Key Points
Untargeted and targeted metabolomics showed association of low plasma acylcarnitines levels with venous thrombosis risk.
Long-chain acylcarnitines are anticoagulants that inhibit factor Xa by binding to factor Xa outside the γ-carboxy glutamic acid domain.
Abstract
In many patients with deep vein thrombosis and pulmonary embolism (venous thromboembolism, VTE), biomarkers or genetic risk factors have not been identified. To discover novel plasma metabolites associated with VTE risk, we employed liquid chromatography-mass spectrometry-based untargeted metabolomics, which do not target any specific metabolites. Using the Scripps Venous Thrombosis Registry population for a case-control study, we discovered that 10:1 and 16:1 acylcarnitines were low in plasmas of the VTE patient group compared with matched controls, respectively. Data from targeted metabolomics studies showed that several long-chain acylcarnitines (10:1, 12:0, 12:2, 18:1, and 18:2) were lower in the VTE group. Clotting assays were used to evaluate a causal relationship for low acylcarnitines in patients with VTE. Various acylcarnitines inhibited factor Xa-initiated clotting. Inhibition of factor Xa by acylcarnitines was greater for longer acyl chains. Mechanistic studies showed that 16:0 acylcarnitine had anticoagulant activity in the absence of factor Va or phospholipids. Surface plasmon resonance investigations revealed that 16:0 acylcarnitine was bound to factor Xa and that binding did not require the γ-carboxy glutamic acid domain. In summary, our study identified low plasma levels of acylcarnitines in patients with VTE and showed that acylcarnitines have anticoagulant activity related to an ability to bind and inhibit factor Xa.
Introduction
Deep vein thrombosis and pulmonary embolism (venous thromboembolism, VTE) are common causes of morbidity and mortality.1 Genetic or acquired biomarkers or risk factors have not been identified in many patients with VTE. Thus, there is a major unmet need to identify new biomarkers and new causal risk factors in patients with VTE. Metabolomics is an unexplored frontier for VTE research. Thus, to discover novel plasma metabolite biomarkers for VTE risk, we performed liquid chromatography-mass spectrometry (LC-MS)-based untargeted metabolomics in a pilot study to analyze plasma metabolites without any specific targeting of known metabolites (supplemental Figure 1A, available on the Blood Web site).2-6 One lipid metabolite family, namely, acylcarnitines (ACs), exhibited association with VTE. When AC lipids were tested in coagulation assays for any potential causality relationship, the novel anticoagulant activity of ACs was discovered and was shown to involve targeting of factor Xa.
Materials and methods
Material
See supplemental Materials and Methods.
VTE registry
The Scripps Venous Thrombosis Registry is an ongoing case-control study of risk factors for VTE, as described.7 Inclusion criteria for this study included age at thrombosis younger than 55 years, more than 3 months since diagnosis of acute thrombosis, a life expectancy of at least 3 years and no lipid-lowering medications or cancer. Blood was collected in the General Clinical Research Center at least 3 months after VTE diagnosis and after 12 hours of fasting. Serum and EDTA plasma were prepared, and plasma was stored at −70°C. Age-matched (± 2 years) healthy male controls were recruited through the General Clinical Research Center’s blood donation program at Scripps. Participants in the blood donation program had normal complete blood count and were negative for HIV, hepatitis B, and hepatitis C. Some were from the community, but most were employees or former employees of Scripps. Clinical data collection included detailed medical history and the presence of any known risk factors for VTE. The protocol was approved by the Institutional Review Board of Scripps Clinic, and subjects provided written informed consent. Forty (82%) of 49 patients with VTE presented with idiopathic VTE, defined as events that did not occur within 90 days after surgery, trauma, or major immobilization. In this study, male patients presenting with idiopathic VTE (n = 40) and age-matched controls (n = 40) were analyzed for untargeted metabolomics. Of 40 subjects, 37 were of European ancestry and 3 were of African-American ancestry. The information of medication is presented in supplemental Table 1. Of the 40 patients with VTE, 33 were receiving warfarin treatment at the time of blood draw.
Warfarin cross-over study
The cross-over study design was ON-warfarin treatment and OFF-warfarin treatment, using a 17-patient cross-over cohort (9 male, 8 female; mean age, 58.1 ± 18.6 years; range, 27-83 years).6 Blood draw was performed 4 to 6 weeks after initiation of warfarin (ON-warfarin). Blood draw was also performed approximately 10 days after discontinuation of the clinically determined course of warfarin (OFF-warfarin). Blood was collected after a 12-hour fast, and EDTA or citrated plasma was stored at −80°C. All the protocols for the Scripps Venous Thrombosis Registry, healthy donors, and this warfarin study were approved by the Institutional Review Board, and subjects provided written informed consent.
Untargeted metabolomics
Plasma metabolites were extracted from 50 μL by cold methanol, and the sample was reconstituted in 50 μL 5% acetonitrile in water for LC-MS analysis, using an Agilent quadrupole time-of-flight mass spectrometer.2,3 A capillary LC (Agilent 1200 series) system was used to perform the 60-minute gradient separation on a 0.5-mm Zorbax SB-C18 column at 20 μL/min. The data were analyzed using the XCMS data analysis software4 (https://xcmsonline.scripps.edu/) to perform nonlinear alignment and to calculate P values for a student t test on the 40 VTE and 40 control samples. Because many of the patients with VTE use warfarin, warfarin metabolites and the plasma metabolite features influenced by warfarin use were excluded on the basis of the analyses obtained from a warfarin cross-over study.6 Tandem MS (MS/MS) was used to identify the metabolites by comparison, with high purity standards and the METLIN MS/MS database5 (https://metlin.scripps.edu/index.php). Various chosen peaks were identified by comparison of MS/MS data and LC retention time after the elemental formula was unambiguously determined, using accurate mass data acquired on the Agilent 6520 quadrupole time-of-flight and Bruker Apex (7.0 Tesla) Fourier-transform ion cyclotron resonance mass spectrometers with internal calibration.
Plasma AC targeted analysis
We performed plasma AC metabolites extraction and protein precipitation by adding 400 μL cold methanol to 100 μL plasma. Samples were then vortex-mixed and stored at −20°C for 1 to 2 hours. The pellet was removed by centrifuging at 13 000 rpm for 10 minutes at 4°C. The supernatant was removed to a clean tube, and the samples were dried in a SpeedVac to dryness. We added 50 μL of acetonitrile/water (5:95), followed by vortex-mixing and sonication in a bath sonicator for 5 minutes. After centrifuge at 13 000 rpm for 10 minutes at 4°C, the supernatant was transferred to LC vials for analysis. LC-electrospray ionization (ESI)/MS/MS Triple Quad analysis was performed to measure plasma ACs. We injected 8 μL processed plasma for each run. Reverse phase chromatography was performed using a 150 × 0.5 mm (diameter) Zorbax C18 column (Agilent) with 5-μm particles at a flow rate of 20 μL/min. Buffer A was water with 0.1% formic acid, and buffer B was acetonitrile with 0.1% formic acid. The column was equilibrated in 5% B for 5 minutes, and the gradient was 5% to 95% B (5-55 minutes). MS was performed in positive ion electrospray (ESI+) modes. The capillary voltage was 3.5 kV, the fragmentor voltage was 135 V, and the collision energy was 4 to 24 V. Multiple reaction monitoring or dynamic range multiple reaction monitoring were used as scan type for collecting AC metabolites data. To convert the obtained mass intensity into the absolute concentrations of 10:0-AC, 10:1-AC, and 10:2-AC (µM), purified 10:0-AC was employed as a standard. Similarly, 12:0-AC, 14:0-AC, 16:0-AC, and 18:0-AC were used as standards to calculate the concentrations for the isomers of 12-AC, 14-AC, 16-AC, and 18-AC, respectively. Plasma samples from the same 17 subjects on and off warfarin therapy (warfarin cross-over study6 ) were also analyzed for the targeted plasma ACs to identify warfarin metabolites.
Preparation of acylcarnitine solutions
ACs were dissolved in water (stock solution: final 5 mM), and the stock solutions were diluted using Tris buffed saline (50 mM Tris⋅HCl at pH 7.4 and 150 mM NaCl; TBS) containing 0.6% bovine serum albumin (BSA).
Clotting assays
The anticoagulant properties of 16:0-AC and acetyl-carnitine were determined using factor Xa-initiated, tissue factor-initiated, and thrombin-initiated clotting assays. Briefly, 7.5 µL pooled plasma was mixed with varying amounts of 16:0-AC or acetyl-carnitine at varying doses and fibrinogen (0.6 mg/mL, final) to give a total volume of 87.5 µL, and then incubated for 3 minutes at 37°C. After the addition of 50 µL containing factor Xa (0.9 nM, final) or 1:60 dilution of recombinant human tissue factor (Innovin) or thrombin (0.43 units/mL, final) containing 30 mM CaCl2, clotting times were recorded using an Amelung KC4 micro coagulometer (Sigma Diagnostics). In some experiments, we reconstituted human factor X-deficient plasma with purified factor X, and clotting was started by addition of Russell's viper venom factor X activator (RVV-X) with 30 mM CaCl2 in the presence of various concentrations of ACs.
Lipid vesicles
Small unilamellar vesicles of phosphatidylcholine/phosphatidylserine (PCPS; 9:1 wt/wt) in TBS were prepared by sonication under a flow of nitrogen, using a microtip sonicator.
Chromogenic substrates
Thrombin or factor Xa amidolytic activities were measured by S2238 or S2222 hydrolysis. The effect of 16:0-AC on factor Xa and thrombin amidolytic activities was tested using factor Xa and thrombin chromogenic substrates S2222 (0.33 mM final) and S2238 (0.33 mM final), respectively.
Activation of prothrombin by prothrombinase complex
The effect of 16:0-AC and acetyl-carnitine on prothrombin activation by prothrombinase was measured in a 2-stage assay. The first step involved the generation of thrombin, which was subsequently measured in the second step, using the thrombin chromogenic substrate S2238 (DiaPharma). The amounts of factor Xa, phospholipids, and prothrombin present in the assay were such that the rate of thrombin generation was linearly proportional to the amount of factor Va present in the reaction mixture. In a typical experiment, varying concentrations of 16:0-AC were incubated with factor Va (0.2 nM, final) in TBS-BSA plus 5 mM CaCl2, prothrombin (0.75 µM final) and PCPS vesicles (6 µM final) in TBS-BSA. Thrombin generation was initiated by the addition of factor Xa (0.1 nM, final). After detectable thrombin was generated, the reaction was quenched by EDTA, and the rate of thrombin formation was quantified by measuring thrombin concentration as the rate of substrate (S2238) hydrolysis. Prothrombin activation in the absence of phospholipids was also determined using factor Xa alone or factor Xa plus factor Va. In the assay with factor Xa plus factor Va, prothrombin was mixed with various concentrations of 16:0-AC at room temperature and then incubated with factor Xa (0.75 nM, final) in the presence or absence of factor Va (15.4 nM, final) for 5 or 120 minutes, respectively. In separate experiments with engineered clotting factors, either DG-prothrombin or DG-factor Xa was used in place of prothrombin or factor Xa, following similar protocols. After detectable thrombin was generated, the prothrombinase reaction was quenched by EDTA, and the rate of thrombin formation was determined. One notes that the critical micelle concentration even in the absence of carrier protein for 16:0 AC is 75 µM (http://avantilipids.com/index.php?option=com_content&view=article&id=2107&Itemid=566&catnumber=870851), and inhibition of prothrombinase activity occurred below this concentration.
Sensorgrams for 16:0-AC interaction with biotinylated proteins coupled to the streptavidin sensor chip
BEGR is a rapidly acting irreversible covalent factor Xa inhibitor. It was used to label the active site of purified factor Xa and factor VIIa (Enzyme Research Laboratories) under manufacturer conditions. A Biacore 3000 instrument was used to monitor binding of 16:0-AC to clotting factors that were captured onto a sensor chip. (See supplemental Materials and Methods for detail.)
Statistical analysis
Statistical analyses including 2-tailed Mann Whitney test and 2-tailed Spearman with 95% confidence interval were performed using Prism 4.03 software (Graph Pad Software Inc.). Prism 4.03 software was used to calculate the odds ratio (OR) for the VTE risk, based on the odds of a VTE event occurring in each of 2 groups; namely, subjects with ACs levels either below the 10th percentile or above the 10th percentile of AC level in control group. The significance of the VTE association with low ACs or categorical parameters listed in supplemental Table 1 was determined using the 2-tailed Fisher’s exact probability test. The difference was considered significant when P was <.05.
Results
Untargeted metabolomics
Untargeted metabolomics data2-5 for 40 male idiopathic adult VTE cases and 40 age-matched male controls (supplemental Table 1) recorded 9400 metabolic features (supplemental Figure 1) for each plasma sample and identified 257 features that had a significant difference between controls and patients with VTE (P < .05, student t-test). Almost 90% of these 257 features were from warfarin-associated metabolites that were previously identified in our warfarin cross-over metabolomics study.6 After warfarin-related metabolite features were eliminated, intensity values for 6 features were significantly higher, whereas intensities for 22 features were significantly lower for patients with VTE compared with controls (supplemental Figure 2). Among those 28 metabolic features, 2 metabolic features with 398.3256 m/z eluting at 39.9 minutes and 314.2326 m/z eluting at 28.7 minutes that were decreased in patients with VTE by 1.7- and 1.6-fold (P = .003 and .02), respectively (Table 1), potentially belonged to the long-chain AC family; namely, palmitoleoyl-carnitine (16:1) and decenoyl-carnitine (10:1), respectively. These ions were confirmed as 16:1-AC and 10:1-AC on the basis of the fragmentation pattern obtained by MS/MS analysis, using 16:0-AC and 10:0-AC as standard, respectively (supplemental Figure 3). Data for the other 26 significantly different features are shown in supplemental Figure 2, where the exact mass and a list of potential metabolites are given. Further identification of these features showing differences was not completed.
Two long-chain ACs were identified as VTE-associated metabolites by untargeted metabolomics
| Name . | Molecular formula . | Molecular mass . | MH+ calculated . | m/z observed . | Retention time, min . | Fold change . | P value . |
|---|---|---|---|---|---|---|---|
| Palmitoleoyl carnitine | C23H43NO4 | 397.3192 | 398.3265 | 398.3340 | 39.9 | 1.7 ↓ | .003 |
| Decenoyl carnitine | C17H31NO4 | 313.2253 | 314.2326 | 314.2337 | 28.7 | 1.6 ↓ | .02 |
| Name . | Molecular formula . | Molecular mass . | MH+ calculated . | m/z observed . | Retention time, min . | Fold change . | P value . |
|---|---|---|---|---|---|---|---|
| Palmitoleoyl carnitine | C23H43NO4 | 397.3192 | 398.3265 | 398.3340 | 39.9 | 1.7 ↓ | .003 |
| Decenoyl carnitine | C17H31NO4 | 313.2253 | 314.2326 | 314.2337 | 28.7 | 1.6 ↓ | .02 |
Among 9400 features, the untargeted metabolomics identified 2 metabolites from the long-chain AC’s family, based on mass accuracy and retention time, which had significantly different levels between VTE and controls. The fold change reflects the average decrease in VTE subjects compared with controls. The observed and calculated m/z are given. P values were obtained using student t test.
Plasma AC targeted analysis
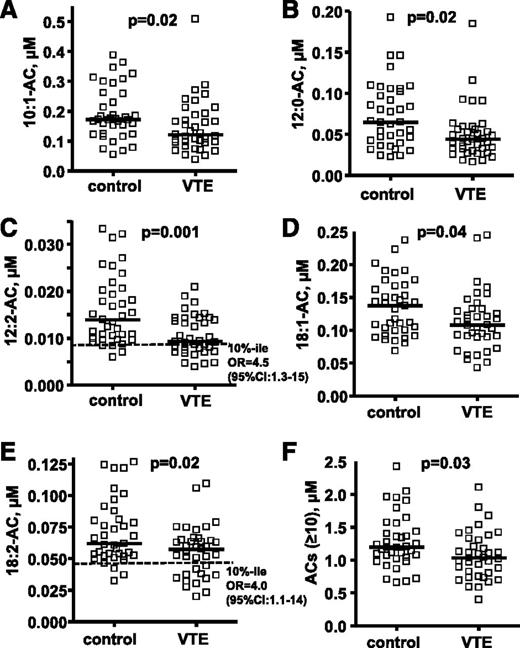
The targeted metabolomics analyses for plasma ACs were performed using optimal LC conditions for long-chain AC analysis to quantify many ACs, including some of the major long-chain ACs (10:0, 12:2, 14:1, 18:1, and 18:2) that could not be detected in the untargeted metabolomics screen. Patients with VTE had significantly lower plasma levels of 10:1-AC, 12:0-AC, 12:2-AC, 18:1-AC, 18:2-AC, and total long-chain ACs (n ≥ 10) than matched controls without VTE (P = .01, .004, .001, .04, .02, and .03, respectively; Figure 1; supplemental Table 2). There was no statistical difference in other AC levels between controls and VTE subjects (supplemental Table 2). The subjects with low levels of 12:2-AC and 18:2-AC level (<10th percentile of control) had increased VTE risk with an OR of 4.5 (95% confidence interval [CI], 1.3-15) and an OR of 4.0 (95% CI, 1.1-14), respectively. There was no statistical difference in 10:0-AC and 16:1-AC levels for controls and VTE, but both showed a trend to be lower in VTE (P = .07 for both ACs).
Targeted metabolomics data for 5 plasma AC levels show differences between 37 patients with VTE and controls. The distribution of plasma AC levels whose median level was significantly lower in patients with VTE compared with controls are shown in (A) 10:1-AC, (B) 12:0-AC, (C) 12:2-AC, (D) 18-1-AC, and (E) 18:2-AC. The sum of concentrations for all ACs with acyl chain length ≥ 10 carbons is shown in F. The plasma levels of ACs are shown as micromoles, and the bar represents the median of each subgroup. The dotted line indicates the 10th percentile of control for each parameter. The difference of median values between VTE patients and controls was calculated by Mann-Whitney test. To evaluate the association of VTE with low AC level (<10th percentile of control), ORs whose values are seen in C and E were calculated according to the odds of VTE occurring in each of 2 groups; namely, those with AC levels either below the 10th percentile or above the 10th percentile of control.
Targeted metabolomics data for 5 plasma AC levels show differences between 37 patients with VTE and controls. The distribution of plasma AC levels whose median level was significantly lower in patients with VTE compared with controls are shown in (A) 10:1-AC, (B) 12:0-AC, (C) 12:2-AC, (D) 18-1-AC, and (E) 18:2-AC. The sum of concentrations for all ACs with acyl chain length ≥ 10 carbons is shown in F. The plasma levels of ACs are shown as micromoles, and the bar represents the median of each subgroup. The dotted line indicates the 10th percentile of control for each parameter. The difference of median values between VTE patients and controls was calculated by Mann-Whitney test. To evaluate the association of VTE with low AC level (<10th percentile of control), ORs whose values are seen in C and E were calculated according to the odds of VTE occurring in each of 2 groups; namely, those with AC levels either below the 10th percentile or above the 10th percentile of control.
Analysis of the targeted metabolomics data set for correlations among long-chain ACs for all subjects showed that plasma levels of 18:2-AC were strongly correlated with those of 18:1-AC, 18:0-AC, 16:1-AC, and 16:0-AC (supplemental Figure 4). This reflects the obvious metabolic relationships among these lipids and helps establish the plausibility of the differences we have discovered. When the plasma levels of these long-chain ACs (16:0, 18:1 and 18:2) were summed, and then those sums were compared for the 37 white patients with VTE vs controls, 7 of 37 patients with VTE had a value below that of the lowest control, and subjects with these very low ACs were associated with VTE (P = .01, 2-tailed Fisher Exact Probability Test) (supplemental Figure 5).
Effect of warfarin on plasma ACs
Because a significant number of patients with VTE were under warfarin treatment, we tested the effect of warfarin on plasma levels of ACs, using plasma samples of the warfarin cross-over study6 (n = 17). There was no statistically significant difference for plasma levels of long-chain ACs (10:0, 10:1, 10:2, 10:3, 12:0, 12:1, 12:2, 14:0, 14:1, 14:2, 16:0, 16:1, 18:0, 18:1, 18:2) between ON-warfarin and OFF-warfarin samples for subjects in our cross-over study (P > .05, 2-tailed paired t-test) (data not shown).
Effect of ACs on plasma clotting times
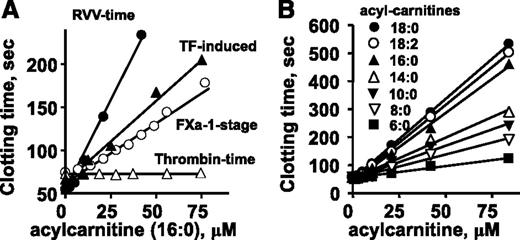
To test the effect of 16:0-AC on plasma clotting reactions, tissue factor (TF)-induced (ie, the prothrombin time assay) and factor Xa-induced clotting assays were performed. 16:0-AC prolonged clotting in both TF-induced and factor Xa-dependent clotting times (Figure 2A). Thrombin-induced clotting of plasma (ie, the thrombin time assay) was not affected by 16:0-AC (Figure 2A), indicating 16:0-AC inhibits the activation of prothrombin, but not the clotting activity of thrombin on fibrinogen. Acetyl-carnitine with a 2-carbon acyl moiety did not prolong clotting times in any of these clotting assays (data not shown).
The anticoagulant effects of long-chain ACs in different clotting assays were determined. Effects of various doses of 16:0-AC are shown for clotting assays in which clotting was induced by endogenously generated factor Xa (RVV-X activated), exogenously added factor Xa, diluted TF, or thrombin. (B) Effect of length of aliphatic side chain of ACs (6:0, 8:0, 10:0, 14:0. 16:0, 18:0, and 18:2) on their anticoagulant activity, measured using RVV-X clotting time.
The anticoagulant effects of long-chain ACs in different clotting assays were determined. Effects of various doses of 16:0-AC are shown for clotting assays in which clotting was induced by endogenously generated factor Xa (RVV-X activated), exogenously added factor Xa, diluted TF, or thrombin. (B) Effect of length of aliphatic side chain of ACs (6:0, 8:0, 10:0, 14:0. 16:0, 18:0, and 18:2) on their anticoagulant activity, measured using RVV-X clotting time.
To probe structure-activity relationships for AC’s anticoagulant activity, lipids with variation in aliphatic chain length and saturation were tested for anticoagulant activity. 18:0-AC and 18:2-AC had similar anticoagulant properties as 16:0-AC in plasma clotting assays, whereas shorter-chain ACs (C14:0, C10:0, C8:0, C6:0) had less anticoagulant potency in a chain length-dependent fashion (Figure 2B).
AC effect on purified prothrombin activation by factor Xa
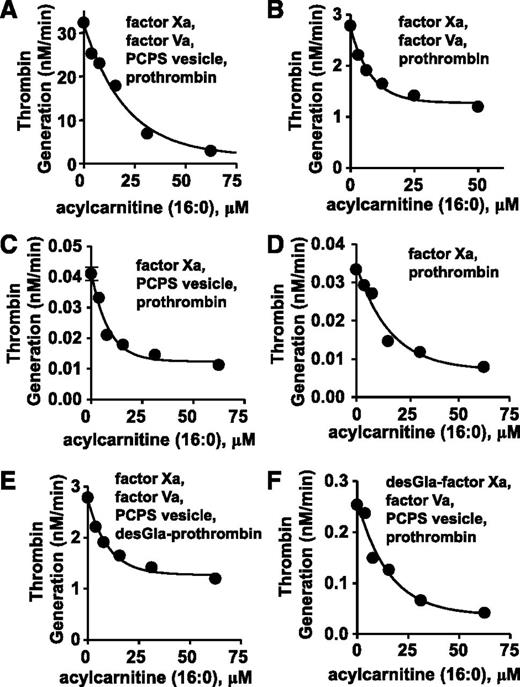
To elucidate mechanisms for anticoagulant activity of long chain-ACs, 16:0-AC was tested for inhibitory activity in purified prothrombinase systems containing prothrombin, factor Xa, factor Va, and/or phospholipid vesicles (PCPS 90:10 wt/wt). In a standard purified prothrombinase system, thrombin generation was inhibited by 16:0-AC (50% inhibition/inhibitory concentration [IC50] = 13 µM) (Figure 3A). For reactant mixtures lacking either phospholipids or factor Va, prothrombin activation by factor Xa was dose-dependently inhibited by 16:0-AC (IC50 = 5.6 µM and 5.8 µM, respectively; Figure 3B-C). Prothrombin activation by factor Xa in the absence of both factor Va and phospholipids was inhibited by 16:0-AC (IC50 = 11 µM; Figure 3D). γ-carboxyglutamic acid (Gla)-domainless (DG)-prothrombin activation by factor Xa and prothrombin activation by DG-factor Xa were each similarly inhibited by 16:0-AC with IC50 values of 11 and 7.0 μM, respectively (Figure 3E-F). In controls, 16:0-AC did not inhibit the amidolytic activity of either factor Xa or thrombin (supplemental Figure 7), indicating the lipid does not inhibit the enzymatic activity.
The anticoagulant effects of 16:0 AC in prothrombinase assay were determined. (A) The effect of 16:0-AC on prothrombin activation by factor Xa, factor Va, and PCPS vesicles. (B) The effect of 16:0-AC on prothrombin activation by factor Xa and factor Va. (C) The effect of 16:0-AC on prothrombin activation by factor Xa and PCPS vesicles. (D) The effect of 16:0-AC on prothrombin activation by factor Xa. (E) The effect of 16:0-AC on desGla-prothrombin activation by factor Xa, factor Va, and PCPS vesicle. (F) The effect of 16:0-AC on prothrombin activation by desGla-factor Xa, factor Va, and PCPS vesicle.
The anticoagulant effects of 16:0 AC in prothrombinase assay were determined. (A) The effect of 16:0-AC on prothrombin activation by factor Xa, factor Va, and PCPS vesicles. (B) The effect of 16:0-AC on prothrombin activation by factor Xa and factor Va. (C) The effect of 16:0-AC on prothrombin activation by factor Xa and PCPS vesicles. (D) The effect of 16:0-AC on prothrombin activation by factor Xa. (E) The effect of 16:0-AC on desGla-prothrombin activation by factor Xa, factor Va, and PCPS vesicle. (F) The effect of 16:0-AC on prothrombin activation by desGla-factor Xa, factor Va, and PCPS vesicle.
Surface plasmon resonance binding studies
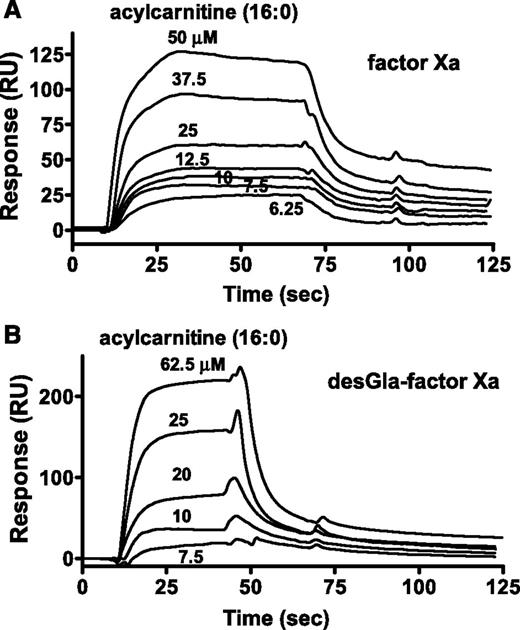
For surface plasmon resonance (SPR) binding studies, biotinylated-glutamyl-glycyl-arginyl-chloromethyl ketone (BEGR) was used to label the active site of factors Xa, DG-factor Xa, factor IXa, and VIIa. 16:0-AC was bound by BEGR-factor Xa and BEGR-DG-factor Xa with similar affinities (10 µM and 23 µM, respectively), whereas no binding of 16:0-AC (60 μM) to BEGR-factor VIIa or BEGR-factor IXa was observed (data not shown). The kinetics of 16:0-AC binding to BEGR-factor Xa and BEGR-DG-factor Xa showed similar binding kinetics and Kd (kon = 5.44 and 3.09 × 103 M−1⋅s−1; koff = 0.0535 and 0.0705 × s−1, respectively; Figure 4A-B; supplemental Table 3). Acetyl carnitine (125 μM) did not show detectable binding to BEGR-factor Xa (data not shown).
The binding of 16:0 AC to factor Xa was determined. SPR was used to monitor binding of 16:0-AC to BEGR-factor Xa and BEGR-DG-factor Xa. (A) Sensorgram depicting the dose-dependent binding of 16:0-AC (from top to bottom; 50, 37.5, 25, 12.5, 10, 7.5, and 6.25 µM) to BEGR-factor Xa. (B) Sensorgram depicting the dose-dependent binding of 16:0-AC (from top to bottom; 62.5, 25, 20, 10, and 7.5 µM) to BEGR-DG-factor Xa. PCPS vesicles exhibited binding to BEGR-factor Xa, but not to BEGR-DG-factor Xa, indicating the proper coupling of the biotinylated proteins (data not shown).
The binding of 16:0 AC to factor Xa was determined. SPR was used to monitor binding of 16:0-AC to BEGR-factor Xa and BEGR-DG-factor Xa. (A) Sensorgram depicting the dose-dependent binding of 16:0-AC (from top to bottom; 50, 37.5, 25, 12.5, 10, 7.5, and 6.25 µM) to BEGR-factor Xa. (B) Sensorgram depicting the dose-dependent binding of 16:0-AC (from top to bottom; 62.5, 25, 20, 10, and 7.5 µM) to BEGR-DG-factor Xa. PCPS vesicles exhibited binding to BEGR-factor Xa, but not to BEGR-DG-factor Xa, indicating the proper coupling of the biotinylated proteins (data not shown).
Discussion
Untargeted metabolomics data analysis of plasma samples identified 28 features that were different (P < .05) in patients with VTE compared with controls. Two plasma long-chain ACs (10:1 and 16:1) were decreased, suggesting an association of decreased plasma long-chain ACs levels with VTE risk. It is noteworthy that not 1, but 2 of the 28 hits belonged to the same long-chain acylcarnitine family. This finding led us to hypothesize that plasma AC levels may be associated with the risk for VTE and to test 15 long-chain ACs with optimal LC conditions for the acylcarnitine family. LC-MS-based targeted metabolomics was used to determine long-chain ACs levels using a European ancestry subset of 37 male VTE cases and 37 matched controls. Three subjects with African-American ancestry were excluded from the analysis because of genetic variations in mitochondrial β-oxidation enzymes related to ACs metabolism.8 Targeted metabolomics data showed that 5 of 15 long-chain ACs (10:1, 12:0, 12:2, 18:1, and 18:2) were lower in plasmas from patients with VTE than in control plasmas (Figure 1; supplemental Table 2).
ACs that heretofore had no known function in blood coagulation reactions consist of a hydrophobic side chain of an acyl moiety that is linked to the carnitine moiety (supplemental Figure 3). ACs circulate in plasma9-12 and play key roles in mitochondrial energy metabolism. Studies were initiated to define procoagulant or anticoagulant properties of ACs. ACs inhibited factor Xa-initiated clotting assays involving addition of purified factor Xa or the factor X activator, RVV-X, to plasma. The anticoagulant activities of various ACs were concentration-dependent and acyl chain length-dependent, such that ACs with longer acyl chains were more potent anticoagulants than ACs with shorter acyl chains (18, 16 > 14, 10 > 6 acyl chain carbons). However, thrombin-induced clotting was not inhibited by ACs, suggesting ACs were acting on the prothrombinase complex.
Total long-chain acylcarnitines (acyl chains ≥ 10 carbons) circulate in plasma, with reported concentrations ranging between 1 and 4 µM,9-11 which agrees with the current report’s plasma levels of 0.4 to 2.5 μM (Figure 1F); moreover, their levels can reach to 10 to 30 µM under certain metabolic conditions.12-15 The positive correlation of long-chain acylcarnitines between serum levels and levels in myocardial cells,11 and the increase of the long-chain acylcarnitines in acute ischemic myocardium (>10 times),16 suggest an increase of long-chain acylcarnitines in ischemic conditions or other metabolic states. The increase of long-chain acylcarnitines after heart transplantation may be an example of this case (eg, >5 µM of total long-chain acylcarnitines).11 Long-chain acylcarnitines could be richer in blood clots where whole-blood components are accumulated; of relevance is the fact that long-chain acylcarnitines in whole blood may be higher than in plasma by ∼5-fold.17,18 Because the effect of acylcarnitines is seen from 2.5 to 5.0 μM in our plasma clotting assays (Figure 2; supplemental Figure 6), the anticoagulant effect of long-chain acylcarnitines could be physiologically relevant, especially when considering concentrations in the local milieu.
Plasma long-chain AC concentrations were well correlated with each other (eg, 18:2-AC correlation with other ACs; supplemental Figure 4). Patients with VTE had significantly lower levels of multiple long-chain ACs (16:0, 16:1, 18:1, and 18:2) with values below the 20th percentile of controls, as seen in supplemental Figure 4, with dotted ellipses, indicating that some patients have multiple AC deficiencies. Moreover, when the signal intensities of 18:2-AC, 18:1-AC, and 16:0-AC, which are the 3 most abundant long-chain plasma ACs, were summed, the summed values for 7 (19%) of 37 patients with VTE were lower than the lowest summed value of any 1 of the control subjects (n = 37; P = .01 by 2-tailed Fisher exact probability test; supplemental Figure 5). These findings suggest that some patients with VTE have a deficiency of plasma anticoagulant lipids resulting from a deficiency of multiple long-chain ACs. However, the limited number of subjects in this pilot study requires future replication studies to confirm and clarify the significance of this finding.
To elucidate mechanisms for anticoagulant activity of long chain-ACs, 16:0-AC was tested for its inhibitory activity in purified prothrombinase systems. Our results indicated that neither factor Va nor phospholipid was required for the anticoagulant property of 16:0-AC, and that this lipid did not inhibit the enzyme active site of factor Xa or thrombin, suggesting this lipid disrupts interactions between factor Xa and prothrombin. Although canonical coagulation paradigms emphasize key roles for lipid-binding sites that are localized in the amino terminal Gla domain of vitamin K-dependent clotting factors, Gla-DG-prothrombin activation by factor Xa and prothrombin activation by DG-factor Xa were each similarly inhibited by 16:0-AC (IC50 values of 11 and 7.0 μM, respectively). Further, SPR studies showed that 16:0-AC bound to factor Xa and to DG-factor Xa with similar affinities (10 and 23 µM, respectively). Thus, these prothrombinase inhibition data and SPR binding data indicate that 16:0-AC binding sites on factor Xa are located outside the Gla domain at binding sites that likely mediate this lipid’s anticoagulant activity.
The long-standing paradigm for such interactions posits that a phosphatidylserine-containing phospholipid membrane provides a surface for the assembly of clotting factor complexes.19,20 Specifically, complex formation involves binding of the Gla-containing domains of clotting factors to membrane surfaces. As an exception, short-chain soluble PS (dicaproyl-PS) is reported to bind to factor Xa and enhance prothrombinase activity with unique binding sites localized in the epidermal growth factor-like and catalytic domains, which are distinct from the N-terminal Gla domain.21-24 However, this soluble lipid is a nonphysiological compound, and it requires 0.2 to 2 mM to exert procoagulant activity.21-24 So the current and long-standing general paradigm emphasizes that interactions of lipids with vitamin K-dependent coagulation factors involves the N-terminal Gla domain. Our finding that the physiologically available lipid, 16:0-AC, binds to the DG factor Xa with the functional consequences of inhibiting thrombin generation is unique and may point to the existence of other functionally significant lipid-binding sites on clotting factors outside their Gla domains.
In summary, first, untargeted and targeted metabolomics data for a pilot VTE case-control study showed that lower plasma AC levels are associated with VTE, and second, mechanistic studies show that the AC, 16:0 acyl-carnitine, has anticoagulant activity in the absence of factor Va or phospholipids that is related to its ability to bind to factor Xa outside its N-terminal Gla domain.
Presented in abstract form at the 56th annual meeting of the American Society of Hematology, San Francisco, CA, December 7, 2014.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Lacthu Tonnu and Phuong M. Nguyen for skillful technical assistance.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants HL021544 (to J.H.G.) and HL101034 (to J.H.G. and H.D.), and UL1RR025774 (Clinical and Translational Science Award, principal investigator: Eric Topol, Scripps).
Authorship
Contribution: J.H.G and H.D. participated in the conception of the study. G.S., S.T., E.K, L.H, and M.T were responsible for untargeted and targeted metabolomics analysis. The statistical analysis was performed by H.D. Y.B., J.A.F., H.D., and S.Y. were responsible for performing experiments using purified proteins (Y.B., J.A.F., and S.Y.) and plasmas (Y.B., J.A.F., and H.D.). D.J.E. was responsible for organizing the Scripps VTE Registry, consenting the patients, and obtaining blood specimens. H.D., Y.B., and J.H.G. were responsible for writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for Y.B. is Department of Biochemistry, Sultan Qaboos University, Muscat, Oman. The current affiliation for S.T. is Small Molecule Mass Spectrometry Facility, Faculty of Arts & Sciences Division of Science, Harvard University, Cambridge, MA. The current affiliation for S.Y. is Bayer HealthCare Pharmaceuticals, US Innovation Center, San Francisco, CA.
Correspondence: John H. Griffin, Department of Molecular and Experimental Medicine, The Scripps Research Institute, MEM180, 10550 North Torrey Pines Rd, La Jolla, CA 92037; e-mail: jgriffin@scripps.edu.
References
Author notes
H.D. and Y.B. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal