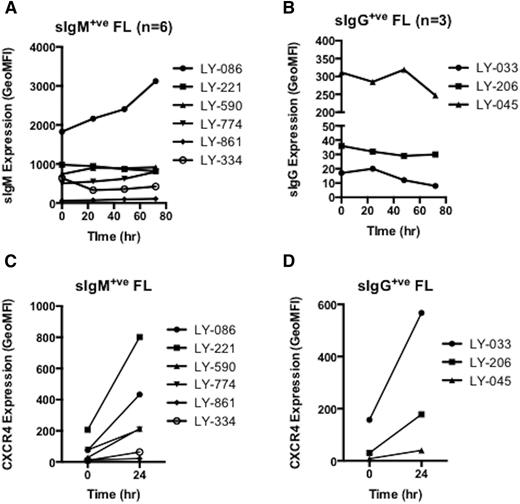
Levels of sIg for FL cells vary but are in the same range as normal memory B cells4 (Figure 1A) and are ∼10 times higher than cells of chronic lymphocytic leukemia (CLL). In CLL, expression of sIgM can be reversibly downregulated in vivo by antigen or in vitro by anti-Ig.16 A second receptor with reversible downregulated expression in CLL is the chemokine receptor, CXCR4.17-20 To investigate potential in vivo downregulation in FL cells, expression of sIg and CXCR4 was tracked over time in vitro in 9 FL samples (6 IgM+, 3 IgG+). Only 1 of 9 showed any recovery of sIg expression, even when starting levels were relatively low (Figure 6A-B). In contrast, most cases (8/9) recovered expression of CXCR4 over a 24-hour period (Figure 6C-D). The high expression of sIg and its stability in vitro argue against downregulation of sIg by antigen in vivo. Recovery of CXCR4 expression reveals that the FL cells were able to reverse the expected chemokine-mediated downmodulation in tissue sites.
FL cells do not increase expression of surface Ig after incubation in vitro. Recovery of surface marker expression of FL samples in vitro (n = 9; 6 IgM+; 3 IgG+). FACS was used to determine expression (GeoMFI) of surface Ig for both (A) IgM-positive and (B) IgG-positive patients along with expression of CXCR4 (C and D) at zero time and at subsequent intervals up to 72 hours. Expression analysis of GeoMFI (CellQuest) was used to plot change of expression over time.
FL cells do not increase expression of surface Ig after incubation in vitro. Recovery of surface marker expression of FL samples in vitro (n = 9; 6 IgM+; 3 IgG+). FACS was used to determine expression (GeoMFI) of surface Ig for both (A) IgM-positive and (B) IgG-positive patients along with expression of CXCR4 (C and D) at zero time and at subsequent intervals up to 72 hours. Expression analysis of GeoMFI (CellQuest) was used to plot change of expression over time.
Engagement of sIg by DC-SIGN fails to lead to endocytosis
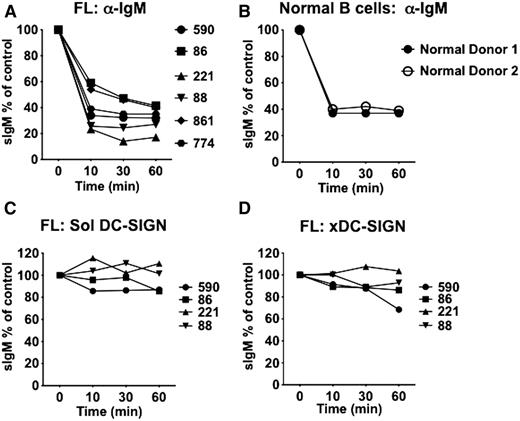
After binding of anti-Ig, sIg of CLL cells undergoes rapid endocytosis.16 The ability of DC-SIGN to induce endocytosis of sIg was first tested by measuring the sIgM remaining at the cell surface after incubation with either DC-SIGN or with anti-Ig. Endocytosis was clearly seen after exposure to anti-IgM as expected in both FL cells (Figure 7A) and normal B cells (Figure 7B) after incubation for 10 to 30 minutes. Efficient removal of sIg was checked using an FITC-labeled secondary anti-goat (Fab′)2 antibody. Assessment of the effects of engagement with DC-SIGN on sIgM expression has to take into account a partial blocking of access of anti-IgM by bound DC-SIGN.4 The initial level of DC-SIGN–engaged sIgM in ice was then assigned a value of 100%. No significant change in this level was detectable after incubation for 60 minutes (Figure 7C), indicating minimal removal by endocytosis. To increase lectin avidity, a crosslinking secondary antibody against Fcγ was added to the DC-SIGN protein before addition to the cells. Even then, no significant endocytosis could be induced (Figure 7D).
DC-SIGN fails to induce endocytosis of surface Ig by FL cells. Analysis of surface IgM internalization in response to DC-SIGN or anti-IgM treatments. Primary FL or normal B cells were treated on ice for 45 minutes with either soluble anti-IgM F(ab′)2 or DC-SIGN before incubation at 37°C to allow internalization. Flow cytometry was used to determine surface IgM expression over time in relation to zero time (100%). (A) FL samples treated with anti-IgM. (B) Normal B cells treated with soluble anti-IgM. (C) FL samples treated with soluble DC-SIGN. (D) FL cells treated with cross-linked (x) DC-SIGN. Expression of sIgM determined by FACS was analyzed using CellQuest.
DC-SIGN fails to induce endocytosis of surface Ig by FL cells. Analysis of surface IgM internalization in response to DC-SIGN or anti-IgM treatments. Primary FL or normal B cells were treated on ice for 45 minutes with either soluble anti-IgM F(ab′)2 or DC-SIGN before incubation at 37°C to allow internalization. Flow cytometry was used to determine surface IgM expression over time in relation to zero time (100%). (A) FL samples treated with anti-IgM. (B) Normal B cells treated with soluble anti-IgM. (C) FL samples treated with soluble DC-SIGN. (D) FL cells treated with cross-linked (x) DC-SIGN. Expression of sIgM determined by FACS was analyzed using CellQuest.
To test whether binding of DC-SIGN, which failed to induce endocytosis, was sufficient to induce a signal, we analyzed 2 FL samples (FL86 and FL590) in depth. The results were very similar and are illustrated for FL86 in supplemental Figure 6. After exposure to DC-SIGN, we measured binding, signaling, and persistence of sIgM on the cell surface (supplemental Figure 6A). It is clear that binding of DC-SIGN was high (supplemental Figure 6B) and was able to mediate phosphorylation of ERK1/2 and especially AKT (supplemental Figure 6C). However, there was no significant loss of expression of sIgM (supplemental Figure 6D) by endocytosis. Comparison of endocytosis estimated by reduction of expression of sIgM at 30 minutes indicated a ∼77% reduction via anti-IgM, but only a ∼29% reduction via DC-SIGN.
Localization of DC-SIGN in FL lymph node
Expression of DC-SIGN in FL lymph node was assessed by immunohistochemistry using anti-CD209. The results (supplemental Figure 7) show a background of CD20+ FL cells with sinusoidlike structures staining for CD209. There were also some mononuclear cells (arrows) that were CD209+.
Discussion
Normal B cells do not linger in the GC, but go through a ruthless process of antigen selection or death. Selected B cells leave the site and, although revisiting does occur,21 their fate is to become memory B cells or plasma cells. In contrast, FL cells remain mainly locked in the GC, partially protected by upregulated BCL-2, and are apparently able to exploit the microenvironment for survival and proliferation. Candidate-supporting cells are likely to be multiple, but appear to include IL-4–producing T-follicular helper cells, tumor-associated macrophages, and stromal cells.22
The distribution and number of macrophages varies in FL, but an overall increase has been associated with a poorer prognosis.23,24 One problem of assessing a prognostic role is that pathogenesis and response to therapy both influence clinical outcome, possibly in opposite directions. Thus macrophages may promote tumor growth but, as mediators of antibody-dependent cellular cytotoxicity, they are required for antibody therapy.25
The positively selected expression of high mannose in the sIg variable regions is a clue to pathogenesis,26 and strongly indicates an essential interaction with a presumed lectin in the lymph node. It appears that mannosylation motifs are acquired at the early stage of lymphomagenesis, termed “follicular lymphoma in situ.”27 Although we cannot be certain which lectin is involved, we have used DC-SIGN, known to recognize mannoses both on pathogens and on self-ligands,11 as a paradigm to probe the consequences of engaging the mannosylated sIg.
It is clear that DC-SIGN can activate intracellular phosphorylation pathways characteristic of sIg engagement, thereby offering a mechanism for antigen-independent B-cell receptor signaling in FL. However, in contrast to anti-Ig, DC-SIGN, even when multimerized, fails to induce endocytosis. This is consistent with the finding that FL cells, unlike CLL cells, show no evidence for downregulation of sIg in vivo or for the anergic phenotype characteristic of CLL. CLL cells have features of cells that reversibly engage antigen in vivo, apparently leading to endocytosis followed by recovery.16 Because the antigen may be cell-bound,28 CLL cells appear capable of “biting” it off cell surfaces as described for normal B cells.29 None of this occurs in FL, but sIg expression remains essential, as does the upregulation of BCL-2. One possibility is that interaction with a lectin could provide a continuous low-level activatory signal for tumor cells.
Expression of DC-SIGN in FL tissue from our cases confirms a dominating high level on lymphatic sinusoidal structures27 but also indicates expression on other nearby mononuclear cells. Because IL-4 is detected in LN tissue, polarization to the M2 type occurs22 and DC-SIGN is upregulated.30 The situation in vivo is more complex. FL cells could encounter DC-SIGN–expressing macrophages as they crawl through the tissue, or the DC-SIGN on lymphatic endothelium could bind FL cells and block egress, acting as a retention signal. Both adhesion and the signaling pathways activated in vitro were blocked by inhibitors of BTK or SYK. However, although it is likely that continuous stimulation by local autolectins is vital, we have recently shown that lectins from certain bacteria can also induce an i[Ca2+] flux,31 raising the possibility of tumor perturbation during infection.
The ability of receptor-associated glycan to modulate signaling pathways has been revealed by the effects of galectins on T or B lymphocytes.32 In that case, binding of galectin to galactose residues on the receptors generates a “lattice” that affects oligomerization and signaling but prevents endocytosis.33 It appears likely that a parallel array of lectins can target V-region mannoses in sIg and that FL cells have exploited this strategy for survival and proliferation. Opportunities for blocking this interaction include either antibodies against high mannoses, which are being developed for targeting HIV,34 or potentially the clinical-grade galactomannans, aimed to release tumor-infiltrating lymphocytes from galectin-mediated suppression in patients with cancer.35
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The method for measuring binding of DC-SIGN was provided by Drs K. Tarte and R. Amin-Ali (Université de Rennes). The authors thank Drs K. McCann and T. Injelevskaya (University of Southampton) for the IGV sequencing; Gavin Babbage, Lisa Boulter, and Dr Kathy Potter (Tissue Bank, Cancer Sciences, University of Southampton) for the processing and storage of FL samples; the Worldwide Cancer Research, Cancer Research UK, and the ECMC for support; Lynsey Block for help with the manuscript preparation; and Drs M. Cragg and S. Beers for generously providing the AT10 antibody.
Authorship
Contribution: A.L. and S.K. performed research and analyzed data; F.K.S., G.P., S.K., and A.L. designed the research and analyzed data; M.P. performed and interpreted the immunohistochemical data; P.W.J. provided patient samples and analyzed clinical data; F.K.S., G.P., and S.K. wrote the initial draft of the manuscript; and all authors contributed to the modification of the draft and approved the final submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Freda Stevenson, University of Southampton Faculty of Medicine, Southampton General Hospital, Tenovus Laboratory, Tremona Rd, Southampton, SO16 6YD, United Kingdom; e-mail: fs@soton.ac.uk.
References
Author notes
A.L. and S.K. contributed equally to this study.
G.P. and F.K.S. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal